The Disproportionate Increase of the Intraoperative Flexion and Extension Gap Space after Posterior Cruciate Ligament Resection in Total Knee Arthroplasty
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Enrollment of the Participants
2.2. Surgical Interventions
2.3. Data Collection of Image Parameters
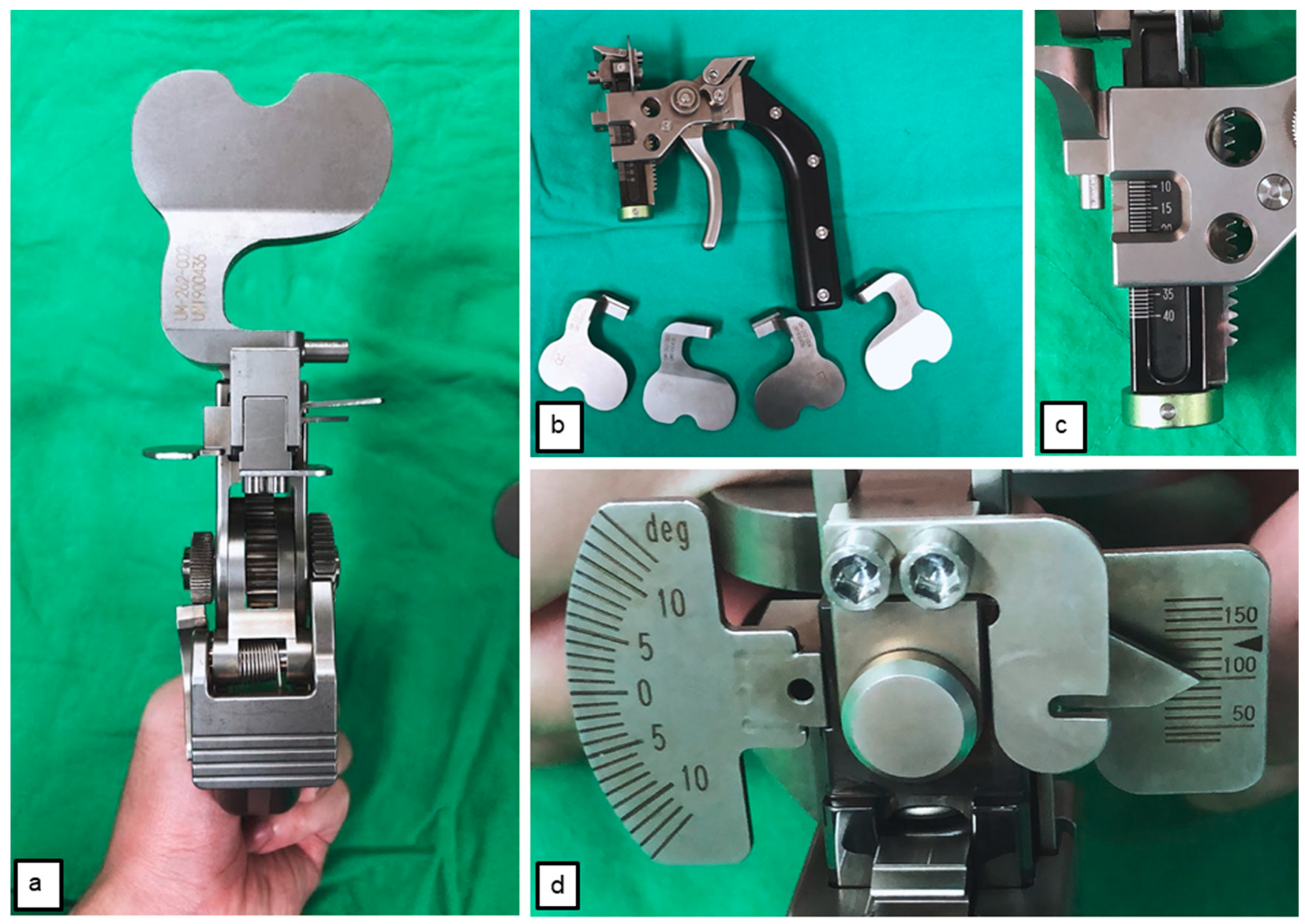
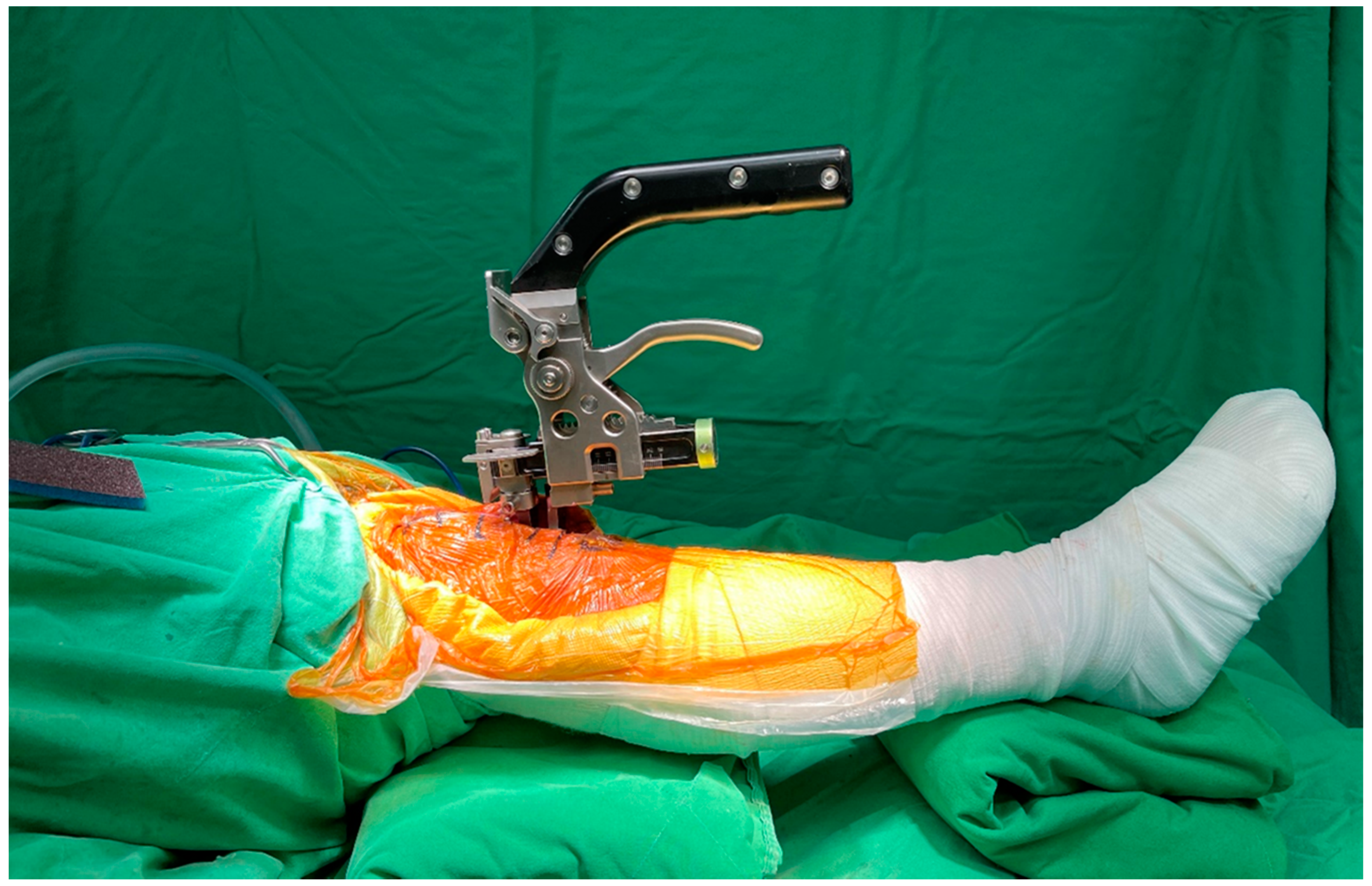
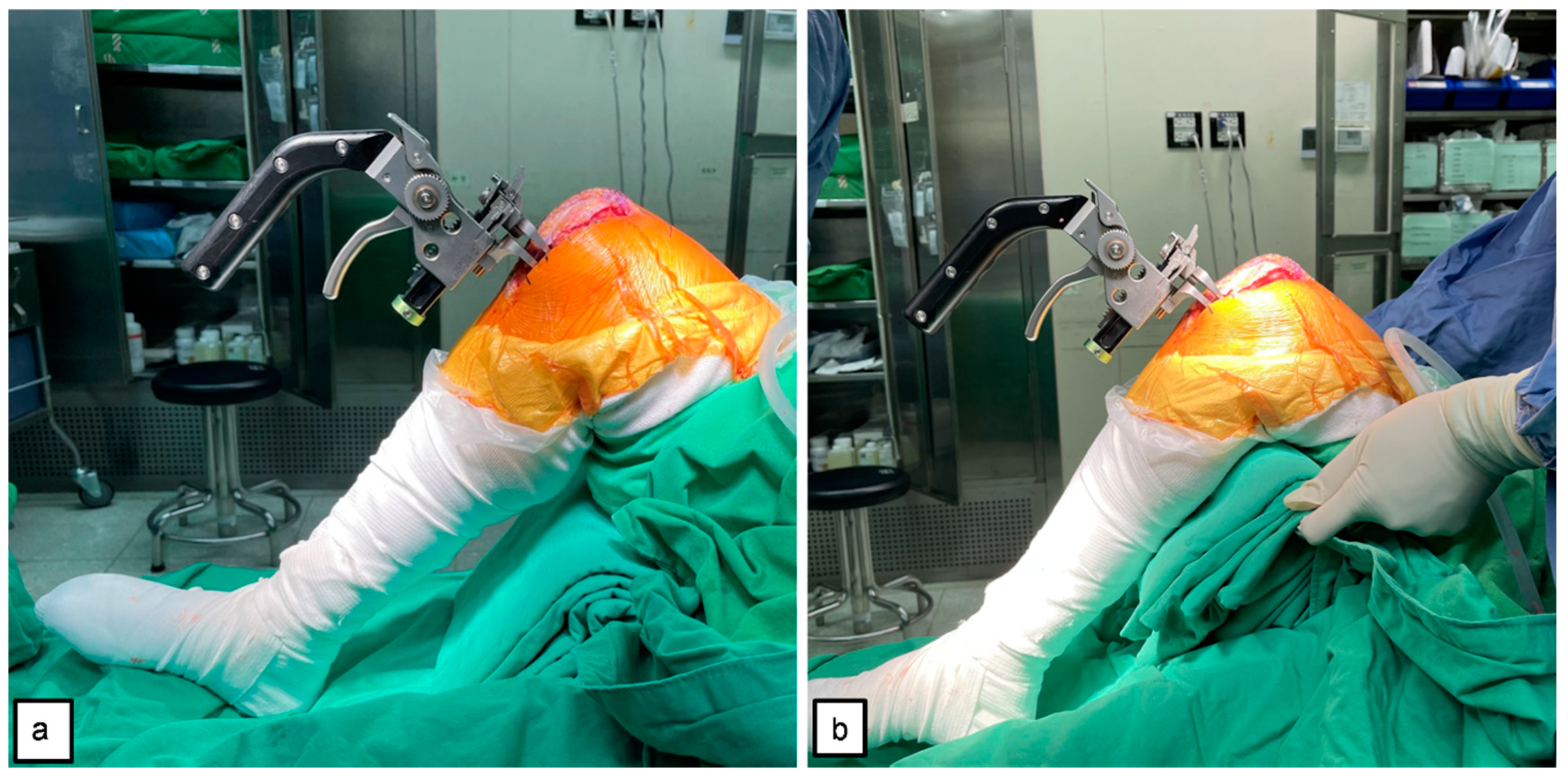
2.4. Tensioner Measurements for Extension–Flexion Gaps
2.5. Data Analysis
3. Results
3.1. Demographics and Characteristics of Enrolled Cases
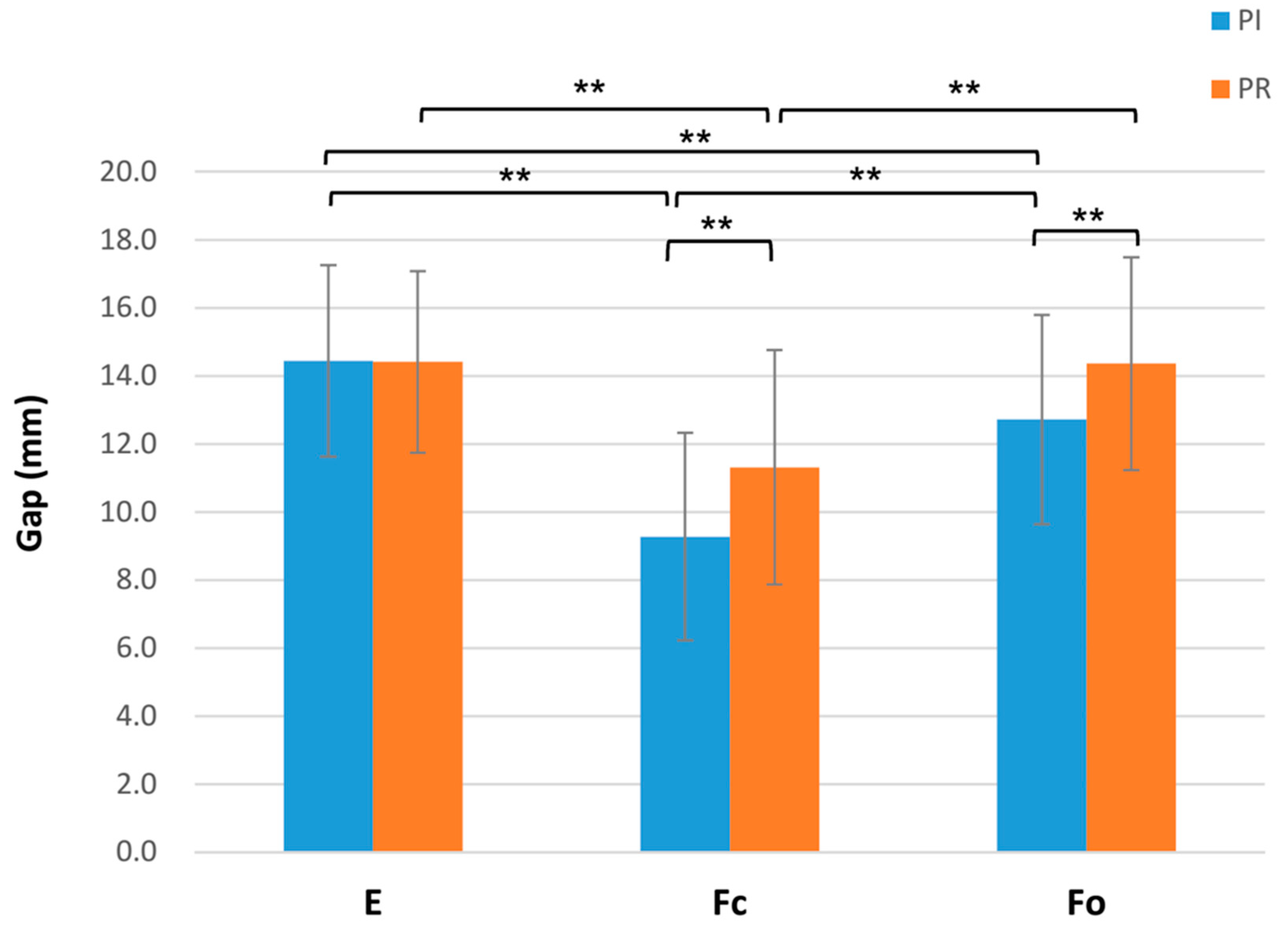
3.2. Gap Space Changes before and after PCL Resection
3.3. Factors Correlated to Flexion Gap Changes with PCL Resection
3.4. Intraclass Correlation (ICC) and Interclass Correlation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbour, K.E.; Moss, S.; Croft, J.B.; Charles, G.; Helmick Kristina, A.; Teresa, J.; Brady Louise, B.; Murphy Jennifer, M.; Hootman Kurt, J.; Greenlund Hua Lu, M.S.; et al. Geographic variations in arthritis prevalence, health-related characteristics, and management—United States, 2015. MMWR Surveill. Summ. 2018, 67, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losina, E.; Thornhill, T.S.; Rome, B.N.; Wright, J.; Katz, J.N. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J. Bone Jt. Surg. Am. 2012, 94, 201. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.M.; Ritter, M.A.; Davis, K.E. Coronal alignment in total knee arthroplasty: Just how important is it? J. Arthroplast. 2009, 24, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Yercan, H.S.; Selmi, T.A.S.; Sugun, T.S.; Neyret, P. Tibiofemoral instability in primary total knee replacement: A review, part 1: Basic principles and classification. Knee 2005, 12, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Fehring, T.K.; Odum, S.; Griffin, W.L.; Mason, J.B.; Nadaud, M. Early failures in total knee arthroplasty. Clin. Orthop. Relat. Res. 2001, 392, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Insall, J.; Tria, A. The total condylar prosthesis type II. Orthop. Trans. 1979, 3, 300–301. [Google Scholar]
- Schnurr, C.; Eysel, P.; Konig, D.P. Is the effect of a posterior cruciate ligament resection in total knee arthroplasty predictable? Int. Orthop. 2012, 36, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Kinsey, T.L.; Mahoney, O.M. Balanced flexion and extension gaps are not always of equal size. J. Arthroplast. 2018, 33, 1062–1068.e5. [Google Scholar] [CrossRef] [Green Version]
- Daines, B.K.; Dennis, D.A. Gap balancing vs. measured resection technique in total knee arthroplasty. Clin. Orthop. Surg. 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Li, N.; Tan, Y.; Deng, Y.; Chen, L. Posterior cruciate-retaining versus posterior stabilized total knee arthroplasty: A meta-analysis of randomized controlled trials. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 556–564. [Google Scholar] [CrossRef]
- Conditt, M.A.; Noble, P.C.; Bertolusso, R.; Woody, J.; Parsley, B.S. The PCL significantly affects the functional outcome of total knee arthroplasty. J. Arthroplast. 2004, 19, 107–112. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Z.; Wang, Y.; Bian, Y.; Feng, B.; Weng, X. Posterior Cruciate Ligament Retention versus Posterior Stabilization for Total Knee Arthroplasty: A Meta-Analysis. PLoS ONE 2016, 11, e0147865. [Google Scholar] [CrossRef]
- Ishii, Y.; Noguchi, H.; Takeda, M.; Sato, J.; Toyabe, S.-I. Prediction of range of motion 2 years after mobile-bearing total knee arthroplasty: PCL-retaining versus PCL-sacrificing. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 2002–2008. [Google Scholar] [CrossRef]
- Hino, K.; Ishimaru, M.; Iseki, Y.; Watanabe, S.; Onishi, Y.; Miura, H.; Khanduja, V. Mid-flexion laxity is greater after posterior-stabilised total knee replacement than with cruciate-retaining procedures: A computer navigation study. Bone Jt. J. 2013, 95, 493–497. [Google Scholar] [CrossRef]
- Kayani, B.; Konan, S.; Horriat, S.; Ibrahim, M.S.; Haddad, F.S. Posterior cruciate ligament resection in total knee arthroplasty: The effect on flexion-extension gaps, mediolateral laxity, and fixed flexion deformity. Bone Jt. J. 2019, 101, 1230–1237. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, W.J.; Nagamine, R. Comparative evaluation of posterior cruciate ligament in total knee arthroplasty. J. Orthop. Surg. 2017, 25, 2309499017690976. [Google Scholar] [CrossRef] [Green Version]
- Matthews, J.; Chong, A.; McQueen, D.; Guinn, J.O.; Wooley, P. Flexion–extension gap in cruciate-retaining versus posterior-stabilized total knee arthroplasty: A cadaveric study. J. Orthop. Res. 2014, 32, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Matziolis, G.; Perka, C. Primary resection of the posterior cruciate ligament does not produce a gap mismatch in the navigated gap technique. Orthopedics 2010, 33, 68–70. [Google Scholar] [CrossRef] [Green Version]
- Baldini, A.; Scuderi, G.R.; Aglietti, P.; Chalnick, D.; Insall, J.N. Flexion—Extension Gap Changes During Total Knee Arthroplasty–Effect of Posterior Cruciate Ligament and Posterior Osteophytes Removal. J. Knee Surg. 2004, 17, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, T.A.; Perry, K.I.; Dobbin, E.; Howard, J.; Lanting, B. Effect of Soft Tissue Releases on Joint Space Opening in Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 2912–2916. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Majima, T.; Iizawa, N.; Hoshikawa, N.; Takahashi, K.; Takai, S. The Influence of Posterior Cruciate Ligament Resection on Tibiofemoral Joint Gap in Varus Osteoarthritic Knees. J. Knee Surg. 2020. [Google Scholar] [CrossRef]
- Nowakowski, A.M.; Majewski, M.; Müller-Gerbl, M.; Valderrabano, V. Development of a force-determining tensor to measure “physiologic knee ligament gaps” without bone resection using a total knee arthroplasty approach. J. Orthop. Sci. 2011, 16, 56–63. [Google Scholar] [CrossRef]
- Kızılgöz, V.; Sivrioğlu, A.; Ulusoy, G.; Yıldız, K.; Aydın, H.; Çetin, T. Posterior tibial slope measurement on lateral knee radiographs as a risk factor of anterior cruciate ligament injury: A cross-sectional study. Radiography 2019, 25, 33–38. [Google Scholar] [CrossRef]
- Kadoya, Y.; Kobayashi, A.; Komatsu, T.; Nakagawa, S.; Yamano, Y. Effects of posterior cruciate ligament resection on the tibiofemoral joint gap. Clin. Orthop. Relat. Res. (1976–2007) 2001, 391, 210–217. [Google Scholar] [CrossRef]
- Nowakowski, A.M.; Majewski, M.; Müller-Gerbl, M.; Valderrabano, V. Measurement of knee joint gaps without bone resection:“physiologic” extension and flexion gaps in total knee arthroplasty are asymmetric and unequal and anterior and posterior cruciate ligament resections produce different gap changes. J. Orthop. Res. 2012, 30, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Mihalko, W.M.; Krackow, K.A. Posterior cruciate ligament effects on the flexion space in total knee arthroplasty. Clin. Orthop. Relat. Res. 1999, 360, 243–250. [Google Scholar] [CrossRef]
- Sang-Jin Park, M.; Seon, J.-K.; Park, J.-K.; Soong, E.-K. Effect of PCL on flexion-extension gaps and femoral component decision in TKA. Orthopedics 2009, 32, 22. [Google Scholar] [CrossRef] [PubMed]
- Kaneyama, R.; Higashi, H.; Yoshii, H.; Shiratsuchi, H.; Sasho, T.; Suzuki, T.; Matsuno, Y.; Nagamine, R.; Weijia, C. The Thigh Weight Influence on the Flexion Gap in Total Knee Arthroplasty: A Cadaveric Study. Orthop. Proc. 2017, 99, 46. [Google Scholar]
- Anderson, C.J.; Ziegler, C.G.; Wijdicks, C.A.; Engebretsen, L.; LaPrade, R.F. Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. JBJS 2012, 94, 1936–1945. [Google Scholar] [CrossRef] [Green Version]
- Foge, D.A.; Baldini, T.H.; Hellwinkel, J.E.; Hogan, C.A.; Dayton, M.R. The Role of Complete Posterior Cruciate Ligament Release in Flexion Gap Balancing for Total Knee Arthroplasty. J. Arthroplast. 2019, 34, S361–S365. [Google Scholar] [CrossRef]
| Characteristics | ||
|---|---|---|
| Case numbers | 33 | |
| Age (years) | 69.39 ± 9.34 | |
| Gender, n (%) | ||
| Male | 20 (60.6%) | |
| Female | 13 (39.4%) | |
| Side, n (%) | ||
| Right | 17 (51.5%) | |
| Left | 16 (48.5%) | |
| BMI | 27.64 ± 3.57 | |
| ASA grade, n (%) | ||
| I | 1 (3.0%) | |
| II | 23 (69.7%) | |
| III | 9 (27.3%) | |
| Preoperative HKA, n (%) | ||
| Varus | 27 (81.8%) | |
| Valgus | 6 (18.2%) | |
| Preoperative HKA (degrees) | 4.09 ± 7.22 | |
| Anesthesia, n (%) | ||
| GA | 15 (45.5%) | |
| SA | 18 (54.5%) | |
| Joint Space (mm) | p Value | |
|---|---|---|
| Extension | 0.838 | |
| PI-E | 14.44 ± 2.82 | |
| PR-E | 14.41 ± 2.67 | |
| ΔE (PR-E—PI-E) | −0.03 ± 0.8 | |
| 90° flexion gap with closed chain (Fc) | <0.001 ** | |
| PI-Fc | 9.27 ± 3.05 | |
| PR-Fc | 11.31 ± 3.44 | |
| ΔFc (PR-Fc—PI-Fc) | 2.04 ± 2.06 | |
| 90° flexion gap with open chain (Fo) | <0.001 ** | |
| PI-Fo | 12.72 ± 3.07 | |
| PR-Fo | 14.36 ± 3.13 | |
| ΔFo (PR-Fo—PI-Fo) | 1.64 ± 1.36 | |
| ΔFc (mm) (Mean ± SD) | p Value | ΔFo (mm) (Mean ± SD) | p Value | ||
|---|---|---|---|---|---|
| Gender | 0.274 | 0.406 | |||
| male | 2.36 ± 2.25 | 1.80 ± 1.38 | |||
| female | 1.55 ± 1.68 | 1.38 ± 1.35 | |||
| BMI | 0.920 | 0.289 | |||
| <25 | 2.09 ± 1.21 | 1.19 ± 1.14 | |||
| ≥25 | 2.02 ± 2.28 | 1.79 ± 1.42 | |||
| ASA grade, n (%) | 0.038 * | 0.138 | |||
| I–II | 2.49 ± 2.11 | 1.42 ± 1.37 | |||
| III | 0.84 ± 1.38 | 2.22 ± 1.21 | |||
| Preoperative deformity | 0.361 | 0.326 | |||
| Varus | 2.20 ± 2.05 | 1.76 ± 1.30 | |||
| Valgus | 1.33 ± 2.10 | 1.14 ± 1.64 | |||
| Anesthesia | 0.521 | 0.125 | |||
| GA | 2.30 ± 2.24 | 2.04 ± 1.39 | |||
| SA | 1.83 ± 1.93 | 1.29 ± 1.28 | |||
| ΔFc | ΔFo | |
|---|---|---|
| Age | 0.060 | 0.232 |
| BMI | −0.249 | 0.163 |
| Pre-op. HKA | 0.166 | 0.165 |
| Post-op. HKA | 0.179 | 0.245 |
| Post-op. tibia slope | 0.094 | 0.104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, K.-C.; Shih, H.-T.; Tang, S.-C.; Lee, C.-H.; Liao, W.-J.; Wang, S.-P. The Disproportionate Increase of the Intraoperative Flexion and Extension Gap Space after Posterior Cruciate Ligament Resection in Total Knee Arthroplasty. J. Clin. Med. 2021, 10, 4228. https://doi.org/10.3390/jcm10184228
Tu K-C, Shih H-T, Tang S-C, Lee C-H, Liao W-J, Wang S-P. The Disproportionate Increase of the Intraoperative Flexion and Extension Gap Space after Posterior Cruciate Ligament Resection in Total Knee Arthroplasty. Journal of Clinical Medicine. 2021; 10(18):4228. https://doi.org/10.3390/jcm10184228
Chicago/Turabian StyleTu, Kao-Chang, Han-Ting Shih, Shih-Chieh Tang, Cheng-Hung Lee, Wei-Jen Liao, and Shun-Ping Wang. 2021. "The Disproportionate Increase of the Intraoperative Flexion and Extension Gap Space after Posterior Cruciate Ligament Resection in Total Knee Arthroplasty" Journal of Clinical Medicine 10, no. 18: 4228. https://doi.org/10.3390/jcm10184228
APA StyleTu, K.-C., Shih, H.-T., Tang, S.-C., Lee, C.-H., Liao, W.-J., & Wang, S.-P. (2021). The Disproportionate Increase of the Intraoperative Flexion and Extension Gap Space after Posterior Cruciate Ligament Resection in Total Knee Arthroplasty. Journal of Clinical Medicine, 10(18), 4228. https://doi.org/10.3390/jcm10184228







