Assessment of Coronary Inflammation by Pericoronary Fat Attenuation Index in Clinically Suspected Myocarditis with Infarct-Like Presentation
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Participants
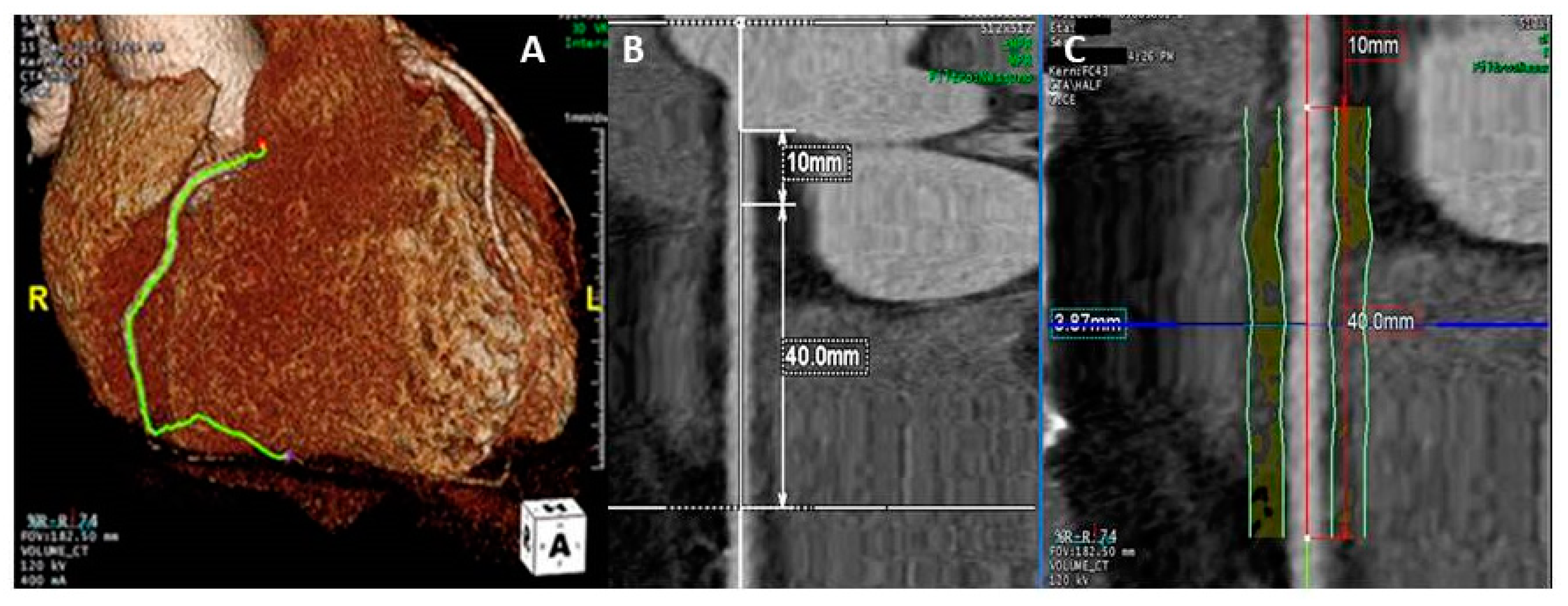
2.2. Coronary Computed Tomography Angiography
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
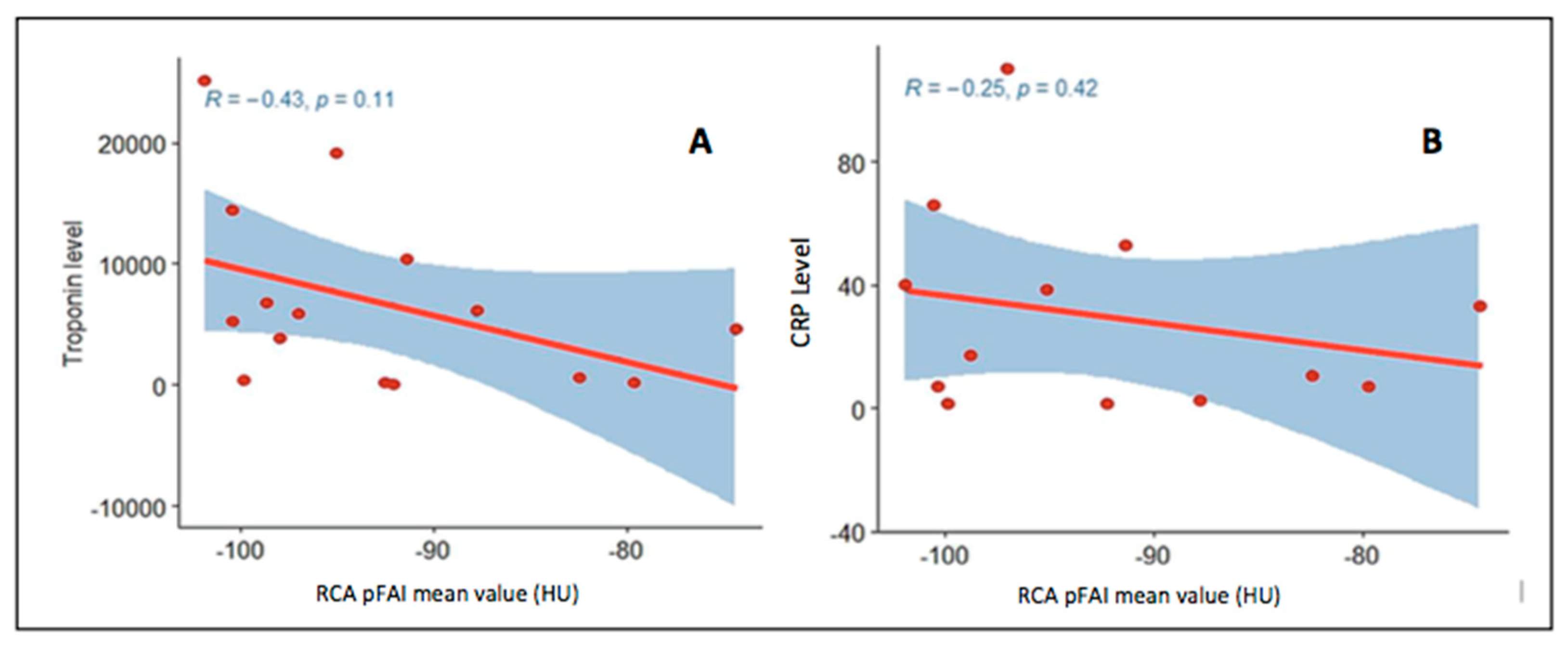
3.2. Analysis of Pericoronary Adipose Tissue
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Gimeno-Bianes, J.; Felix, S.B.; Fu, M.; Helio, T.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Calzolari, V.; Calabrese, F.; Boffa, G.M.; Maddalena, F.; Chioin, R.; Thiene, G. Myocarditis mimicking acute myocardial infarction: Role of endomyocardial biopsy in the differential diagnosis. Heart 2000, 84, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caforio, A.L.P.; Calabrese, F.; Angelini, A.; Tona, F.; Vinci, A.; Bottaro, S.; Ramondo, A.; Carturan, E.; Iliceto, S.; Thiene, G.; et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007, 28, 1326–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budoff, M.J.; Achenbach, S.; Blumenthal, R.S.; Carr, J.J.; Goldin, J.G.; Greenland, P.; Guerci, A.D.; Lima, J.A.C.; Rader, D.J.; Rubin, G.D.; et al. Assessment of coronary artery disease by cardiac computed tomography: A scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006, 114, 1761–1791. [Google Scholar] [PubMed] [Green Version]
- Antoniades, C.; Kotanidis, C.P.; Berman, D.S. State of the art review article. Atherosclerosis affecting fat: What can we learn by imaging perivascular adipose tissue? J. Cardiovasc. Comput. Tomogr. 2019, 13, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.-M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef] [Green Version]
- Mancio, J.; Oikonomou, E.K.; Antoniades, C. Perivascular adipose tissue and coronary atherosclerosis. Heart 2018, 104, 1654–1662. [Google Scholar] [CrossRef]
- Lin, A.; Dey, D.; Wong, D.T.L.; Nerlekar, N. Perivascular Adipose Tissue and Coronary Atherosclerosis: From Biology to Imaging Phenotyping. Curr. Atheroscler. Rep. 2019, 21, 47. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Kepiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [Green Version]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing (4.0.2) [Computer Software]. R Foundation for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 16 September 2020).
- Gamer, M.; Lemon, J.; Singh, I.F.P. “irr”: Various Coefficients of Interrater Reliability and Agreement. 2019. Available online: https://CRAN.R-project.org/package=irr (accessed on 16 September 2020).
- Sugiyama, T.; Kanaji, Y.; Hoshino, M.; Yamagughi, M.; Hada, M.; Misawa, T.; Sumino, Y.; Nogami, K.; Ueno, H.; Kakuta, T. Prognostic value of fat attenuation index of pericoronary adipose tissue surrounding left anterior descending artery on coronary computed tomography angiography. Eur. Heart J. 2020, 41 (Suppl. S2), ehaa946.1346. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Martini, C.; Botti, A.; Pinazzi, A.; Bottazzi, B.; Palumbo, A.A. Coronary Inflammation by Computed Tomography Pericoronary Fat Attenuation in MINOCA and Tako-Tsubo Syndrome. J. Am. Heart Assoc. 2019, 8, e013235. [Google Scholar] [CrossRef]
- Ma, R.; Ties, D.; van Assen, M.; Pelgrim, G.J.; Sidorenkov, G.; van Ooijen, P.M.A.; van der Harst, P.; van Dijk, R.; Vliegenthart, R. Towards reference values of pericoronary adipose tissue attenuation: Impact of coronary artery and tube voltage in coronary computed tomography angiography. Eur. Radiol. 2020, 30, 6838–6846. [Google Scholar] [CrossRef] [PubMed]
- Balcer, B.; Dykun, I.; Schlosser, T.; Forsting, M.; Rassaf, T.; Mahabadi, A.A. Pericoronary fat volume but not attenuation differentiates culprit lesions in patients with myocardial infarction. Atherosclerosis 2018, 276, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Deng, J.; Yu, M.; Lu, Z.; Shen, C.; Zhang, J. Perivascular fat attenuation index and high-risk plaque features evaluated by coronary CT angiography: Relationship with serum inflammatory marker level. Int. J. Cardiovasc. Imaging 2020, 36, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary adipose tissue computed tomography attenuation and high-risk plaque characteristics in acute coronary syndrome compared with stable coronary artery disease. JAMA Cardiol. 2018, 3, 858–863. [Google Scholar] [CrossRef] [Green Version]
- Pergola, V.; Previtero, M.; Cecere, A.; Storer, V.; Castiello, T.; Baritussio, A.; Cabrelle, G.; Mele, D.; Motta, R.; Caforio, A.L.P.; et al. Clinical value and time course of pericoronary fat inflammation in patients with angiographically nonobstructive coronaries: A preliminary report. J. Clin. Med. 2021, 10, 1786. [Google Scholar] [CrossRef]
- Woudstra, L.; Juffermans, L.J.M.; van Rossum, A.C.; Niessen, H.W.M.; Krijnen, P.A.J. Infectious myocarditis: The role of the cardiac vasculature. Heart Fail. Rev. 2018, 23, 583–595. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, T.; Monma, N.; Satodate, R.; Segawa, I.; Oyama, K.; Kawana, R.; Kurata, T. Myocardial lesions by Coxsackie virus B3 and cytomegalovirus infection in infants. Heart Vessels Suppl. 1985, 1, 167–172. [Google Scholar] [CrossRef]
- Bültmann, B.D.; Klingel, K.; Sotlar, K.; Bock, C.T.; Baba, H.A.; Sauter, M.; Kandolf, R. Fatal parvovirus B19-associated myocarditis clinically mimicking ischemic heart disease: An endothelial cell-mediated disease. Hum. Pathol. 2003, 34, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Kühl, U.; Rohde, M.; Lassner, D.; Gross, U.M.; Escher, F.; Schultheiss, H.P. miRNA as activity markers in Parvo B19 associated heart disease. Herz 2012, 37, 637–643. [Google Scholar] [CrossRef] [PubMed]
| Clinically Suspected Myocarditis Undergoing CCTA n = 15 | Clinically Suspected Myocarditis Not Undergoing CCTA n = 450 | p-Value | |
|---|---|---|---|
| Sex, female | 5 (33) | 127 (28) | 0.67 |
| Age at diagnosis, years | 30 ± 10 | 36 ± 15 | 0.16 |
| Family history of immune-mediated disease | 3 (20) | 63 (14) * | 0.53 |
| Acute viral infection 6 months before | 3 (21) ** | 225 (51) * | 0.032 |
| Immune-mediated diseases | 0 (0) | 51 (11) | 0.17 |
| Diabetes | 0 (0) | 6 (1) | 0.65 |
| Clinical presentation | |||
| Arrhythmias | 1 (7) # | 28 (6) | 0.94 |
| Heart failure | 1 (7) # | 29 (6) | 0.97 |
| Infarct-like | 13 (87) | 380 (84) | 0.81 |
| Asymptomatic | 0 (0) | 10 (2) | 0.56 |
| Symptoms | |||
| Palpitations | 2 (13) | 31 (7) *** | 0.35 |
| Syncope | 3 (20) | 16 (4) *** | 0.002 |
| Chest pain | 9 (64) ** | 250 (56) ^ | 0.56 |
| Embolism | 1 (7) | 1 (0.2) *** | <0.001 |
| NYHA class | 0.031 | ||
| I | 12 (80) | 419 (94) ^^ | |
| II-IV | 3 (20) ## | 28 (6) | |
| Peak hs-Troponin I level, ng/L | 5197 (364–8469) | 331 (88–1181) | 0.12 |
| Peak C reactive protein level, mg/dL | 16.4 (6.1–40.0) ** | 25.6 (7.5–63.8) ° | 0.27 |
| AHA positivity | 4/10 (40) | 184/374 (49) | 0.57 |
| LA antero-posterior diameter, mm | 50.8 ± 8.5 ^^^ | 41.1 ± 11.0 °° | 0.007 |
| LVEDV Echo, mL/m2 | 65 ± 13 °°° | 63 ± 16 § | 0.43 |
| LVEF, % | 56.4 ± 9.5 ** | 56.2 ± 9.5 §§ | 0.80 |
| RVEDA, cm2 | 22.2 ± 3.4 §§§ | 20.3 ± 4.1 ¶ | 0.097 |
| FAC, % | 42.6 ± 7.8 §§§ | 45.1 ± 7.6 ¶¶ | 0.68 |
| LVEDV CMR, mL/m2 | 86 ± 13 °°° | 86 ± 18 ¶¶¶ | 0.96 |
| LVESV CMR, mL/m2 | 36.8 ± 6.6 °°° | 37.0 ± 12.0 ¶¶¶ | 0.74 |
| RVEDV, mL/m2 | 87 ± 13 °°° | 81 ± 17 ¶¶¶ | 0.16 |
| RVESV, mL/m2 | 38.8 ± 8.4 °°° | 33.2 ± 9.7 ¶¶¶ | 0.058 |
| LVEF CMR, % | 57.3 ± 4.0 °°° | 57.6 ± 6.6 ¶¶¶ | 0.49 |
| RVEF CMR, % | 56.0 ± 4.7 °°° | 60.0 ± 6.8 ¶¶¶¶ | 0.048 |
| Myocardial oedema | 9 (60) | 225/357 (63) | 0.47 |
| Myocardial LGE | 12 (86) ** | 319/347 (92) | 0.41 |
| Observer 1 | Observer 2 | ICC pFAI Value | ICC Vessel Diameter | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pFAI Value (HU) | Vessel Diameter (mm) | pFAI Value (HU) | Vessel Diameter (mm) | ICC | 95% CI | p-Value | ICC | 95% CI | p-Value | |
| RCA | −92.8 ± 8.4 | 3.38 ± 0.74 | −92.9 ± 8.3 | 3.51 ± 0.97 | 0.994 | 0.981–0.998 | <0.01 | 0.851 | 0.623–0.947 | <0.01 |
| LAD | −90.3 ± 6.1 | 4.15 ± 0.88 | −91.1 ± 6.2 | 3.91 ± 0.86 | 0.945 | 0.839–0.982 | <0.01 | 0.851 | 0.623–0.947 | <0.01 |
| LCx | −86.4 ± 9.0 | 3.74 ± 1.01 | −86.1 ± 9.4 | 3.70 ± 0.59 | 0.958 | 0.780–0.993 | <0.01 | 0.825 | 0.253–0.968 | <0.01 |
| Group 1 pFAI Values < RCA Median pFAI Value n = 7 | Group 2 pFAI Values ≥ RCA Median pFAI Value n = 8 | p-Value | |
|---|---|---|---|
| Sex, male | 4 (57) | 6 (75) | 0.46 |
| Age at diagnosis, years | 32.7 ± 10.5 | 27.6 ± 9.9 | 0.34 |
| Family history of immune-mediated disease | 3 (43) | 0 (0) | 0.038 |
| Acute viral infection 6 months before | 2 (29) | 1 (14) | 0.52 |
| Symptoms before diagnosis | 5 (71) | 4 (50) | 0.4 |
| Palpitations | 1 (14) | 1 (12) | 0.92 |
| Syncope | 1 (14) | 2 (25) | 0.6 |
| Chest pain | 5 (71) | 4 (57) | 0.58 |
| Embolism | 1 (14) | 0 (0) | 0.3 |
| NYHA class | 0.63 | ||
| I | 6 (86) | 6 (75) | |
| II-IV | 1 (14) | 2 (24) | |
| Peak hs-Troponin I level, ng/L | 5884 (4438–10,554) | 2442 (146–7078) | 0.26 |
| C reactive protein level at diagnosis, mg/dL | 28.2 (8.7–59.0) | 10.0 (4.0–35.2) | 0.50 |
| AHA positivity | 1 (5) * | 3 (60) * | 0.20 |
| LVEDV Echo, mL/m2 | 62 ± 15 | 68 ± 11 | 0.46 |
| LVEF Echo, % | 60.6 ± 6.4 | 52.3 ± 10.8 | 0.095 |
| LVEDV CMR, mL/m2 | 82 ± 14 | 90 ± 12 ** | 0.18 |
| LVESV CMR, mL/m2 | 33.5 ± 4.0 | 39.6 ± 7.3 ** | 0.038 |
| LVEF CMR, % | 58.5 ± 4.4 | 56.3 ± 3.7 ** | 0.32 |
| RVEDV CMR, mL/m2 | 83 ± 13 | 91 ± 13 ** | 0.34 |
| RVESV, mL/m2 | 32.8 ± 3.2 | 43.9 ± 8.2 ** | 0.024 |
| RVEF CMR, % | 58.8 ± 3.3 | 53.6 ± 4.4 ** | 0.038 |
| LGE presence | 6 (86) | 6 (75) ** | 0.38 |
| Pleural effusion | 1 (17) | 1 (14) ** | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baritussio, A.; Vacirca, F.; Ocagli, H.; Tona, F.; Pergola, V.; Motta, R.; Marcolongo, R.; Lorenzoni, G.; Gregori, D.; Iliceto, S.; et al. Assessment of Coronary Inflammation by Pericoronary Fat Attenuation Index in Clinically Suspected Myocarditis with Infarct-Like Presentation. J. Clin. Med. 2021, 10, 4200. https://doi.org/10.3390/jcm10184200
Baritussio A, Vacirca F, Ocagli H, Tona F, Pergola V, Motta R, Marcolongo R, Lorenzoni G, Gregori D, Iliceto S, et al. Assessment of Coronary Inflammation by Pericoronary Fat Attenuation Index in Clinically Suspected Myocarditis with Infarct-Like Presentation. Journal of Clinical Medicine. 2021; 10(18):4200. https://doi.org/10.3390/jcm10184200
Chicago/Turabian StyleBaritussio, Anna, Francesco Vacirca, Honoria Ocagli, Francesco Tona, Valeria Pergola, Raffaella Motta, Renzo Marcolongo, Giulia Lorenzoni, Dario Gregori, Sabino Iliceto, and et al. 2021. "Assessment of Coronary Inflammation by Pericoronary Fat Attenuation Index in Clinically Suspected Myocarditis with Infarct-Like Presentation" Journal of Clinical Medicine 10, no. 18: 4200. https://doi.org/10.3390/jcm10184200
APA StyleBaritussio, A., Vacirca, F., Ocagli, H., Tona, F., Pergola, V., Motta, R., Marcolongo, R., Lorenzoni, G., Gregori, D., Iliceto, S., & Caforio, A. L. P. (2021). Assessment of Coronary Inflammation by Pericoronary Fat Attenuation Index in Clinically Suspected Myocarditis with Infarct-Like Presentation. Journal of Clinical Medicine, 10(18), 4200. https://doi.org/10.3390/jcm10184200










