Abstract
COVID-19 exerts deleterious cardiopulmonary effects, leading to a worse prognosis in the most affected. This retrospective multi-center observational cohort study aimed to analyze the trajectories of key vitals amongst hospitalized COVID-19 patients using a chest-patch wearable providing continuous remote patient monitoring of numerous vital signs. The study was conducted in five COVID-19 isolation units. A total of 492 COVID-19 patients were included in the final analysis. Physiological parameters were measured every 15 min. More than 3 million measurements were collected including heart rate, systolic and diastolic blood pressure, cardiac output, cardiac index, systemic vascular resistance, respiratory rate, blood oxygen saturation, and body temperature. Cardiovascular deterioration appeared early after admission and in parallel with changes in the respiratory parameters, showing a significant difference in trajectories within sub-populations at high risk. Early detection of cardiovascular deterioration of COVID-19 patients is achievable when using frequent remote patient monitoring.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a multi-system disease with a wide range of clinical manifestations, from asymptomatic patients to a simple influenza-like illness, or to a fulminant disease comprised of acute respiratory distress syndrome (ARDS) and pulmonary insufficiency [1]. As the understanding of the disease evolved, it was shown that COVID-19 damages epithelial and endothelial cells in numerous tissues leading to SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2)-related multi-organ failure [1,2,3,4]. Previous studies have demonstrated substantial damage to the cardiovascular system with systemic hemodynamic and direct cardiac effects, a negative prognostic sign leading to increased morbidity in individuals with underlying cardiovascular diseases [1,5,6]. Respiratory parameters such as respiratory rate (RR) and blood oxygen saturation (SpO2) are key measurements in the assessment and prognosis of COVID-19 patients [7]. So far, these were carried out by infrequent spot measurements, mainly due to the capabilities of currently used devices.
Novel technologies are leading us to the next generation of care provision, as they broaden the capacity to remotely monitor patients, providing optimized support and protection both for COVID-19 patients and for health care providers by reducing direct contact without compromising the treatment given to isolated patients, as well as lightening the strain on the medical teams and providing early detection of patient deterioration [8,9,10,11,12,13]. Moreover, frequent monitoring of multiple vital physiological parameters may allow better care for patients who are acutely ill, for hospital-at-home of stable patients or those in imminent danger, and early discharge of admitted patients [5,9]. It is also accepted that such a system would be of benefit if it frequently and automatically measures numerous vital signs, improves patient surveillance, and, ultimately, improves patient outcomes [8,14,15,16]. Frequent remote patient monitoring (RPM) systems are potentially better equipped to detect and alert of changes since the vital sign measurements are taken continuously and for longer periods [8,9]. Combining advanced wearable devices with machine learning in various clinical settings may allow reliable detection of changes in population health status, including follow-up of COVID-19 patients and at an earlier stage, helping to control the spread of the pandemic [9,17,18].
Several reports so far have identified differences in COVID-19 infection rates, symptom severity, and mortality between sex, age, and body mass index (BMI) categories [19,20,21,22]; however, whether the physiological response of key cardiovascular and respiratory parameters during the course of hospitalization is different between individuals remains unknown. This knowledge may help in further understanding the clinical course of the disease and preventing clinical deterioration, with highlighted importance due to the rapid deterioration often seen in COVID-19 patients [1].
This retrospective observational multi-center study aimed to determine the trajectory of nine physiological parameters amongst COVID-19 patients admitted to isolation units in Israeli medical centers. A special emphasis was given to characterizing the disease progression among these patients, and to the identification of differences in physiological responses over time of sub-groups according to age, sex, and BMI.
2. Materials and Methods
2.1. Study Design
This retrospective observational and non-interventional multi-center study was conducted between 3 March 2020, and 22 May 2020. We included COVID-19 patients 18 years and older that were admitted into five COVID-19 dedicated isolation units in Israel, and continuously monitored using non-invasive, wearable, and wireless photoplethysmography (PPG)-based chest-monitors (BB-613WP, Biobeat Technologies Ltd., Petah Tikva, Israel). The monitors were attached and activated upon admission, with measurements automatically taken every 15 min and transmitted in real-time to a cloud-based web application used by the medical staff (Figure 1). Big data analysis was conducted using advanced AI and bioinformatics tools.
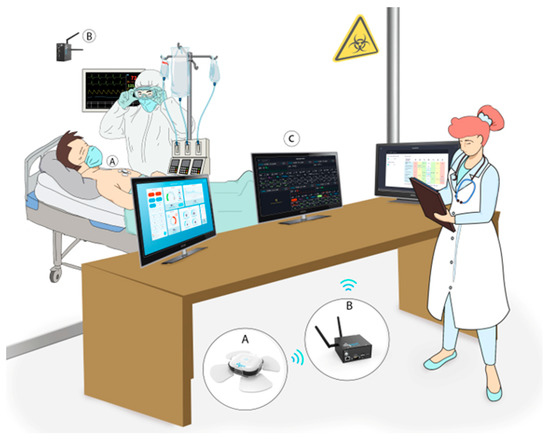
Figure 1.
The remote patient monitoring (RPM) system is deployed in isolation units. The chest-patch sensor was attached to all moderate to severe COVID-19 patients included in the study. This RPM system provides automatic measurement of 15 vital signs every 5 to 15 min. Data is transmitted through Bluetooth low energy (BLE) from the sensors (A) to gateways (B) installed in the isolation units and from there through Wi-Fi or a SIM card to a cloud-based medical management application available to health care providers on any web platform (C), allowing to monitor all admitted patients at once. The monitor shows the vital signs in real-time, provides alerts and has an integrated early warning score system.
Designated gateways (BB-SGWB Smart Gateway Box/BB-90000300100, Biobeat Technologies Ltd., Petah Tikva, Israel) were installed in the isolation units to ensure continuous monitoring and data transmission of all measurements (Figure 1). Physiological parameters included in the analysis were heart rate (HR), SpO2, RR, cuff-less non-invasive blood pressure (BP), stroke volume (SV), cardiac output (CO), cardiac index (CI), systemic vascular resistance (SVR), and body temperature.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria were male and female adults above the age of 18 years, with a positive COVID-19 PCR test, admitted to designated COVID-19 isolation units in a moderate to a severe condition as defined by the local health care providers, but with no need for admission in an intensive care unit (ICU). Exclusion criteria were pregnancy, the need for ventilation, and admission to an ICU.
2.3. The Remote Patient Monitoring (RPM) System
The FDA-cleared chest-monitor device used in this study (Figure 1) utilizes a unique reflective PPG technology, in which specific wavelengths of light are transmitted onto the skin, and the reflected light is collected by a photodiode detector positioned near the light source transmitter. The sensor tracks vital signs derived from changes in the pulse contour, following a simple offset baseline trimonthly calibration process using an approved non-invasive, cuff-based device, and is based on Pulse Wave Transit Time (PWTT) technology combined with Pulse Wave Analysis (PWA) (for error% levels please see validation results in [23,24,25,26]). Patients were monitored throughout hospitalization, and sensors were replaced if hospitalization lasted longer than the 6-day battery life. The same sensors were not used to monitor different patients.
2.4. Ethical Approval
All methods were carried out under relevant guidelines and regulations and approved by the institutional review boards as follows: approval 0077-20-WOMC was provided by the Wolfson Medical Center’s IRB, approval 0048-20-POR was provided by the Padeh Poriya Medical Center’s IRB, approval 0506-20-RMB was provided by the Rambam Medical Center’s IRB, approval 0193-20-KMC was provided by the Kaplan Medical Center’s IRB, and approval 0421-20-SOR was provided by the Soroka Medical Center’s IRB. Informed consent was waived by all IRBs.
2.5. Statistical Analysis
Global outliers were picked using PCA and histogram examination. On a per-patient level, outlier observations with a difference from the mean that was greater than Q3 + 3xIQR were discarded. Differences between the studied groups were determined using an independent t-test when the data satisfied test requirements. Equal variances were assumed if Barlett and Levene’s tests came out significant. Wilcox test was performed for metrics with non-normal distribution. For key physiological variables recorded during the first 2 h of admission, normality was assessed using the Shapiro test QQ-plots, allowing the removal of extreme outliers. Then, differences between groups were determined using repeated-measures ANOVA. Where a significant main effect was found, a Tukey’s post hoc test was performed. The data were fitted to a linear mixed model with nlme 3.1, with sex, age range, and BMI as coefficients, then tested using ANOVA. Pairwise Wilcox was used for post hoc testing when the sample sizes were sufficiently large (n > 50). Trend estimation figures with less than 1000 observations were performed using LOESS, while larger trend data was performed using GAM. All other results are presented as means ± SD. Statistical analyses were considered significant if p < 0.05. All statistical analyses were made using R version 3.6.3 (GBIF.org, Copenhagen, Denmark) [27].
3. Results
Initially, 571 patients participated in the study. Subjects with less than 24 h of continuous tracking data were excluded, with 492 patients and a total of 3,215,334 measurements remaining. As can be seen in Figure 2a–c, the number of patients decreased throughout the monitoring period due to patients’ discharge from the hospital, transfer to ICUs, or death. As a result, we focused our final analysis on the first five days (120 h) after admission. The raw, unfiltered data included measurements collected during an average monitoring period of 75.26 h (range 0–455), with 245.67 ± 226.39 observations (±standard deviation) per patient. The analyzed filtered data included patients with at least 24 h of tracking and included observations from the first five days only. Measurements were collected during an average monitoring period of 74.78 h (range 24–120) per patient (174.89 ± 110.58).

Figure 2.
The number of subjects per group throughout the entire monitoring period. (a) division by sex; (b) division by age groups (young: 18–40 years; middle age: 40–60 years; elderly: 60–80 years; >80 years); (c) division by body mass index (BMI; normal weight: <24.9 kg∙min−2; overweight: 25–29.9 kg∙min−2; obese: >30 kg∙min−1). The breakdown of patients’ numbers by day was 492 at 24 h, 408 at 48 h, 294 at 72 h, 204 at 86 h, 130 at 120 h, 92 at 144 h, 66 at 168 h, 49 at 192 h, 43 at 216 h, 33 at 240 h, and 21 at 264 h.
Mean values during the first 2 h of monitoring (average of 8 measurements on admission) served as a baseline monitoring period for each subject. For the entire study population, baseline measurements were 82 ± 10 for HR, 95 ± 2 for SpO2, 129 ± 17 for SBP, 74 ± 11 for DBP, 70 ± 12 for SV, 5.7 ± 1.3 for CO, 1368 ± 296 for SVR, and 35.7 ± 3.6 for body temperature.
Subject characteristics, along with mean values during the baseline monitoring period for each subject for body temperature, SpO2, RR, HR, systolic BP (SBP), diastolic BP (DBP), CO, CI, and SVR for each sub-group are presented in Table 1.

Table 1.
Patient characteristics and average values by sub-groups for the first 2 h of monitoring.
For baseline physiological measurements, an ANOVA analysis revealed a significant (p < 0.01) difference between men and women for body temperature, SpO2, HR, DBP, and CO. When analyzing age groups, significant (p < 0.003) interactions were seen for all physiological measures recorded during the first 2 h of admission. Lastly, only SpO2, RR, CO, and CI were significantly (p < 0.01) different between BMI groups upon admission.
Figure 3 provides an overall description of the nine vital signs in the 130 patients that fully completed five days of continuous monitoring. Overall, within the first 24 h, we found a significant increase in temperature, RR, and SVR, and a significant decrease in SpO2, DBP, CO, and CI (p < 0.01 for all). These changes all appeared at the same time. For HR, SBP, CO, and CI, the changes appeared in a repetitive pattern.
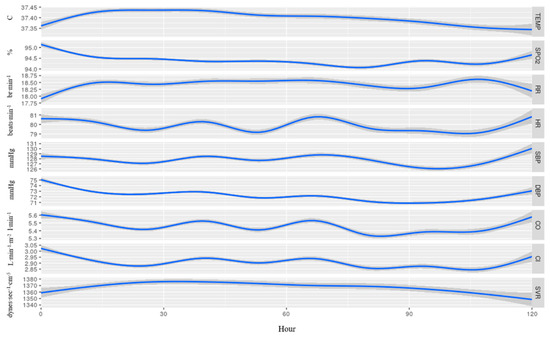
Figure 3.
Average recorded measurements among 130 patients that were continuously monitored for 5 days from admission, without separation to sub-groups. Temp, temperature; SpO2, blood oxygen saturation; RR, respiratory rate; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; CO, cardiac output; CI, cardiac index; SVR, systemic vascular resistance. The blue line represents the mean value of each vital and the 95% confidence interval appears in gray.
Further analysis is provided in Figure 4, where we show the results of repeated-measures ANOVA tests performed to determine differences between groups during the same timeframe. In both males and females, the temperature increased during the first 24 h of monitoring, reaching significantly higher values among males (p < 0.001). From the second day, females showed a significant decrease in temperature until the fifth day (p < 0.001). In the age group, temperature among the elderly was higher (p < 0.001) during the whole 5 days.
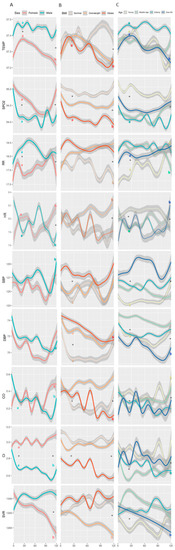
Figure 4.
Measured vital signs by (A) sex, (B) BMI, and (C) age group, over the course of the first 5 days of tracking. Definitions of BMI and age groups are provided in Figure 2. Temp, temperature; SpO2, blood oxygen saturation; RR, respiratory rate; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; CO, cardiac output; CI, cardiac index; SVR, systemic vascular resistance. Each line represents mean values of the vitals, and the 95% confidence interval appears in gray. * Significant (p < 0.05) difference between groups for a given time-point; a significant (p < 0.05) difference between 24 h and baseline within a group; b significant (p < 0.05) difference between 120 h and baseline within a group.
SpO2 decreased in both males and females during the first 48 h (p < 0.001 for both), with a higher decrease among males. Starting from 72 h since admission, and throughout the next two days, females showed a quicker return to the baseline levels, while males remained with lower values (p < 0.03 between sex). In the age group, the young maintained SpO2 levels throughout the five days of hospitalization with a slight reduction (p = 0.044), while other sub-groups showed dynamic changes with lower SpO2 values.
Within the first 48 h from admission, RR increased significantly in males, obese patients, and in all age sub-groups (p < 0.01) except the young. Throughout the whole five days of hospitalization, the overweight and obese sub-groups had higher RR as compared to the normal weight sub-group (p < 0.01). The young showed a significant increase (p = 0.044) in RR.
During the first 48 h, HR dropped among men, while it increased among women (p < 0.01 in both). From then on, the dynamics of HR behaved in opposite manners between sex, until day 5, in which both showed an increase, with females showing a significant increase from baseline (p < 0.01).
Changes in SBP over five days of hospitalization were significantly different between age groups (p = 0.002). Both sexes showed a decrease in DBP values during the first 4 days, women to a higher extent than men (p < 0.001), and in the fifth day both showed an increase with values among women returning to the baseline level. Patients over 80 years showed relatively lower DBP values during the 5 days, reaching a nadir at day 4 (p < 0.001). The young and elderly showed a decreasing trend in the first 3 days (p < 0.01), returning close to baseline levels by day 5. Normal weight patients had a sharp decrease during the first 24 h (p < 0.001), maintaining this level until the 4th day, followed by a sharp increase during the 5th day to baseline levels. Both overweight and obese had a milder decrease during the first 4 days with overweight returning to baseline levels on the 5th day, and the obese returning to a lower level than the baseline (p = 0.02).
Males had a sharp decrease in CO in the first 24 h (p < 0.01), kept stable over the next 48 h, followed by a sharp decrease in the 4th day, and an increase in the 5th day to a level below the baseline. Females showed dynamic changes reaching peak high levels on each of days one to three, followed by a drop shortly after every peak, followed by a constant increase until reaching the highest peak on day 5 (p < 0.01). Patients over 80 years had a dynamic pattern of changes with peaks at 24 h and 48 h from admission (p < 0.01 and p = 0.02, respectively), followed by lower peaks and increasing again at day 5 after admission. Similar changes were found in SV for the entire population and within the sub-groups.
Both sexes started with the same CI values at baseline. Shortly after, a sharp increase was evident amongst females, reaching its peak at 48 h after admission (p < 0.001), while amongst males a sharp decrease was seen after 24 h (p < 0.001), maintained until day 4, in which a further decrease was evident, and a moderate increase appeared on day 5, to levels lower than baseline (p < 0.001). Both middle-aged and elderly showed decreases within the first 24 h (p < 0.001 in both). Amongst the elderly, a further decrease was evident on day 4, increasing slightly on day 5 (p < 0.001). Unlike other age sub-groups, the over 80 years started with a sharp increase in CI during the first 24 h, followed by a sharp decrease and immediately followed with a higher increase by 48 h after admission (p < 0.001). This was followed by an unstable decrease over the next two days, and a sharp increase at day 5 (p < 0.001). Obese patients had a lower baseline level of CI, further decreasing during the first 24 h, and reaching a nadir at day 5 (p < 0.001).
Females had a higher SVR value at baseline as compared with males, and during the 5 days showed a consistent decrease until the end of monitoring (p < 0.001). Males started with an increase during the first 24 h (p < 0.001) and remained relatively stable until day 5. Middle-aged patients had an increase in the first 24 h (p < 0.01) followed by a continuous decrease until day 5, returning to baseline level. Overweight patients showed a continuous decrease during the five days (p < 0.01).
4. Discussion
In this retrospective multi-center study, we continuously monitored key vitals amongst hospitalized COVID-19 patients using a non-invasive chest-patch sensor. Early after admission, cardiovascular and respiratory parameters deteriorated in parallel, but significant differences in trajectories were found between age, sex, and BMI groups.
While prior studies employed periodic and infrequent biomarkers and echocardiography focusing on cardiac damage, we used continuous monitoring of advanced cardiac parameters using a novel and non-invasive technology, previously showing its capabilities with similar measurements compared to invasive techniques [28]. By using continuous remote patient monitoring combined with health AI we show early cardiovascular changes in COVID-19 patients. Our findings strengthen the notion that frequently measured vital signs, including advanced cardiovascular parameters such as CO, CI, and SVR, might have future implications in the understanding of the progression of COVID-19 in humans, and in particular in high-risk sub-groups [19,20,21,22].
We found that during the first 24 h from admission the changes in the cardiovascular parameters appeared in parallel to the changes in the respiratory parameters, and were more prominent amongst males, patients older than 80 years, and obese patients. Moreover, the decrease found in DBP throughout most of the monitoring period with a concomitant decrease in CO and CI, and increase in SVR, might correlate with prior reports on diastolic dysfunction resulting from COVID-19 damage to the heart [29,30], yet this hypothesis is still to be validated since we did not perform echocardiography. In parallel to the decrease in the cardiovascular parameters, we found an increased average temperature in males, while lower SpO2 and increased RR were evident in males, elderly patients, and overweight patients. This could also strengthen previous findings that showed patients with pre-existing respiratory and cardiovascular diseases have an increased risk of severe morbidity and mortality [1,19,31,32,33,34,35].
Additional observations were repetitive patterns of HR, SBP, CO, and CI (Figure 3 and Figure 4). This might be part of a circadian rhythm, yet again, we do not have enough data to substantiate this observation, and it should be further studied.
Significant changes between the studied groups were already apparent during the 2-h baseline period, emphasizing that physiological effects of COVID-19 are different among the various groups beyond the expected naturally occurring differences.
Although statistically significant changes were found in some vitals among the groups, they were slight and are currently not considered to be of clinical significance. However, we think that as advanced monitoring tools will keep developing and being introduced into clinical practice, amplified by advanced AI and machine learning analysis tools, we may find that even these slight changes could have significance and warrant clinical attention, especially when developing early warning score systems in the context of complex patients at high risk of deterioration.
Operationally, the small wearable, wireless RPM device continuously and automatically collected and transferred the data, in real-time, to the medical staff. This reduced the direct contact between medical staff and patients without compromising the medical care provided, an important feature highly required during a pandemic. Moreover, by using this technology with COVID-19 patients we now have an opportunity to define a novel COVID-19 score for the accurate early detection of deterioration. This might also serve as an important component of medical care in the ambulatory and out-of-hospital environments, with early identification of symptomatic and pre-symptomatic infected individuals especially valuable during this period [28,36,37,38].
A limitation of this study is that we did not have the clinical data records and outcomes of these patients. All were admitted to isolation units in a moderate to severe condition, and some were later transferred to COVID-19 dedicated ICUs. We have no information regarding outcomes, administration of supplemental oxygen, vasopressors, and specific therapeutics against COVID-19. However, they all received advanced medical care, and the number of data points of multiple physiological parameters collected was large and frequent, still allowing to have insights of clinical significance. Ongoing studies are now conducted to allow the parallel analysis of collected vitals and clinical data records.
5. Conclusions
Frequent monitoring approach using a remote patient monitoring system and advanced bioinformatic tools shows early cardiovascular changes among hospitalized COVID-19 patients. These changes appear in parallel to changes in respiratory parameters, further emphasizing the cardiorespiratory effects of COVID-19 over time, with differential physiological responses noted between sex, BMI, and age groups. This may serve to improve early detection of clinical deterioration of COVID-19 patients, especially important in times of overwhelmed health care systems, helping to reduce direct contact between health care providers and COVID-19 patients without compromising medical care.
Author Contributions
Conceptualization, A.E., Y.M., A.B.I. and Y.G.; methodology, A.E., D.N., R.L. (Ran Levyand) and Y.G.; software, N.G. and A.B.I., validation, A.E. and Y.G.; formal analysis, A.E., K.C., N.G., D.N., R.L. (Ran Levyand), G.S., N.S., E.S. and Y.G.; investigation, A.E., Y.M., M.H., N.A.H., R.G., E.R., E.L., O.C., A.B.I., R.M., M.F. and R.L. (Romi Littman); resources, A.E.; data curation, A.E., K.C., N.G., D.N., R.L. (Romi Littman) and Y.G.; writing—original draft preparation, A.E., K.C. and Y.G.; writing—review and editing, A.E., Y.M., K.C., N.G., D.N., R.L. (Romi Littman) and Y.G.; visualization, A.E. and Y.G.; supervision, A.E. and Y.G.; project administration, A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Boards of the Wolfson Medical Center (protocol code 0077-20-WOMC, 06/04/2020), the Padeh Poriya Medical Center (0048-20-POR, 15/06/2020), the Rambam Medical Center (0506-20-RMB, 30/11/2020), the Kaplan Medical Center (0193-20-KMC, 08/12/2020) and the Soroka Medical Center (0421-20-SOR, 12/12/2020).
Informed Consent Statement
Patient consent was waived due to lack of any Personally Identifiable Information and the time elapsed from the monitoring phase.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We wish to thank all medical and para-medical staff in the COVID-19 isolation departments for their hard work, personal sacrifice, and life-saving efforts. We thank Yazid Zoabi from Shomron’s Lab at the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel, for his assistance.
Conflicts of Interest
A.E., A.B.I., E.S., R.M., M.F., and R.L. are employees at Biobeat Technologies Ltd. They all report no additional financial interests. All other authors declare no conflict of interest.
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Tikellis, C.; Thomas, M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012, 2012, 256294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in critically ill patients in the Seattle region—Case series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Keesara, S.; Jonas, A.; Schulman, K. Covid-19 and health care’s digital revolution. N. Engl. J. Med. 2020, 382, e82. [Google Scholar]
- Russo, V.; Bottino, R.; Carbone, A.; Rago, A.; Papa, A.A.; Golino, P.; Nigro, G. COVID-19 and Heart: From Clinical Features to Pharmacological Implications. J. Clin. Med. 2020, 9, 1944. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe Covid-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Posthuma, L.M.; Downey, C.; Visscher, M.J.; Ghazali, D.A.; Joshi, M.; Ashrafian, H.; Khan, S.; Darzi, A.; Goldstone, J.; Preckel, B. Remote wireless vital signs monitoring on the ward for early detection of deteriorating patients: A case series. Int. J. Nurs. Stud. 2020, 104, 103515. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, D.R.; Davies, E.V.; Harlow, E.R.; Hsu, J.J.; Knighton, S.C.; Walker, T.A.; Voos, J.E.; Drummond, C.K. Wearable Sensors for COVID-19: A Call to Action to Harness Our Digital Infrastructure for Remote Patient Monitoring and Virtual Assessments. Front. Digit. Health 2020, 2, 8. [Google Scholar] [CrossRef]
- Russo, V.; Cassini, R.; Caso, V.; Donno, C.; Laezza, A.; Naddei, M.; Fiorelli, A.; Golino, P.; Nigro, G. Nursing Teleconsultation for the Outpatient Management of Patients with Cardiovascular Disease during COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 2087. [Google Scholar] [CrossRef]
- Russo, V.; Nigro, G.; D’Onofrio, A. COVID-19 and cardiac implantable electronic device remote monitoring: Crocodile tears or new opportunity? Expert Rev. Med. Devices 2020, 17, 471–472. [Google Scholar] [CrossRef]
- Magnocavallo, M.; Bernardini, A.; Mariani, M.V.; Piro, A.; Marini, M.; Nicosia, A.; Adduci, C.; Rapacciuolo, A.; Saporito, D.; Grossi, S.; et al. Home delivery of the communicator for remote monitoring of cardiac implantable devices: A multicenter experience during the covid-19 lockdown. Pacing Clin. Electrophysiol. 2021, 44, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Radin, J.M.; Quer, G.; Jalili, M.; Hamideh, D.; Steinhubl, S.R. The hopes and hazards of using personal health technologies in the diagnosis and prognosis of infections. Lancet Digit. Health 2021, 3, e455–e461. [Google Scholar] [CrossRef]
- Watkinson, P.J.; Barber, V.S.; Price, J.D.; Hann, A.; Tarassenko, L.; Young, J.D. A randomised controlled trial of the effect of continuous electronic physiological monitoring on the adverse event rate in high risk medical and surgical patients. Anaesthesia 2006, 61, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Adhikari, R.; Edelson, D.P. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016, 102, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taenzer, A.H.; Perreard, I.M.; MacKenzie, T.; McGrath, S.P. Characteristics of desaturation and respiratory rate in postoperative patients breathing room air versus supplemental oxygen: Are they different? Anesth. Analg. 2018, 126, 826–832. [Google Scholar] [CrossRef]
- Lonini, L.; Shawen, N.; Botonis, O.; Fanton, M.; Jayaraman, C.; Mummidisetty, C.K.; Shin, S.Y.; Rushin, C.; Jenz, S.; Xu, S.; et al. Rapid Screening of Physiological Changes Associated With COVID-19 Using Soft-Wearables and Structured Activities: A Pilot Study. IEEE J. Transl. Eng. Health Med. 2021, 9, 1–11. [Google Scholar] [CrossRef]
- Russo, V.; Rapacciuolo, A.; Pafundi, P.C.; de Divitiis, M.; Volpicelli, M.; Ruocco, A.; Rago, A.; Uran, C.; Nappi, F.; Attena, E.; et al. Cardiac implantable electronic devices replacements in patients followed by remote monitoring during COVID-19 lockdown. Eur. Heart J. Digit. Health 2021, 2, 171–174. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Ogedengbe, O.; Agarwal, P.; Money-Coomes, S.; Abdurrahman, A.Z.; Mohammed, S.; Kalra, P.A.; Rothwell, N.; Pradhan, S. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting-a cohort study. BMC Geriatr. 2020, 20, 409. [Google Scholar]
- Hussain, A.; Mahawar, K.; Xia, Z.; Yang, W.; Shamsi, E.-H. Obesity and mortality of COVID-19. Meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 295–300. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Olsson, P.-E. Sex differences in severity and mortality from COVID-19: Are males more vulnerable? Biol. Sex. Differ. 2020, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Nachman, D.; Constantini, K.; Poris, G.; Wagnert-Avraham, L.; Gertz, D.S.; Littman, R.; Eisenkraft, A.; Gepner, Y. Wireless, Non-Invasive, Wearable Device for Continuous Remote Monitoring of Hemodynamic Parameters in a Swine Model of Controlled Hemorrhagic Shock. Sci. Rep. 2020, (in press). [Google Scholar] [CrossRef] [PubMed]
- Nachman, D.; Gepner, Y.; Goldstein, N.; Kabakov, E.; Ishay, A.B.; Littman, R.; Azmon, Y.; Jaffe, E.; Eisenkraft, A. Comparing blood pressure measurements between a photoplethysmography-based and a standard cuff-based manometry device. Sci. Rep. 2020, 10, 16116. [Google Scholar] [CrossRef]
- Nachman, D.; Gilan, A.; Goldstein, N.; Constantini, K.; Littman, R.; Eisenkraft, A.; Grossman, E.; Gepner, Y. Twenty-Four-Hour Ambulatory Blood Pressure Measurement Using a Novel Noninvasive, Cuffless, Wireless Device. Am. J. Hypertens. 2021, hpab095. [Google Scholar] [CrossRef]
- Kachel, E.; Constantini, K.; Nachman, D.; Carasso, S.; Littman, R.; Eisenkraft, A.; Gepner, Y. A Pilot Study of Blood Pressure Monitoring After Cardiac Surgery Using a Wearable, Non-invasive Sensor. Front. Med. 2021, 8, 693926. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014.
- Chau, N.V.V.; Lam, V.T.; Dung, N.T.; Yen, L.M.; Minh, N.N.Q.; Ngoc, N.M.; Dung, N.T.; Man, D.N.H.; Nguyet, L.A.; Ny, N.T.H. The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. medRxiv 2020. [Google Scholar] [CrossRef]
- Kim, J.; Volodarskiy, A.; Sultana, R.; Pollie, M.P.; Yum, B.; Nambiar, L.; Tafreshi, R.; Mitlak, H.W.; RoyChoudhury, A.; Horn, E.M.; et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 1965–1977. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Naqvi, T.Z. The Right Ventricle: A Target in COVID-19 Cardiac Insult; American College of Cardiology Foundation: Washington, DC, USA, 2020. [Google Scholar]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.-P. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Pinney, S.P.; Lala, A.; Reddy, V.Y.; Johnston-Cox, H.A.; Mechanick, J.I.; Halperin, J.L.; Fuster, V. Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia. J. Am. Coll. Cardiol. 2020, 76, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.-L.; Liu, M.-J.; Zhang, Z.-B.; Fang, L.-Q.; Yuan, J.; Zhang, A.-R.; Dean, N.E.; Luo, L.; Ma, M.-M.; Longini, I. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: A retrospective cohort study. Lancet Infect. Dis. 2020, 20, 1141–1150. [Google Scholar] [CrossRef]
- Quer, G.; Radin, J.M.; Gadaleta, M.; Baca-Motes, K.; Ariniello, L.; Ramos, E.; Kheterpal, V.; Topol, E.J.; Steinhubl, S.R. Wearable sensor data and self-reported symptoms for COVID-19 detection. Nat. Med. 2020, 27, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, L.; Wymant, C.; Kendall, M.; Zhao, L.; Nurtay, A.; Abeler-Dörner, L.; Parker, M.; Bonsall, D.; Fraser, C. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 2020, 368, eabb6936. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).