High Oxygen Does Not Increase Reperfusion Injury Assessed with Lipid Peroxidation Biomarkers after Cardiac Arrest: A Post Hoc Analysis of the COMACARE Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design, Participants, and Inclusion Criteria
2.2. Blood Samples and Sample Analysis
2.3. Outcome Measures
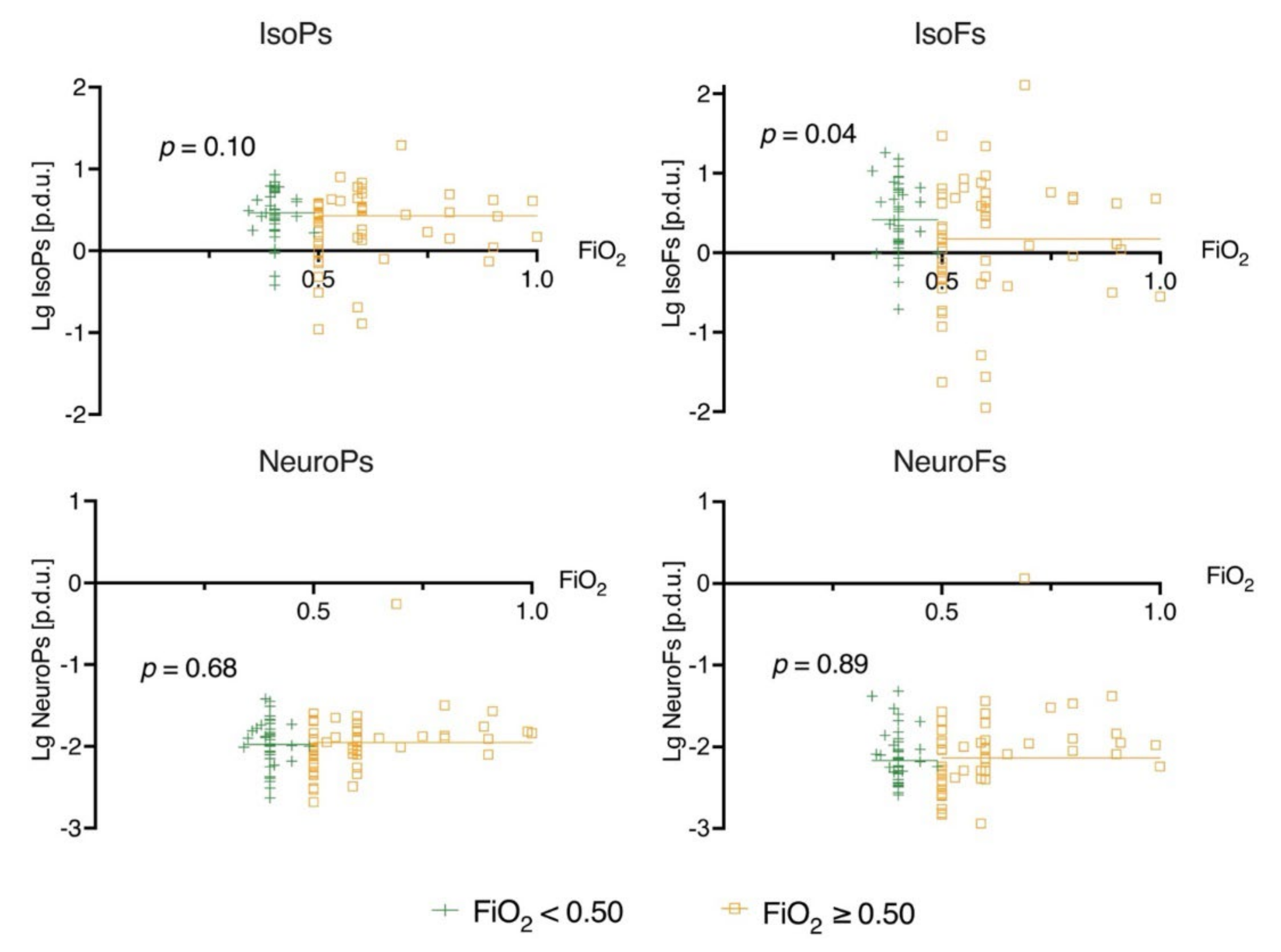
2.4. Exposure to Oxygen
2.5. Statistical Methods
3. Results
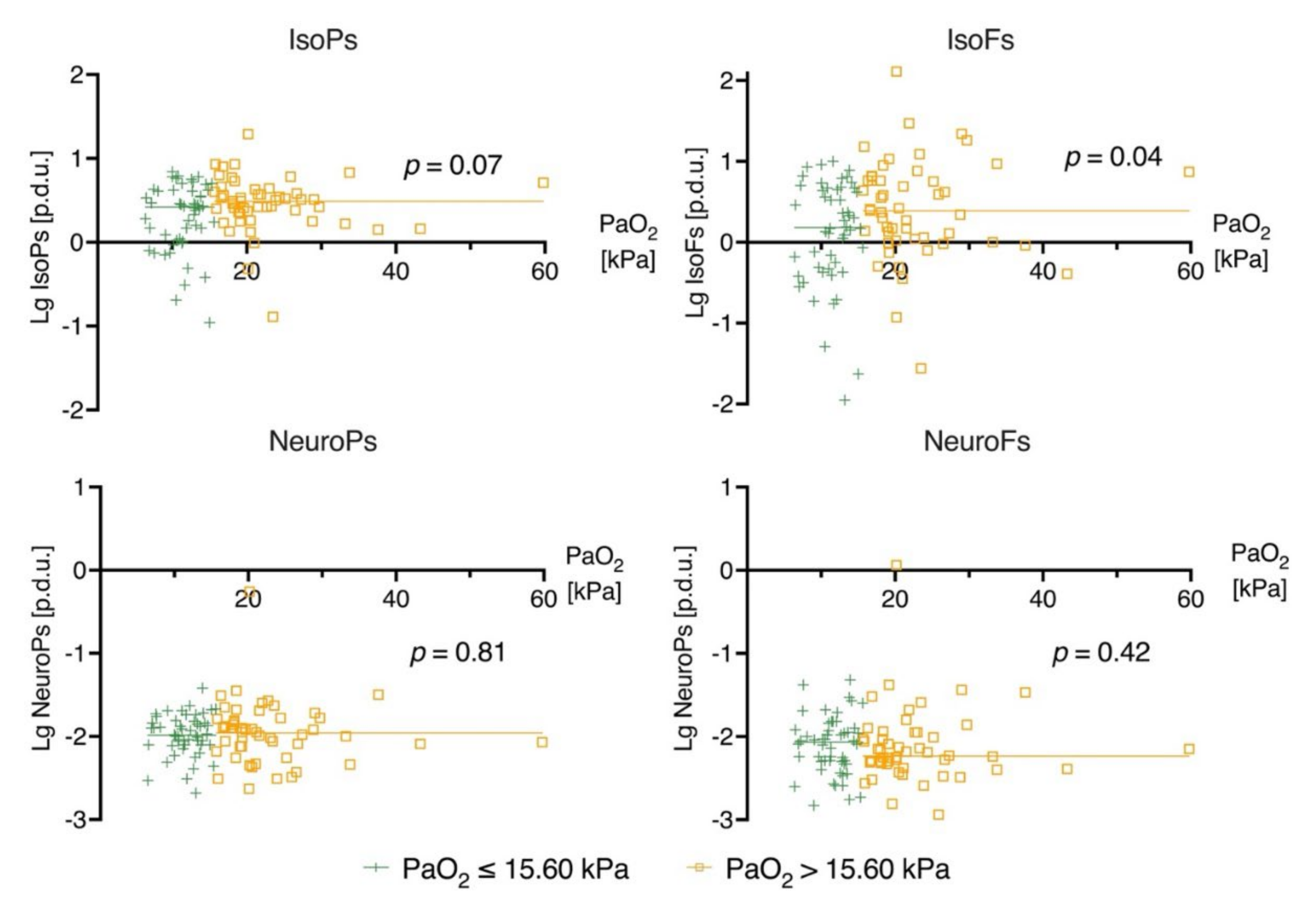
3.1. Biomarker Levels on Admission (0 h) and Prehospital Oxygen Exposure
3.2. Biomarker Levels at 24 h and Oxygen Exposure
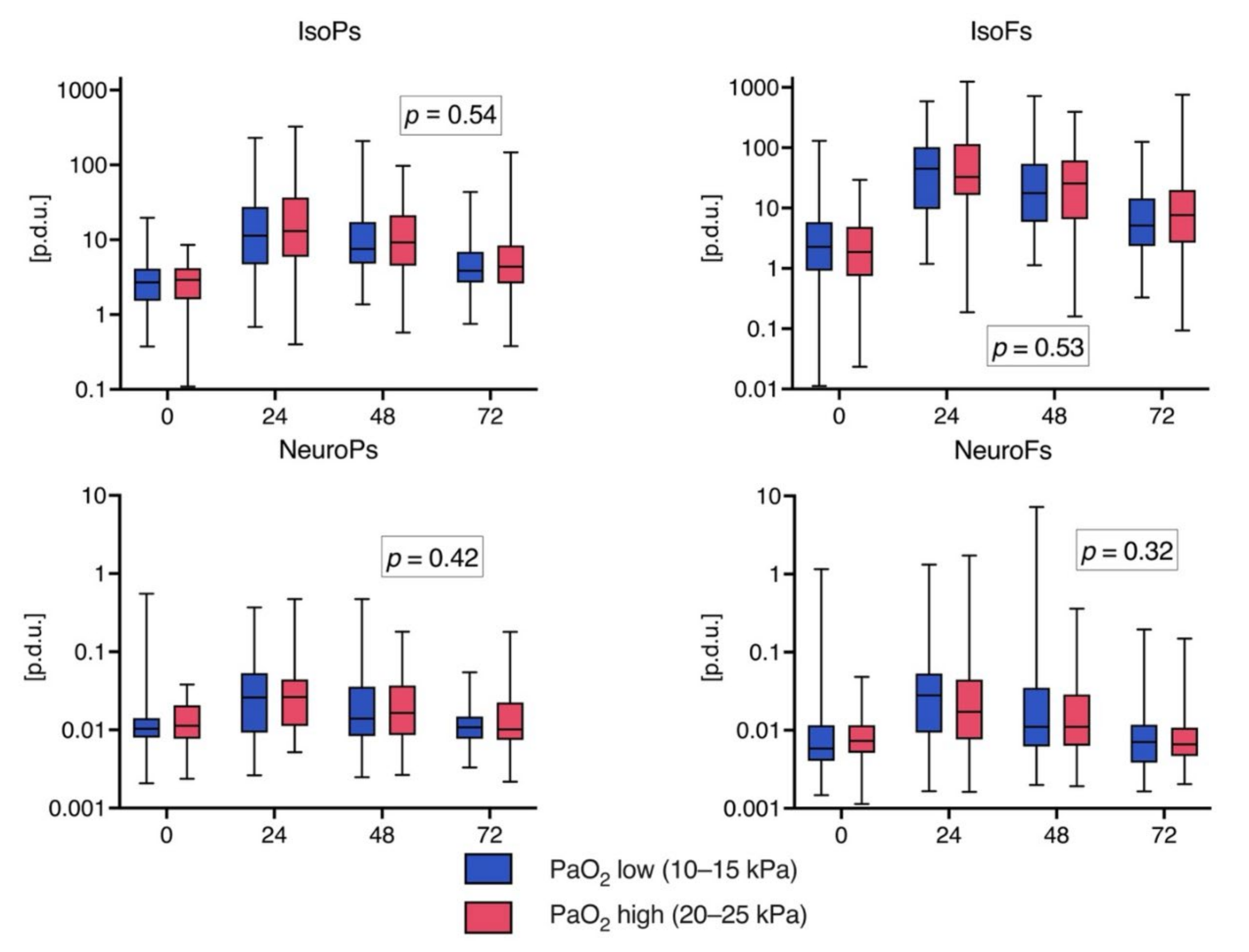
3.3. Biomarker Levels over Time
3.4. Linear Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherry, B.H.; Sumien, N.; Mallet, R.T. Neuronal injury from cardiac arrest: Aging years in minutes. Age 2014, 36, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J.Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [Green Version]
- Roberts, L.; Fessel, J.P. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Brain Pathol 2004, 128, 173–186. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med. Cell. Longev. 2017, 2017, 1–32. [Google Scholar] [CrossRef]
- Vigor, C.; Bertrand-Michel, J.; Pinot, E.; Oger, C.; Vercauteren, J.; Le Faouder, P.; Galano, J.-M.; Lee, J.C.-Y.; Durand, T. Non-enzymatic lipid oxidation products in biological systems: Assessment of the metabolites from polyunsaturated fatty acids. J. Chromatogr. B 2014, 964, 65–78. [Google Scholar] [CrossRef]
- Milne, G.; Dai, Q.; Roberts, L.J. The isoprostanes—25 years later. Biochim. Biophys. Acta 2014, 1851, 433–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.-L.; Lawson, J.A.; Reilly, D.; Rokach, J.; Chang, C.-T.; Giasson, B.; FitzGerald, G.A. Neurofurans, Novel Indices of Oxidant Stress Derived from Docosahexaenoic Acid. J. Biol. Chem. 2008, 283, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Torres-Cuevas, I.; Llorca, A.P.; Sánchez-Illana, L.; Nuñez-Ramiro, A.; Kuligowski, J.; Cháfer-Pericás, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Jakkula, P.; COMACARE study group; Reinikainen, M.; Hästbacka, J.; Loisa, P.; Tiainen, M.; Pettilä, V.; Toppila, J.; Lähde, M.; Bäcklund, M.; et al. Targeting two different levels of both arterial carbon dioxide and arterial oxygen after cardiac arrest and resuscitation: A randomised pilot trial. Intensive Care. Med. 2018, 44, 2112–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vento, M.; Sastre, J.; Asensi, M.A.; Vina, J. Room-Air Resuscitation Causes Less Damage to Heart and Kidney than 100% Oxygen. Am. J. Respir. Crit. Care Med. 2005, 172, 1393–1398. [Google Scholar] [CrossRef]
- Liu, Y.; Rosenthal, R.E.; Haywood, Y.; Miljkovic-Lolic, M.; Vanderhoek, J.Y.; Fiskum, G. Normoxic Ventilation After Cardiac Arrest Reduces Oxidation of Brain Lipids and Improves Neurological Outcome. Stroke 1998, 29, 1679–1686. [Google Scholar] [CrossRef] [Green Version]
- Angelos, M.G.; Kutala, V.K.; Torres, C.A.; He, G.; Stoner, J.D.; Mohammad, M.; Kuppusamy, P. Hypoxic reperfusion of the ischemic heart and oxygen radical generation. Am. J. Physiol. Circ. Physiol. 2006, 290, H341–H347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakkula, P.; Reinikainen, M.; Hästbacka, J.; Pettilä, V.; Loisa, P.; Karlsson, S.; Laru-Sompa, R.; Bendel, S.; Oksanen, T.; Birkelund, T.; et al. Targeting low- or high-normal Carbon dioxide, Oxygen, and Mean arterial pressure After Cardiac Arrest and REsuscitation: Study protocol for a randomized pilot trial. Trials 2017, 18, 507. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Illana, A.; Shah, V.; Piñeiro-Ramos, J.D.; Di Fiore, J.M.; Quintás, G.; Raffay, T.M.; Macfarlane, P.M.; Martin, R.J.; Kuligowski, J. Adrenic acid non-enzymatic peroxidation products in biofluids of moderate preterm infants. Free Radic. Biol. Med. 2019, 142, 107–112. [Google Scholar] [CrossRef]
- Sánchez-Illana, A.; Thayyil, S.; Montaldo, P.; Jenkins, D.; Quintás, G.; Oger, C.; Galano, J.-M.; Vigor, C.; Durand, T.; Vento, M.; et al. Novel free-radical mediated lipid peroxidation biomarkers in newborn plasma. Anal. Chim. Acta 2017, 996, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Kaviarasan, S.; Muniandy, S.; Qvist, R.; Ismail, I.S. F2-Isoprostanes as Novel Biomarkers for Type 2 Diabetes: A Review. J. Clin. Biochem. Nutr. 2009, 45, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummins, R.O.; A Chamberlain, D.; Abramson, N.S.; Allen, M.; Baskett, P.J.; Becker, L.; Bossaert, L.; Delooz, H.H.; Dick, W.F.; Eisenberg, M.S. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: The Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation 1991, 84, 960–975. [Google Scholar] [PubMed] [Green Version]
- Neuhäuser, M. Wilcoxon–Mann–Whitney Test. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1656–1658. [Google Scholar]
- McHugh, M.L. The Chi-square test of independence. Biochem. Medica 2013, 23, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Schober, P.; Vetter, T.R. Linear Regression in Medical Research. Anesth. Analg. 2020, 132, 108–109. [Google Scholar] [CrossRef]
- A Duricki, D.; Soleman, S.; Moon, L.D.F. Analysis of longitudinal data from animals with missing values using SPSS. Nat. Protoc. 2016, 11, 1112–1129. [Google Scholar] [CrossRef] [Green Version]
- Saugstad, O.D.; Ramji, S.; Soll, R.F.; Vento, M. Resuscitation of Newborn Infants with 21% or 100% Oxygen: An Updated Systematic Review and Meta-Analysis. Neonatology 2008, 94, 176–182. [Google Scholar] [CrossRef]
- Zhao, S.; Qian, J.; Wang, J.; Gong, P.; Yang, Z.; Cahoon, J.; Wu, X.; Duggal, N.; Lin, C.; Tang, W. Effects of Oxygen Concentrations on Postresuscitation Myocardial Oxidative Stress and Myocardial Function in a Rat Model of Cardiopulmonary Resuscitation. Crit. Care Med. 2015, 43, e560–e566. [Google Scholar] [CrossRef]
- Helmerhorst, H.J.F.; Roos-Blom, M.-J.; Van Westerloo, D.J.; De Jonge, E. Association Between Arterial Hyperoxia and Outcome in Subsets of Critical Illness. Crit. Care Med. 2015, 43, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Bailey, M.; Eastwood, G.M.; Nichol, A.; Pilcher, D.; Hart, G.K.; Reade, M.C.; Egi, M.; Cooper, D.J.; the Study of Oxygen in Critical Care (SOCC) Group. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit. Care 2011, 15, R90. [Google Scholar] [CrossRef] [Green Version]
- Young, P.J.; Bailey, M.; Bellomo, R.; Bernard, S.; Bray, J.; Jakkula, P.; Kuisma, M.; Mackle, D.; Martin, D.; Nolan, J.P.; et al. Conservative or liberal oxygen therapy in adults after cardiac arrest. Resuscitation 2020, 157, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, J.; Aguar, M.; Rook, D.; Lliso, I.; Cuevas, I.T.; Escobar, J.; Quintás, G.; Brugada, M.; Sánchez-Illana, A.; van Goudoever, J.; et al. Urinary Lipid Peroxidation Byproducts: Are They Relevant for Predicting Neonatal Morbidity in Preterm Infants? Antioxid. Redox Signal. 2015, 23, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vento, M.; Moro, M.; Escrig, R.; Arruza, L.; Villar, G.; Izquierdo, I.; Roberts, L.J.; Arduini, A.; Escobar, J.; Sastre, J.; et al. Preterm Resuscitation With Low Oxygen Causes Less Oxidative Stress, Inflammation, and Chronic Lung Disease. Pediatrics 2009, 124, e439–e449. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar]
- Wiklund, L.; Patnaik, R.; Sharma, A.; Miclescu, A.; Sharma, H.S. Cerebral Tissue Oxidative Ischemia-Reperfusion Injury in Connection with Experimental Cardiac Arrest and Cardiopulmonary Resuscitation: Effect of Mild Hypothermia and Methylene Blue. Mol. Neurobiol. 2017, 55, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Tissier, R.; Chenoune, M.; Pons, S.; Zini, R.; Darbera, L.; Lidouren, F.; Ghaleh, B.; Berdeaux, A.; Morin, D. Mild hypothermia reduces per-ischemic reactive oxygen species production and preserves mitochondrial respiratory complexes. Resuscitation 2013, 84, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Ahn, J.H.; Lee, T.-K.; Kim, B.; Tae, H.-J.; Park, J.H.; Shin, M.C.; Cho, J.H.; Won, M.-H. Therapeutic hypothermia reduces inflammation and oxidative stress in the liver after asphyxial cardiac arrest in rats. Acute Crit. Care 2020, 35, 286–295. [Google Scholar] [CrossRef]
- Diao, M.-Y.; Zheng, J.; Shan, Y.; Xi, S.; Zhu, Y.; Hu, W.; Lin, Z. Hypothermia prevents hippocampal oxidative stress and apoptosis via the GSK-3β/Nrf2/HO-1 signaling pathway in a rat model of cardiac arrest-induced brain damage. Neurol. Res. 2020, 42, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Alva, N.; Carbonell, T.; Palomeque, J. Hypothermic protection in an acute hypoxia model in rats: Acid–base and oxidant/antioxidant profiles. Resuscitation 2010, 81, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, C. Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J. Mol. Cell. Cardiol. 1980, 12, 797–808. [Google Scholar] [CrossRef]
- Kogure, K.; Watson, B.D.; Busto, R.; Abe, K. Potentiation of lipid peroxides by ischemia in rat brain. Neurochem. Res. 1982, 7, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.B.; Davies, M.J.; Hearse, D.J.; Slater, T.F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ. Res. 1987, 61, 757–760. [Google Scholar] [CrossRef] [Green Version]
- Solberg, R.; Andresen, J.H.; Escrig, R.; Vento, M.; Saugstad, O.D. Resuscitation of Hypoxic Newborn Piglets With Oxygen Induces a Dose-Dependent Increase in Markers of Oxidation. Pediatr. Res. 2007, 62, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoran, T.B.; Mas, E.; Barden, A.E.; Durand, T.; Galano, J.-M.; RobertsII, L.J.; Phillips, M.; Ho, K.M.; Mori, T.A. Are Isofurans and Neuroprostanes Increased After Subarachnoid Hemorrhage and Traumatic Brain Injury? Antioxid. Redox Signal. 2011, 15, 2663–2667. [Google Scholar] [CrossRef] [Green Version]
- VanRollins, M.; Woltjer, R.L.; Yin, H.; Morrow, J.D.; Montine, T.J. F2-Dihomo-isoprostanes arise from free radical attack on adrenic acid. J. Lipid Res. 2008, 49, 995–1005. [Google Scholar]
- Sakamoto, H.; Corcoran, T.B.; Laffey, J.G.; Shorten, G.D. Isoprostanes—Markers of Ischaemia Reperfusion Injury. Eur. J. Anaesthesiol. 2002, 19, 550–559. [Google Scholar]
- Idris, A.; Roberts, L.J.; Caruso, L.; Showstark, M.; Layon, A.J.; Becker, L.B.; Hoek, T.V.; Gabrielli, A. Oxidant injury occurs rapidly after cardiac arrest, cardiopulmonary resuscitation, and reperfusion. Crit. Care Med. 2005, 33, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Haid, M.; Muschet, C.; Wahl, S.; Römisch-Margl, W.; Prehn, C.; Möller, G.; Adamski, J. Long-Term Stability of Human Plasma Metabolites during Storage at −80 °C. J. Proteome Res. 2017, 17, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Vento, M.; Asensi, M.; Sastre, J.; Lloret, A.; García-Sala, F.; Viña, J. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J. Pediatr. 2003, 142, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Chafer-Pericas, C.; Cernada, M.; Rahkonen, L.; Stefanovic, V.; Andersson, S.; Vento, M. Preliminary case control study to establish the correlation between novel peroxidation biomarkers in cord serum and the severity of hypoxic ischemic encephalopathy. Free Radic. Biol. Med. 2016, 97, 244–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solberg, R.; Longini, M.; Proietti, F.; Vezzosi, P.; Saugstad, O.D.; Buonocore, G. Resuscitation with supplementary oxygen induces oxidative injury in the cerebral cortex. Free Radic. Biol. Med. 2012, 53, 1061–1067. [Google Scholar] [CrossRef]
| PaO2 on Intensive Care Unit Admission | Randomized PaO2 Target Groups | ||||||
|---|---|---|---|---|---|---|---|
| All n = 112 | ≤15.6 kPa, n = 56 | >15.6 kPa, n = 56 | p-Value 1 | 10–15 kPa, n = 57 | 20–25 kPa, n = 55 | p-Value 1 | |
| Median (IQR)/count (%) | Median (IQR)/count (%) | ||||||
| Age (in years) | 62 (53–68) | 65 (54–69) | 58 (52–67) | 0.12 | 62 (53–68) | 62 (54–69) | 0.69 |
| Male gender | 92 (82) | 43 (77) | 49 (88) | 0.14 | 47 (83) | 45 (82) | 0.93 |
| BMI | 26.3 (23.8–29.4) | 27.8 (23.7–30.9) | 25.5 (23.8–27.8) | 0.015 | 26.2 (23.4–29.1) | 26.5 (24.2–30.9) | 0.39 |
| Smoker (yes) 2 | 35 (35%) | 15 (29) | 20 (42) | 0.20 | 17 (34) | 18 (37) | 0.78 |
| Bystander resuscitation (yes) | 93 (83) | 48 (86) | 45 (80) | 0.45 | 47 (83) | 46 (84) | 0.87 |
| ROSC time (min) | 21 (16–26) | 22 (17–28) | 21 (15–25) | 0.12 | 20 (16–25) | 22 (17–27) | 0.38 |
| Delay to first unit (min) | 7 (6–9) | 8 (6–10) | 6.5 (5–9) | 0.008 | 7 (6–9) | 7 (6–9) | 0.68 |
| 30-day mortality | 36 (32) | 19 (34) | 17 (30) | 0.69 | 17 (30) | 19 (35) | 0.59 |
| 180-day mortality | 37 (33) | 20 (36) | 17 (30) | 0.55 | 17 (30) | 20 (36) | 0.46 |
| CPC 1–2 at 180 days | 73 (65) | 35 (63) | 38 (68) | 0.55 | 39 (69) | 34 (62) | 0.46 |
| R2 | B | 95% CI for B | p-Value | ||
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Lg IsoFs at 24 h | 0.08 | ||||
| Age (years) | −0.01 | −0.02 | 0 | 0.045 | |
| Bystander CPR | −0.22 | −0.48 | 0.05 | 0.10 | |
| PaO2 (10–15 kPa/20–25 kPa) | 0.12 | −0.07 | 0.31 | 0.22 | |
| PaCO2 (4.5–4.7 kPa/5.8–6.0 kPa) | −0.11 | −0.30 | 0.08 | 0.25 | |
| MAP (65–75 mmHg/80–100 mmHg) | 0.01 | −0.18 | 0.21 | 0.89 | |
| Lg IsoPs at 24 h | 0.12 | ||||
| Age (years) | −0.01 | −0.02 | −0.004 | 0.006 | |
| Bystander CPR | −0.37 | −0.70 | −0.04 | 0.03 | |
| PaO2 | 0.05 | −0.19 | 0.29 | 0.68 | |
| PaCO2 | −0.12 | −0.36 | 0.12 | 0.34 | |
| MAP | 0.06 | −0.18 | 0.31 | 0.61 | |
| Lg NeuroPs at 24 h | 0.12 | ||||
| Age (years) | −0.01 | −0.02 | 0 | 0.04 | |
| Bystander CPR | −0.36 | −0.59 | −0.11 | 0.005 | |
| PaO2 | 0.01 | −0.17 | 0.18 | 0.96 | |
| PaCO2 | 0.02 | −0.16 | 0.20 | 0.82 | |
| MAP | 0.09 | −0.09 | 0.27 | 0.34 | |
| Lg NeuroFs at 24 h | 0.14 | ||||
| Age (years) | −0.01 | −0.02 | −0.001 | 0.04 | |
| Bystander CPR | −0.53 | −0.84 | −0.22 | 0.001 | |
| PaO2 | −0.14 | −0.37 | 0.08 | 0.21 | |
| PaCO2 | −0.07 | −0.30 | 0.15 | 0.53 | |
| MAP | 0.04 | −0.20 | 0.27 | 0.76 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humaloja, J.; Vento, M.; Kuligowski, J.; Andersson, S.; Piñeiro-Ramos, J.D.; Sánchez-Illana, Á.; Litonius, E.; Jakkula, P.; Hästbacka, J.; Bendel, S.; et al. High Oxygen Does Not Increase Reperfusion Injury Assessed with Lipid Peroxidation Biomarkers after Cardiac Arrest: A Post Hoc Analysis of the COMACARE Trial. J. Clin. Med. 2021, 10, 4226. https://doi.org/10.3390/jcm10184226
Humaloja J, Vento M, Kuligowski J, Andersson S, Piñeiro-Ramos JD, Sánchez-Illana Á, Litonius E, Jakkula P, Hästbacka J, Bendel S, et al. High Oxygen Does Not Increase Reperfusion Injury Assessed with Lipid Peroxidation Biomarkers after Cardiac Arrest: A Post Hoc Analysis of the COMACARE Trial. Journal of Clinical Medicine. 2021; 10(18):4226. https://doi.org/10.3390/jcm10184226
Chicago/Turabian StyleHumaloja, Jaana, Maximo Vento, Julia Kuligowski, Sture Andersson, José David Piñeiro-Ramos, Ángel Sánchez-Illana, Erik Litonius, Pekka Jakkula, Johanna Hästbacka, Stepani Bendel, and et al. 2021. "High Oxygen Does Not Increase Reperfusion Injury Assessed with Lipid Peroxidation Biomarkers after Cardiac Arrest: A Post Hoc Analysis of the COMACARE Trial" Journal of Clinical Medicine 10, no. 18: 4226. https://doi.org/10.3390/jcm10184226
APA StyleHumaloja, J., Vento, M., Kuligowski, J., Andersson, S., Piñeiro-Ramos, J. D., Sánchez-Illana, Á., Litonius, E., Jakkula, P., Hästbacka, J., Bendel, S., Tiainen, M., Reinikainen, M., & Skrifvars, M. B. (2021). High Oxygen Does Not Increase Reperfusion Injury Assessed with Lipid Peroxidation Biomarkers after Cardiac Arrest: A Post Hoc Analysis of the COMACARE Trial. Journal of Clinical Medicine, 10(18), 4226. https://doi.org/10.3390/jcm10184226








