Abstract
Endothelial cells that cover the lumen of all blood vessels have the inherent capacity to express both pro and anticoagulant molecules. However, under normal physiological condition, they generally function to maintain a non-thrombogenic surface for unobstructed blood flow. In response to injury, certain stimuli, or as a result of dysfunction, endothelial cells release a highly adhesive procoagulant protein, von Willebrand factor (VWF), which plays a central role in formation of platelet aggregates and thrombus generation. Since VWF expression is highly restricted to endothelial cells, regulation of its levels is among the most important functions of endothelial cells for maintaining hemostasis. However, with aging, there is a significant increase in VWF levels, which is concomitant with a significant rise in thrombotic events. It is not yet clear why and how aging results in increased VWF levels. In this review, we have aimed to discuss the age-related increase in VWF, its potential mechanisms, and associated coagulopathies as probable consequences.
Keywords:
von Willebrand factor; endothelial cells; aging; thrombosis; platelet aggregates; ADAMTS13; COVID-19 1. Introduction
A major contributing factor to aging-associated morbidity and mortality is the increased prevalence of thrombogenicity and consequently thrombotic disorders [1]. Thrombosis is the formation of blood clots within a blood vessel, and complications of thrombosis may cause acute life-threatening or chronic conditions [2]. Thrombotic events in large vessels, such as coronary arteries and cerebral blood vessels, could lead to acute catastrophic events, for instance, heart attack and stroke respectively. Moreover, microvascular thrombosis may contribute to the target organ(s)’ suboptimal functioning as a result of perfusion hindrance. Microvascular thrombosis is the hallmark of a number of vascular diseases, including thrombotic microangiopathy, disseminated intravascular coagulation, and sickle cell disease among others [2,3]. Although many genetic and environmental risk factors contribute to thrombotic events in the general population, aging is a universal and non-modifiable risk factor for thrombosis [4]. Epidemiological studies have indicated an association between age and higher risk incidents of thrombotic cardiovascular complications, including acute myocardial infarction, stroke, and venous thromboembolism [5,6,7]. Aging considerably increases the incidence of venous and arterial thrombotic events, with an annual rate of venous thromboembolism (VTE) from 1 per 10,000 in young, to 1 in 100 among the elderly population over the age of 80 [8]. It has been reported that after the age of 45, the incidence of atherothrombotic brain infarction and myocardial infarction doubles after every 10 years of life [7,9]. The cause of this sharp increase in thrombosis with aging is not yet clearly understood.
The dramatic increase in thrombogenicity in the elderly population may reflect the pathophysiology of aging. Aging-associated development of molecular and anatomical abnormalities have been detected that could contribute to this process. These include increase in diameter and thickness of vessel walls, fragmentation of internal elastic lamina, and vascular smooth muscle cells hypertrophy within the vessel walls that results in endothelial injury, which is a key factor in promoting thrombosis [10,11,12,13]. Aging is also associated with the development of a pro-inflammatory phenotype, a well-established risk factor for thrombosis [14,15,16]. Furthermore, several clinical studies have reported that aging alters the levels of various circulating and vessel wall-associated factors, leading to an imbalance in the ratio of procoagulants to anticoagulants in favour of procoagulants, and consequently a prothrombotic state [17,18].
Procoagulant protein von Willebrand factor (VWF) is among factors that display an age-associated increase in circulation [19]. VWF is fundamental to the process of thrombus generation since it initiates the first step of the process, specifically adherence of platelets to endothelial/subendothelial surfaces [20]. It also mediates the subsequent step of platelet-platelet interactions that is necessary for the formation of platelet aggregates [20]. The circulating levels of VWF are not only elevated with aging in healthy population, but are also influenced by multiple pathologies associated with the process of aging, such as insulin resistance, obesity, and various risk factors that are associated with cardiovascular disorders [21,22]. Thus, it stands to reason that increased production of VWF, without a proportional increase in anticoagulant proteins, may contribute significantly to increased thrombogenicity with aging [16,19]. In this review, we aim to discuss the impact of aging on the VWF levels, its consequences under physiological/pathophysiological conditions, and potential mechanism(s) that may instigate aging-associated elevation of VWF.
2. VWF Role in Physiology
VWF is an adhesive multimeric glycoprotein that plays a pivotal role in normal hemostasis and thrombosis [23]. It was first identified by Dr. Eric von Willebrand during the treatment of a severe bleeding disorder [24]. The gene coding for VWF is located on chromosome 12, spans 180 kb and contains 52 exons [25,26]. VWF expression is strictly restricted to two cell types, endothelial cells (ECs) and megakaryocytes (precursor of platelets) [25]. VWF biosynthesis is a complex process that starts with the translation of an approximately 9 kb VWF mRNA to generate a precursor protein that is subjected to a wide range of post-translational modifications. These include dimerization, multimerization, and cleavage, in addition to extensive glycosylation and sulfation [27,28]. Cleavage of the precursor protein generates two components, pro-peptide (VWFpp) and mature subunit (VWF), which remain non-covalently associated until secreted into the bloodstream [27]. Multimerized VWF is stored in specialized organelles known as Weibel-Palade bodies (WPB) and alpha granules in endothelial cells and platelets, respectively [27]. While alpha-granules release VWF upon activation of platelets, endothelial cells release VWF by two pathways, namely constitutive and regulatory secretions. Dimer/smaller multimers of VWF are secreted continuously from endothelial cells (consecutive secretion), while most biologically active ultra-large WPB-stored multimers (UL-VWF) are secreted through regulatory pathway in response to inducers [29]. Biosynthesis, structure, processing, and secretion of VWF have been subjects of many excellent reviews periodically [27,29,30].
VWF that is secreted from endothelial cells either enters circulation or is deposited in the subluminal extracellular matrix, which when exposed in response to injury, provides the binding sites for platelets [2,6]. Due to its highly adhesive nature that allows association with various extracellular matrix proteins, circulating VWF can also interact with other proteins (such as collagen) in exposed extracellular matrix at the site of vascular injury [31]. When the shear rate is elevated, globular VWF is unfurled and its various binding sites are exposed, mediating a transient interaction between VWF and platelet [32]. This leads to initial platelet captures and slows platelets movement, which is then stabilized through the interaction of various other proteins, in addition to VWF (Figure 1) [33,34]. Once initially captured platelets are stabilized and activated to release their VWF, VWF and other proteins on adherent activated platelets provide recruitment and attachment sites for additional circulating platelets [35,36]. Activated platelets provide a major site for prothrombin complexes assembly and enhance thrombin generation, thus directly participating in thrombosis. This physiological function of subluminal VWF is critical for maintaining vessel integrity while the repair of the vessel wall proceeds at the site of injury. Upon completion of the repair process, VWF cleaving enzymes, mainly metalloproteinase ADAMTS13 (although other proteases that target VWF, i.e., ADAMTS28, have also been identified [37]), as well as other thrombolytic enzymes, mediate the disassembly of platelet aggregates, leading to thrombus resolution and restoration of unobstructed blood flow (Figure 1). However, VWF is also shown to bind to the endothelial cell membrane in the intact luminal surface [38]. Membrane-bound VWF could also mediate platelet adhesion to the intact endothelial surface under permissive conditions (Figure 1), and consequently, this may be a contributing factor towards undesired thrombus formation [38].
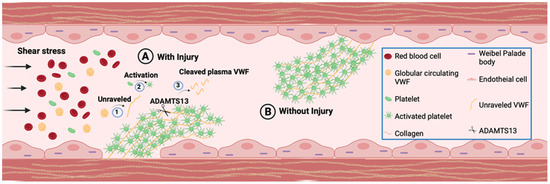
Figure 1.
VWF Function. VWF is stored in Weibel-Palade bodies (WPB) and deposited in extracellular matrix of vascular endothelial cells, as well as circulating in plasma as globular form. (A) At the site of injury in presence of shear stress globular VWF attach to the exposed subendothelial collagen layer and (1) unravel to form the most adhesive form of VWF. The extracellular matrix deposited VWF is similarly exposed to bind platelets. Platelets captured by unraveled VWF are (2) activated and form VWF-platelet aggregates to seal the damaged vessel wall and prevent blood leakage while repair. Following the completion of repair process ADAMTS13 (Scissors) will (3) cleave UL VWF to smaller VWF multimers to resolve platelet aggregates. (B) External stimuli induce secretion of VWF, which may bind to endothelial luminal surface membrane and mediate platelet aggregate formation through a similar process as that observed at the site of vascular injury.
VWF also performs additional hemostatic functions by binding and stabilizing FVIII (a vital coagulation protein of the intrinsic clotting pathway) in circulation. Furthermore, VWF has been recently shown to participate in a number of other physiological and pathophysiological processes, such as inflammation, angiogenesis, and cancer metastasis, which are the subject of many recent excellent reviews and will not be discussed here [39,40,41,42].
Plasma levels of VWF are influenced by a combination of environmental, genetic, and pathological factors, although what is considered as normal levels constitute a wide range (50–200%) in a healthy population [35]. It is estimated that about 30% of the variation in the plasma VWF levels is related to genetic factors through pedigree analyses, while twins’ studies have suggested this value to be 65% [35,36,43,44]. Another major contributing factor to the variation in circulating VWF levels is the ABO blood group, which is proposed to account for approximately 30% of the observed variability [35,45]. Blood Group O individuals have 25% lower plasma VWF levels than non-O blood group individuals [45,46]. The presence of AB antigens coincides with a protective function against VWF clearance. This is proposed as the main reason for higher VWF levels in the non-O blood group; although recent evidence suggesting a significantly decreased levels of cellular VWF protein in lung endothelial cells of the O blood group individuals compared to non-O have also been presented [47].
3. Coagulopathies Associated with von Willebrand Factor
Considering the central role of VWF in hemostasis and thrombosis, both decreased and increased levels of VWF (outside the normal range) are associated with coagulopathies. Deficiencies of VWF, either quantitative or qualitative, are associated with the most common inherited bleeding disorder known as Von Willebrand disease (VWD) [48,49,50]. There are four main types of VWD based on phenotypic analysis of the VWF: Type 1, Type 2, Type 3, and platelet-type [51,52,53]. The most common and mildest form of VWD is Type 1 (quantitative) that is caused by low VWF production or accelerated clearance [53]. Type 2 VWD (qualitative) is characterized by defects in VWF (abnormal function), and it accounts for 20–40% of VWD cases [51]. Whereas Type 3 (qualitative) VWD is a total quantitative deficiency of VWF and is the most severe form of VWD that represents 5% of VWD cases [53]. In platelet-type VWD, a gain of function mutation within platelet surface receptor for VWF leads to exaggerated platelet-VWF interaction, resulting in thrombocytopenia and consequently prolonged bleeding time [52].
In contrast to VWF deficiency, increased plasma VWF levels and functional activity is a significant risk factor for thrombosis [54]. The thrombotic event may involve large vessels, leading to acute consequences such as stroke or heart attack [55]; or implicate microvasculature, leading to various chronic diseases such as thrombotic microangiopathy [56]. Significantly elevated levels of VWF are observed in thrombotic thrombocytopenic purpura (TTP), which results from a profound deficiency of ADAMTS13. It is the leading cause of thrombotic microangiopathy characterized by the formation of platelets aggregates in arterioles, capillaries, and venules [57,58]. Several other pathological conditions including vascular dementia [58,59], Alzheimer’s disease [59,60], traumatic brain injury [61], acute respiratory distress syndrome [62], and kidney failure [63] are also associated with increased plasma levels of VWF. Such disorders may arise or be exacerbated potentially as a consequence of increased microvascular occlusion.
4. Influence of Aging on VWF and ADAMTS13 in a Healthy Population
Age-associated increases in plasma levels of VWF have been reported in studies using animal models [64,65,66], but also well-established in the human population (Table 1). A study of 74 centenarians and 110 individuals aged 45–86 years old has shown that VWF antigen levels, and its functional activity, were markedly elevated in centenarians compared to the younger cohort [67]. The study did not find any significant difference between Blood Group O and non-O [67]. Another cross-sectional study on a cohort of 207 individuals aged 1–87 years demonstrated that the levels of circulating VWF increased significantly in the elderly (with an overall increase of 1.56-fold), and that the increase was more significant in the non-O blood group individuals [68]. Although the influence of the ABO blood group was not significant in young individuals, it became more pronounced with advancing age [68]. Furthermore, the age-related increase in VWF levels was accompanied by a 2.03-fold increase in VWF functional activity in older individuals [68]. This increase in VWF levels was followed by simultaneous elevation in FVIII coagulant activity and antigen levels (reaching up to 1.5-fold by later life in healthy individuals) [68,69]. A recent cross-sectional study of 2923 individuals also reported elevation of VWF:Ag as well as FVIII coagulation activity per decade of age after adjustment for comorbidities including body mass index, inflammation and hormone use; and the increase was higher for non-O blood group individuals [69]. However, the influence of aging and plasma VWF levels on FVIII level and coagulant activity in mild Hemophilia A patients is scarcely studied. One study reported a significant positive correlation between FVIII coagulant activity and aging without amelioration of bleeding [70]; whereas another study identified only minor influence of aging on FVIII levels/activity [71]. A cross-sectional study of 3616 Japanese participants, aged 30 to 79 years reported that VWF levels tend to increase (significantly influenced by ABO blood groups), whereas ADAMTS13 activity was decreased (not significantly influenced by ABO blood groups) with advancing age, leading to an increased ratio of plasma VWF levels -to-ADAMTS13 activity with aging in both men and women [72]. It is worth noting that this study reported a lower level of ADAMTS13 activity in men compared to women, suggesting that elderly males are at a higher risk of developing hypercoagulable state and consequently thrombosis [72]. Another study of the healthy Arab population revealed that VWF levels increase with aging, while ADAMTS13 antigen levels remain unaffected but ADAMTS13 activity decreases with aging [73]. Inconsistent findings have been reported concerning the impact of biological sex on rising VWF levels with aging. The large study of blood donors in South Wales (n = 5052) reported that there was a minor but significant difference between VWF levels among men and women (approximately 114 IU for men vs. 109 IU for women) [73]. In contrast, the study of the healthy Arab population found lower plasma levels of VWF in females compared to males, but only in Blood Group O females [73]. ADAMTS13 activity and antigen levels were unaffected by blood groups in both genders of the Arab population [73]. Collectively these reports suggest that the trend of rising VWF levels with aging is similar in both women and men. While it is reported that women have a higher rate of increase in the VWF plasma levels during middle age (41–50 years), this may be attributed to changes in hormone levels during pregnancy, menstrual cycle, hormone-based contraceptives, and menopause, all of which can influence VWF levels [74,75,76].
From several studies, it is clearly demonstrated that circulating VWF levels tend to increase gradually with aging in humans, at a 1–2% rate per year in adults, but the absolute increase is greater with advancing age [72,77,78]. However, a higher rate of elevation in VWF levels with age occurs after midlife (above 40 years of age) in both men and women [21,74]. It is reported that among different variables such as age, gender, blood type, and use of medications, only age was significantly predictive of VWF level, and exhibited age-related elevation [74]. Since VWFpp and mature VWF have different half-lives (2 h and around 8–12 h for each respectively), VWFpp levels have been used as a marker for endothelial cell activation and VWF release [68,79]. VWFpp and mature VWF are cleared independently, hence multiple studies have utilized the ratio between VWFpp and VWF antigen (VWF:Ag) levels to evaluate the synthesis and clearance of VWF [80,81]. These analyses have demonstrated that in addition to upregulated VWF secretion, a reduction in the ratio of the VWFpp:VWF antigen is observed with aging, suggesting an age-associated decrease in VWF clearance and consequently its increased half-life in circulation [68]. However, a report of immunohistochemical analysis of cellular VWF expression in lung tissues of 64 pulmonary neoplasia patients [26 children (mean age 3.5 years) and 38 adults (mean age 55 years)], demonstrated a significantly elevated levels of cellular VWF in arterioles, capillaries, and venules of adult lungs compared to children [82]. This observation suggests that in addition to increased secretion and decreased clearance, a potential increase in VWF biosynthesis, specifically in smaller vessels, maybe a significant contributing factor to the elevated VWF levels in response to aging. Furthermore, another potential source of the age-associated increase in VWF may involve alteration in platelets activity and consequently increased release of VWF from platelets alpha-granules. For instance, aging is associated with increased content of basal phosphoinositide in the platelet plasma membrane [83], which is important for activating the second messenger signaling pathway and triggering platelet activity. This suggests that aging may alter platelet transmembrane signaling pathways and consequently its propensity for activation and as a result platelet aggregate formation. Furthermore, studies have shown an age-related reduction in nitric oxide (NO) levels [84,85,86], contributing to abnormal platelet aggregation. Collectively, procoagulant alterations in endothelial cells’ phenotype and platelets activities with aging, may contribute to enhanced VWF levels and decreased ADAMTS13 activities, providing the foundation for thrombosis.

Table 1.
Effect of age on Von Willebrand factor in the healthy population.
Table 1.
Effect of age on Von Willebrand factor in the healthy population.
| Location | Population Characteristics | Results | Author |
|---|---|---|---|
| Italy | 184 healthy individuals included 74 centenarians (100–107 years), 55 younger controls (<45 years), 55 older controls (>45 years) | centenarians VWF:Ag level 245 (U/dL) in O blood group and 285 (U/dL) in non-O blood group; older controls VWF:Ag level 99 (U/dL) in O blood group and 152 (U/dL) in non-O blood group; younger controls VWF:Ag level 77 (U/dL) in O blood group and 115 (U/dL) in non-O blood group; VWF activity level centenarians > older controls > younger controls | Coppola et al., 2003 [67] |
| Canada | 207 healthy individuals included 113 old (55–87 years), 42 middle-age (30–49 years), 52 young (1–17 years) | Plasma VWF level increased with age reaching a 1.71-fold by old age in non-O and 1.25-fold in O blood group. VWF activity reached 2.03-fold by old age and VWFpp level (as a marker of VWF secretion) elevated to 1.26-fold in older individuals with blood type non-O than blood type O. | Albanez et al., 2016 [68] |
| United Kingdom | Cohort of 5052 healthy individuals | VWF:Ag level increase minor and non-significant up to age of 40 years but elevated significantly above age >40 years. For the whole cohort absolute increase between each age group: 2 IU/dL between <20 years and 21–30 years, 3 IU/dL between 21–30 years and 31–40 years, 7 IU/dL between 31–40 years and 41–50 years, 8 IU/dL between 41–50 years and 51–60 years, and 15 IU/dL between 51–60 years and 61–70 years. | Davies et al., 2012 [74] |
| Japan | Cohort of 3616 healthy Japanese population between the age range of 30–79 years | VWF:Ag increased with advancing age and the linear regression coefficient being 1.37 and 1.30 in men and women respectively. Plasma ADMATS13 activity decreased with age, significantly after 60 years and regression coefficient was −0.642 resulted in increased ratio of VWF:Ag-to-ADAMTS13 activity with age. | Kokame et al., 2011 [72] |
| Kuwait | 200 healthy individuals | Plasma VWF level significantly higher in older individuals and ADAMTS-13 activity decreased with age, however, ADAMTS13 level not affected by age. | Al-Awadhi et al., 2014 [73] |
| The Netherlands | Cohort of 2923 individuals between the age range of 18–70 years | VWF:Ag increase per decade of age 18 IU/dL and for FVIII activity 12 IU/dL. After adjustment for acquired factors (comorbidities, body mass index, reduced kidney function, hormone use, and inflammation), the increase per decade 13 IU/dL for VWF:Ag and 9 IU/dL for FVIII activity. Increase was higher in blood group non-O. | Biguzzi et al., 2021 [69] |
5. Impact of Aging on von Willebrand Disease
The normal reference range of plasma VWF levels is between 50 and 200 IU/dL in a healthy population [87]. However, as described in the previous section, quantitative (Types 1 and 3) and qualitative (Type 2) deficiencies of VWF occurs that result in von Willebrand disease (VWD), characterized by excessive mucocutaneous bleeding. Although VWF levels in the range of 30–50 IU/dL have been generally considered as an indication of VWD, currently this range is considered as a low normal VWF level, which is nevertheless a risk factor for developing bleeding disorders [87]. The observation of an age-associated increase in plasma VWF levels of a healthy population suggests that aging may also impact elderly VWD patients. While Type 2 and Type 3 VWD are diagnosed due to qualitative deficiencies or severe reductions in VWF levels, diagnosing and differentiating mild forms of VWD—Type 1 from low levels of VWF is more challenging. Regardless of classification into normal but low levels, or Type I mild VWD, this population might be a target group that could benefit from the age-related rise in VWF levels. Consistent with this hypothesis, VWF levels were shown to rise with aging in milder forms of VWD, but not in severe forms, such as Type 2 and 3 VWD [22,88,89]. Furthermore, normalization of VWF levels with aging may affect symptoms, severity, and diagnosis, in mild forms of VWD. A retrospective study of 126 patients with Type 1 VWD or ‘low VWF’ indicated a significant increase in VWF levels and activity in elderly VWD Type 1 patients, leading to complete VWF levels normalization in 27.8% of participants, although amelioration of bleeding symptoms was not shown [90]. Another retrospective study of Type 1 VWD patients aged 16–60 for eleven years found normalization of VWF antigen levels, activity, and FVIII levels in 18 patients out of 31 [91]. Additionally, Willebrand in the Netherlands (WiN-) study of 664 VWD patients aged 16–85 years found that with aging VWF levels fell within the normal reference range in mild Type 1 VWD, while remained unchanged in Type 2 VWD [92]. Increased VWF levels were also followed by elevated VWF activity and FVIII levels in Type 1 VWD, but not in Type 2 VWD [92]. Very few studies have been performed involving Type 2 VWD patients, whereas none of the studies have included Type 3 VWD patients. This may reflect the expectation that qualitative deficiencies associated with Type 2 VWD will not be ameliorated with increased levels; and that the nature of extensive deficiency associated with Type III VWD (i.e., gene deletion), is incompatible with potential increases in VWF levels.
To evaluate the effect of comorbidities in VWD patients, a cross-sectional study was performed that included 536 VWD Type 1 and Type 2 patients aged 16–83 year [22]. It was found that hypertension, diabetes mellitus, thyroid dysfunction and cancer were associated with increased VWF levels in Type 1 VWD compared to VWD patients without these comorbidities, whereas no association was found in Type 2 VWD patients [22]. Lowered VWFpp:VWF-antigen ratio indicated that reduced clearance of VWF in circulation was a potential cause of elevated VWF levels in these comorbidities [22]. It is vital to investigate and establish the effect of aging on VWD patients since it has serious consequences on the treatment of VWD generally, as well as in conjunction with other age-related comorbidities.
A recent study has described in details the impact of aging on VWF levels and presented an in-depth discussion of its association with bleeding symptoms in VWD patients [89]. However, despite accumulating evidence in support of age-associated increase in VWF levels and function, whether this results in amelioration of bleeding symptoms in VWD patients remains unclear. The uncertainty and conflicting reports are proposed to be due to differences in the study designs and procedures, patients’ characteristics, or presence of comorbidities. It is also hypothesized that with aging there may be a requirement for higher levels of VWF for optimal hemostasis; thus, amelioration of bleeding symptoms may not be observed in aging VWD patients [89].
6. Potential Mechanisms of Age-Associated Increase in VWF
An increase in plasma levels of VWF may result from increased regulated and/or constitutive secretion. Numerous factors including thrombin, histamine, nitric oxide (NO), calcium, vasopressin, epinephrine, leukotrienes, superoxide anions, sphingosine-1-phosphate, endothelin-1 (ET-1), prostacyclin and shear stress were shown to affect VWF plasma levels [93,94,95,96,97,98]. While some factors may alter the regulation of VWF secretions, others may affect VWF levels by altering VWF gene transcription/post-transcriptional activity [95,96,97]. A combination of alterations in secretion and production levels may contribute to increase circulating VWF levels.
Although the underlying mechanism of age-associated increase in VWF remains unclear, age-related alterations in the circulatory and vascular physiology/pathophysiology could provide some insights. Elevated VWF levels with aging may be an intrinsic property of aged endothelial cells, may occur in response to various age-related vascular dysfunctions, and/or increased inflammation. Aging generally leads to the development of a pro-inflammatory phenotype in the elderly population [99,100], and inflammation is a major contributing factor to increased thrombogenicity [101]. Pathogenicity of inflammation-induced thrombosis is complex, and interactions between inflammation and thrombosis is a bidirectional process: inflammation promotes platelets aggregation and coagulation, while activated platelets and coagulation factors promote inflammation [99,100]. Vascular injury is the key factor that associates inflammation with thrombosis; however, inflammation-induced thrombosis may also develop without endothelial injury [100]. Elevated levels of pro-inflammatory markers have been reported in many older individuals, even in the absence of pre-existing health conditions or related risk factors [102,103]. Observation of elevated inflammatory mediators, leading to chronic low-grade inflammation in the aged population, is known as “inflammaging”; and it promotes endothelial dysfunction [102]. VWF plasma level is known as a useful marker for endothelial dysfunction and may be relevant to the disease process. An age-related alteration in endothelium and surrounding microenvironment may also contribute to age-associated increase in VWF levels and thrombogenicity. Aging alters the levels of vasodilator NO and vasoconstrictor endothelin1 (ET-1), which are both regulators of VWF secretion [104]. Nitric oxide (NO) inhibits exocytosis of WPB and platelets’ alpha granules; hence, it inhibits VWF release [93,105]. Aging is shown to result in decreased NO bioavailability and sensitivity to NO, thus ameliorating/reducing its’ inhibitory effect on exocytosis and consequently promoting increased VWF secretion from storage organelles [106,107]. In contrast, ET-1 that increases VWF expression in endothelial cells, and shown to cause elevated circulating VWF levels when infused in men [108], exhibits an elevated levels with aging.
Aging also affects circadian rhythm and is associated with fragmentation or loss of rhythm [106]. VWF promoter activity is under direct regulation of major circadian transcriptional regulators CLOCK/Bmal1 [109]. Bmal1 deficiency not only directly increases VWF mRNA and protein levels, but also downregulates nitric oxide synthase (eNOS) activity and consequently decreases NO production; thus, indirectly contributing to VWF release as well [109,110]. However, it should be noted that the reported age-associated decrease in Bmal1 expression was based on analyses of the master circadian pacemaker in the suprachiasmatic nucleus (SCN), the hippocampus, and cingulate cortex [111]. Further studies are required to determine whether aging similarly downregulates Bmal1 in other tissues/cell types, including endothelial cells to directly establish that decreased Bmal1 with aging increases VWF levels.
Another potential contributor to the age-related increase in VWF could be related to alteration in osmolarity. In-vivo studies in mice demonstrated that water restriction and elevated plasma sodium concentration, above normal physiological range, significantly increased VWF mRNA and protein levels [112]. This process was shown to be mediated through binding of the tonicity-regulated transcription factor NFAT5 to the VWF promoter [112]. With aging water intake decreases and the risk of dehydration increases. Numerous studies on the elderly have reported that aging decreases perceived thirst and water intake in response to hypovolemia, hypertonicity, and hormonal stimulus [113,114,115]. Thus, dehydration in older people increases sodium concentration and this could lead to an increase in VWF production as well as secretion.
In addition to increased VWF levels, aging is also reported to be associated with a decrease in the level and activity of VWF cleaving enzyme ADAMTS13 [67,72]. Thus, a combination of these two events cumulatively contributes to the generation and persistence of ultra-large VWF multimers in circulation [67,72]. Under the condition of high shear stress, ADAMTS13 mediates increased proteolysis of high molecular weight (HMW) [116], but aging is reported to be also associated with reduced shear stress, thus further contributing to a condition that promotes persistence of high molecular weight VWF in the circulation and elevated platelets aggregation [117]. Together these age-associated alterations directly and/or indirectly increase VWF production, secretion, and stability, potentially leading to the generation of platelets aggregates and consequently increased thrombotic events (Figure 2).
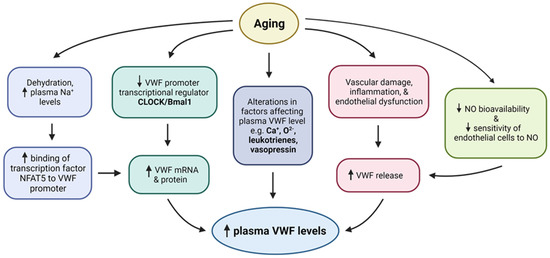
Figure 2.
Potential mechanisms of age associated increase in VWF levels. Upward and downward arrows depict increase and decrease respectively.
7. VWF and COVID-19
Various infectious diseases including HIV [118], malaria [119], scrub typhus [120], and Dengue virus [121] infection have been associated with thrombosis and increased circulating VWF levels. The COVID-19 disease caused by the SARS-CoV-2 (CoV-2) virus starts with an early infectious stage, but it may be followed by viral pneumonia and systemic inflammation that could potentially lead to respiratory failure and multiple organ dysfunction [122,123,124,125]. It is now well established that the COVID-19 is also associated with hypercoagulation and severe thrombotic complications—‘Corona virus-associated coagulopathy’ (CAC) [126,127,128]. Several studies have reported incidence of ischemic stroke, deep vein thrombosis, pulmonary thromboembolism, and thrombosis in small brain vessels leading to cerebral microbleeds in 20–30% of COVID-19 patients admitted to the intensive care unit (ICU). The incidences of these coagulopathies are significantly higher among aging population of COVID-19 patients and encompass more than 50% of elderly non-survivors [129,130,131,132]. CoV-2 infection is reported to facilitate vascular inflammation (vasculitis) and enhance the prothrombotic state, potentially leading to organ dysfunction due to the presence of microangiopathy and microthrombi in vascular beds of multiple organs [133,134,135].
Multiple hematological abnormalities are observed in COVID-19 patients including elevated levels of fibrinogen and D-Dimer in critically ill patients [127,136,137]. However, hematological analyses of these patients have also found that VWF levels and activities were elevated five to six folds beyond the upper normal limit [128,138]. In a cohort of 150 elderly COVID-19 patients with a medical history of cardiovascular, respiratory, and multiple organ abnormalities, median VWF levels were reported at 455% of normal [128]. In some cases, VWF elevation is augmented by a deficiency of the VWF cleavage enzyme ADAMTS-13 or reduced ADAMTS13 activity in the blood [126,139,140]. Considering the age-associated increase in VWF, it is plausible to hypothesize that elevated basal levels of VWF in elderly may increase their susceptibility towards development of CAC complications as a result of CoV-2 infection. Consistent with this hypothesis are the observations that microangiopathy in the lung is highly prevalent among COVID-19 patients with CAC [125,141,142,143], and that age-associated increase, specifically in cellular expression of VWF, is observed in lung microvasculature [82].
CoV-2 infection is not only associated with increased plasma levels of VWF but also was reported to have a qualitative impact on the VWF activity, specifically enhancing its adhesive properties, such as collagen binding [140]. While more investigation is required, emerging data suggest that COVID-19 is associated with increased consumption of HMWM VWF [140,144,145], which could be indicative of increased platelets aggregte formation. Consistent with this, lower platelet counts were observed in some COVID-19 patients, which could be due to hyperactivation of platelets and aggregate formation following platelet interaction with VWF [128,146].
Regarding ADAMTS13 levels in COVID-19 patients, the reports are less consistent. Some studies reported normal levels of ADAMTS13 while few reported decreased levels of ADAMTS13[126,147,148]. There have also been reports of a significantly reduced ratio of ADAMTS13/VWF, which suggest that while ADAMTS13 may or may not be reduced, a very high concentration of VWF could overwhelm the capacity of ADAMTS13 [145]. It is also noteworthy that neutrophil extracellular traps (NETs) are found in the microvascular thrombi present in the heart, lung, or kidney of COVID-19 patients [149]. It is reported that contents released from NETs might reduce enzymatic activity of ADAMTS13, consequently increasing VWF levels [150]. Summary of the literature reports of investigation into the levels and activities of VWF and ADAMTS13 in critically ill COVID-19 patients with and without coexisting comorbidities is presented in Table 2. While the mechanism for increased VWF levels in COVID-19 patients with the hypercoagulable state remains unclear, it is noteworthy that elevated VWF levels are predominantly reported in critically ill patients with health conditions/comorbidities that are also associated with increased VWF levels, including elderly and/or those with hypertension and diabetes mellitus. Elevated VWF levels associated with various chronic diseases and natural aging may generate a heightened prothrombotic state. This, combined with CoV-2 induced increase in VWF levels, could significantly tip the balance of pro- and anti- thrombotic state in favor of thrombosis.
CoV-2 may directly infect endothelial cells, and/or indirectly stimulate endothelial cells to release VWF as a result of enhanced inflammatory environment. The lungs were reported as the main target of CoV-2, which can bind to Angiotensin-converting enzyme 2 (ACE2), on the alveolocytes [151]. ACE2 receptor, which belongs to the renin-angiotensin system and has a major role in regulating blood pressure, is also expressed on the endothelial surfaces of the heart, lung, kidney, and systemic vessels [152]. ACE2 is proposed to mediate CoV-2 entry into endothelial cells [153,154], independently, or potentially in combination with other proteins, which have been also identified as receptors for CoV-2 on endothelial cell surface [155]. However, interaction of virus particles with ACE2 may also lead to a loss of ACE2 enzymatic function and consequently decrease plasma level of vaso-protective angiotensin [156,157,158]. This in turn could result in acute-phase inflammatory responses leading to endothelialitis (vascular endothelial inflammation) and hypersecretion of VWF [159,160,161]. Proinflammatory conditions arising as a result of cytokine storm generation further contribute to prolonged endothelial stimulation and activation of complement cascade; both of which stimulate VWF release [159,160,161]. Inflammation, endothelial injury, and dysregulated immune response instigate assembly of membrane attack complex (MAC) on endothelial cells, which increases endothelial cytosolic Ca2+ concentration that stimulates VWF release from Weible-Palade bodies [162]. Inflammatory cytokine TNF-alpha can regulate VWF expression indirectly by decreasing NO synthesis through inhibition of eNOS expression in endothelial cells [163,164]. Consistent with this, significantly decreased NO levels has been reported as a result of CoV-2 infection [165,166]. Additionally, significantly increased IL-8 and IL-6 inflammatory cytokines also promote VWF secretion in a concentration-dependent manner. Furthermore, IL-6 has been reported to also decrease ADAMTS13 activity, which further increases accumulation of ultra-large VWF levels with higher activity [167].

Table 2.
VWF and ATDAMTS13 levels ad activity reported in critically ill COVID-19 patients with and without preexisting comorbidities. Normal reference VWF antigen range (%) 42–136 a. Normal reference VWF activity range (%) 42–168 b. Normal ADAMTS13 antigen reference range (%) 40–130 c. Normal ADAMTS13 activity reference range (%) 50–150 d. The value is reported as median (interquartile range) e.
Table 2.
VWF and ATDAMTS13 levels ad activity reported in critically ill COVID-19 patients with and without preexisting comorbidities. Normal reference VWF antigen range (%) 42–136 a. Normal reference VWF activity range (%) 42–168 b. Normal ADAMTS13 antigen reference range (%) 40–130 c. Normal ADAMTS13 activity reference range (%) 50–150 d. The value is reported as median (interquartile range) e.
| Location | Patient Characteristics (n) | Mean Age (years) | VWF Antigen (%) a Mean (Min–Max) | VWF Activity (%) b Mean (Min–Max) | ADAMTS13 (%) c Mean (Min–Max) | ADAMTS13 Activity (%) d Mean (Min–Max) | Author |
|---|---|---|---|---|---|---|---|
| Italy | Intubated COVID-19 patients (11) | – | 529 (210–863) | 387 (195–550) | – | – | Panigada et al., 2020 [146] |
| The Netherlands | COVID-19 patients admitted to ICU (12) | 61.8 | 408.0 | 374.0 | 48.0 | – | Huisman et al., 2020 [168] |
| France | Patients with radiological signs of interstitial pneumonia (212) | 63.9 | 361.0 | – | – | – | Rauch et al., 2020 [169] |
| Germany e | COVID-19 patients with high severity (150) | 63.0 | 455 (350–521) | 328 (212–342) | – | – | Helms et al., 2020 [128] |
| Italy e | COVID-19 patients with high severity (19) | 59.0 | 476 (381–537) | 388 (328–438) | – | 55 (42–68) | Mancini et al., 2021 [144] |
| United Kingdom e | Intubated ICU patients (24) | 65.0 | 350 (302–433) | – | – | – | Ladikou et al., 2020 [170] |
| Ireland | COVID-19 patients admitted to ICU (28) | 55.0 | 690.2 (467–848.4) | – | – | – | Ward et al., 2021 [171] |
| France e | Patients with critical form of COVID-19 in ICU (89) | 62.0 | 507 (428–596) | 399 (333–537) | – | – | Philippe et al., 2021 [172] |
| France e | COVID-19 patients with Venous thromboembolic events (38) | 63.0 | 522 (411–672) | – | – | 59 (38.8–70.5) | Delrue et al., 2021 [173] |
| Italy | Intubated ICU patients (6) | 62.0 | 634 (455–772) | 450 (339–496) | 37.3 (24–56) | – | Morici et al., 2020 [148] |
| United States | Intubated ICU patients (48) | 64.0 | 565.0 | 390.0 | – | – | Goshua et al., 2020 [138] |
| Italy | Patients with COVID-19 pneumonia (37) | 61.8 | 280.8 | 265.1 | – | – | Taus et al., 2020 [174] |
| Spain | ICU patients with the history of hypertension and diabetes mellitus (22) | 68.0 | 368.6 | – | – | 38.9 | Marco et al., 2021 [175] |
| Italy e | Patients with novel coronavirus pneumonia (10) | 61.0 | 324.1 | 341.5 | 69.0 | – | De Cristofaro 2021 [176] |
| Spain e | COVID-19 patients with cardiovascular disease and diabetes (23) | 64.0 | 306.0 | – | 47.3 | – | Blasi et al., 2020 [177] |
| Germany | COVID-19 patients with mild to moderate severity (75) | 66.0 | 403.0 | – | 67.8 | – | Doevelaar et al., 2021 [145] |
| Italy | Patients with the history of hypertension and diabetes (19) | 69.0 | 331.4 | 321.7 | – | – | Ruberto et al., 2020 [178] |
| United States e | COVID-19 non-survivors (90) | 72.5 | 441.0 | 321.0 | – | 48.8 | Sweeney et al., 2020 [179] |
| Spain e | Severe COVID-19 patients (50) | – | 355 (267–400) | – | 53.2 (38.8–65.3) | – | Rodríguez et al., 2021 [180] |
| Italy | COVID-19 non-survivors (9) | 72.0 | 395.5 | – | 32.2 | – | Bazzan et al., 2020 [126] |
| Belgium | COVID-19 patients admitted to ICU (9) | 57–64 | 475.0 | 429.0 | 45.0 | – | Hardy et al., 2020 [181] |
| United States e | 17 | 42–58 | 448 (362–529) | 313 (190–347) | – | – | Masi et al., 2020 [182] |
| Sweden e | Patients with COVID-19 high care (12) | 53–69 | 425 (321–465) | – | 57 (42–62) | – | Meijenfeldt et al., 2021 [183] |
| Germany | COVID-19 non-survivors (5) | 78.0 | 260.4 | 217.6 | – | 43.3 | De Jongh et al., 2021 [184] |
| France e | COVID-19 patients admitted to ICU (22) | – | 456 (402–493) | 355 (297–416) | 458 (364–615) | – | Pascreau et al., 2021 [185] |
Another factor that may also contribute to elevated levels of VWF in COVID-19 patients is development of hypoxic conditions. COVID-19 patients may suffer from acute lung injury that causes acute respiratory distress syndrome (ARDS) and decreases tissue oxygenation, leading to hypoxic condition. Hypoxia was shown to be a mediator of VWF release from WPB [186]. Furthermore, we have previously demonstrated that hypoxia also induces VWF transcriptional upregulation, and consequently contributes to increased platelet aggregates formation [187,188]. Specifically, in the lungs of mice, hypoxia altered the VWF expression pattern, leading to expression of VWF in a significant proportion of lung microvascular endothelial cells that generally do not exhibit VWF expression under normoxic conditions [187]. Thus, COVID-19 induced hypoxia may upregulate VWF levels, and this has been supported by the clinical observation of rising VWF levels with increased oxygen requirements in a few COVID-19 patients [169]. Additionally, it is noteworthy that elevated VWF levels has been reported only in COVID-19 positive pneumonia patients [176]. This suggests that synergistic effects of hypoxia with other COVID-19 related conditions might be responsible for increase in VWF levels during the disease progression. Thus, pre-existing factors, such as aging and comorbidities, in combination with hypoxic, immunologic, and proinflammatory alterations that ensue in COVID-19 infection, may collectively contribute to a significant increase in VWF levels and consequently increased thrombogenicity.
COVID-19 does not affect all individuals in a similar manner, and this is also reflected in development of hypercoagulability and thrombosis. This, however, is consistent with highly variable basal levels of VWF in population [35], which may play a role in predisposing certain individuals to increased risk of CAC development. For instance, individuals with high basal levels of VWF, when aged and/or have comorbidities, if infected with CoV-2 may reach a VWF threshold that leads to the development of thrombosis, while people with low basal levels of VWF may not. Consistent with this hypothesis, some studies have indicated that COVID-19 patients with Blood Group O (25% lower VWF levels than other blood groups) are significantly underrepresented in thrombogenic complications [189,190]. Similarly, Type I VWD patients with significantly lower VWF levels are also underrepresented in groups of COVID-19 patients with thrombogenic consequences. These observations suggest that determining the level and activity of VWF in COVID-19 patients may provide a useful prognostic marker towards COVID-19 morbidity and mortality as well as identifying patient populations that are at the risk of developing thrombotic complications.
8. Conclusions
Aging is associated with increased risk of arterial, venous, and microvascular thrombosis in the elderly population. Elevated levels of VWF in circulation, as well as cellular levels, are also reported with aging. Considering the central role of VWF in thrombus formation, a likely association between this rise in VWF and thrombogenicity is conceivable. Various external factors, specifically alterations in inflammatory and immunological landscape may significantly contribute to the increased production of VWF with aging. However, age-associated increase in VWF occurs in healthy population and exhibits a gradual constant increase. Thus, it is also conceivable that inherent alterations in endothelial cell phenotype may occur with aging that culminates in increased production of VWF. Such possibility may be specifically important in relation to microvascular endothelial cells, which do not normally exhibit VWF expression. Testing of this hypothesis will provide significant insights towards determining the underlying molecular basis of age-associated increase in VWF. It will also contribute to potential development of targeted therapies to combat various age-associated macro- and microvascular diseases in elderly population with and without comorbidities.
Therapeutic approaches that are developed or under consideration include strategies to interfere with binding of platelets to VWF, or reduction in the levels of ULVWF multimers. For instance, some clinical treatment options include blocking of VWF and platelet interaction using anti VWF antibody such as AJW200 [191,192,193] or a humanized anti-VWF antibody fragment (nanobody) caplacizumab, which bind to VWF A1 domain [194,195]. In addition, anfibatide, a snake-venom derived compound that is an antagonist of GPIb receptor on platelets, can inhibit platelet-VWF interaction [196,197]. Reduction in the levels of ULVWF may be achieved through restoration/increase of ADAMTS13 function by introduction of recombinant ADAMTS13 (rADAMTS13) [198] or through the action of N-acetylcysteine (NAC) that disrupts disulfide bonds in the VWF A1 domain [199,200]. In addition, aptamers that are DNA or RNA single-stranded nucleic acids, which bind to their target protein with high affinity and specificity, could present as novel potential therapeutic agents. DNA aptamer TAGX-0004 [201] and RNA aptamer BT200 [202] are inhibitors of the VWF A1 domain that may be considered as potential inhibitors of VWF function.
Author Contributions
Conceptualization, N.J., P.A. and A.M.R.; literature survey, P.A. and A.M.R. writing—original draft preparation, P.A. and A.M.R.; writing—review and editing, N.J.; supervision, N.J. funding acquisition, N.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Sciences and Engineering Research Council of Canada Discovery Grant (NSERC-DG) RGPIN-2019-04903, (N.J.); and the University Hospital Foundation Marshall Eliuk Grant (N.J.).
Acknowledgments
Figures were created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilkerson, W.R.; Sane, D.C. Aging and thrombosis. Semin. Thromb. Hemost. 2002, 28, 555–568. [Google Scholar] [CrossRef]
- Paramo, J.A. Microvascular thrombosis and clinical implications. Med. Clin. 2021, 156, 609–614. [Google Scholar] [CrossRef]
- Bray, M.A.; Sartain, S.E.; Gollamudi, J.; Rumbaut, R.E. Microvascular thrombosis: Experimental and clinical implications. Transl. Res. 2020, 225, 105–130. [Google Scholar] [CrossRef]
- Cushman, M. Epidemiology and risk factors for venous thrombosis. Semin. Hematol. 2007, 44, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.; Adams, R.; Carnethon, M.; De Simone, G.; Ferguson, T.B.; Flegal, K.; Ford, E.; Furie, K.; Go, A.; Greenlund, K.; et al. Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009, 119, e21–e181. [Google Scholar] [CrossRef] [PubMed]
- Yazdanyar, A.; Newman, A.B. The burden of cardiovascular disease in the elderly: Morbidity, mortality, and costs. Clin. Geriatr. Med. 2009, 25, 563. [Google Scholar] [CrossRef]
- Kelly-Hayes, M. Influence of age and health behaviors on stroke risk: Lessons from longitudinal studies. J. Am. Geriatr. Soc. 2010, 58 (Suppl. 2), S325–S328. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch. Intern. Med. 1998, 158, 585–593. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Thijssen, D.H.; Carter, S.E.; Green, D.J. Arterial structure and function in vascular ageing: Are you as old as your arteries? J. Physiol. 2016, 594, 2275–2284. [Google Scholar] [CrossRef]
- Najjar, S.S.; Scuteri, A.; Lakatta, E.G. Arterial aging: Is it an immutable cardiovascular risk factor? Hypertension 2005, 46, 454–462. [Google Scholar] [CrossRef]
- Hemmeryckx, B.; Hoylaerts, M.F.; Deloose, E.; Van Hove, C.E.; Fransen, P.; Bult, H.; Lijnen, H.R. Age-associated pro-inflammatory adaptations of the mouse thoracic aorta. Thromb. Haemost. 2013, 110, 785–794. [Google Scholar] [CrossRef]
- Fleenor, B.S.; Eng, J.S.; Sindler, A.L.; Pham, B.T.; Kloor, J.D.; Seals, D.R. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell 2014, 13, 576–578. [Google Scholar] [CrossRef]
- Morrisette-Thomas, V.; Cohen, A.A.; Fülöp, T.; Riesco, É.; Legault, V.; Li, Q.; Milot, E.; Dusseault-Bélanger, F.; Ferrucci, L. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech. Ageing Dev. 2014, 139, 49–57. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef]
- Schlaudecker, J.; Becker, R. Inflammatory response and thrombosis in older individuals. Semin. Thromb. Hemost. 2014, 40, 669–674. [Google Scholar] [CrossRef]
- Gleerup, G.; Winther, K. The effect of ageing on platelet function and fibrinolytic activity. Angiology 1995, 46, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Mari, D.; Mannucci, P.M.; Coppola, R.; Bottasso, B.; Bauer, K.A.; Rosenberg, R.D. Hypercoagulability in centenarians: The paradox of successful aging. Blood 1995, 85, 3144–3149. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, C.; Palomo, I.; Fuentes, E. Primary and secondary haemostasis changes related to aging. Mech. Ageing Dev. 2015, 150, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Arya, M.; Anvari, B.; Romo, G.M.; Cruz, M.A.; Dong, J.F.; McIntire, L.V.; Moake, J.L.; López, J.A. Ultralarge multimers of von Willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein Ib-IX complex: Studies using optical tweezers. Blood 2002, 99, 3971–3977. [Google Scholar] [CrossRef] [PubMed]
- Konkle, B.A. Von Willebrand factor and aging. Semin. Thromb. Hemost. 2014, 40, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Atiq, F.; Meijer, K.; Eikenboom, J.; Fijnvandraat, K.; Mauser-Bunschoten, E.P.; van Galen, K.P.M.; Nijziel, M.R.; Ypma, P.F.; de Meris, J.; Laros-van Gorkom, B.A.P.; et al. Comorbidities associated with higher von Willebrand factor (VWF) levels may explain the age-related increase of VWF in von Willebrand disease. Br. J. Haematol. 2018, 182, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Sadler, J.E. von Willebrand factor. J. Biol Chem 1991, 266, 22777–22780. [Google Scholar] [CrossRef]
- Von Willebrand, E.A. Hereditary pseudohaemophilia. Haemophilia 1999, 5, 223–231. [Google Scholar] [CrossRef]
- Collins, C.J.; Underdahl, J.P.; Levene, R.B.; Ravera, C.P.; Morin, M.J.; Dombalagian, M.J.; Ricca, G.; Livingston, D.M.; Lynch, D.C. Molecular cloning of the human gene for von Willebrand factor and identification of the transcription initiation site. Proc. Natl. Acad. Sci. USA 1987, 84, 4393–4397. [Google Scholar] [CrossRef]
- Ginsburg, D.; Handin, R.I.; Bonthron, D.T.; Donlon, T.A.; Bruns, G.A.; Latt, S.A.; Orkin, S.H. Human von Willebrand factor (vWF): Isolation of complementary DNA (cDNA) clones and chromosomal localization. Science 1985, 228, 1401–1406. [Google Scholar] [CrossRef]
- Lenting, P.J.; Christophe, O.D.; Denis, C.V. von Willebrand factor biosynthesis, secretion, and clearance: Connecting the far ends. Blood 2015, 125, 2019–2028. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Wagner, D.D. von Willebrand factor biosynthesis and processing. Ann. N. Y. Acad. Sci. 1991, 614, 153–166. [Google Scholar] [CrossRef]
- Xiang, Y.; Hwa, J. Regulation of VWF expression, and secretion in health and disease. Curr. Opin. Hematol. 2016, 23, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.A. Von Willebrand factor processing. Hamostaseologie 2017, 37, 59–72. [Google Scholar] [CrossRef]
- Ruggeri, Z.M.; Mendolicchio, G.L. Adhesion mechanisms in platelet function. Circ. Res. 2007, 100, 1673–1685. [Google Scholar] [CrossRef]
- Lancellotti, S.; Sacco, M.; Basso, M.; Cristofaro, R.D. Mechanochemistry of von Willebrand factor. Biomolecular. Concepts 2019, 10, 194–208. [Google Scholar] [CrossRef]
- Aird, W.C. Endothelium in health and disease. Pharmacol. Rep. 2008, 60, 139–143. [Google Scholar] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Swystun, L.L.; Lillicrap, D. Genetic regulation of plasma von Willebrand factor levels in health and disease. J. Thromb. Haemost. 2018, 16, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- de Lange, M.; Snieder, H.; Ariens, R.A.; Spector, T.D.; Grant, P.J. The genetics of haemostasis: A twin study. Lancet 2001, 357, 101–105. [Google Scholar] [CrossRef]
- Mochizuki, S.; Soejima, K.; Shimoda, M.; Abe, H.; Sasaki, A.; Okano, H.J.; Okano, H.; Okada, Y. Effect of ADAM28 on carcinoma cell metastasis by cleavage of von Willebrand factor. J. Natl. Cancer Inst. 2012, 104, 906–922. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Goerge, T.; Schneider, S.W.; Wagner, D.D. Formation of platelet strings and microthrombi in the presence of ADAMTS-13 inhibitor does not require P-selectin or beta3 integrin. J. Thromb. Haemost. 2007, 5, 583–589. [Google Scholar] [CrossRef]
- Randi, A.M.; Laffan, M.A. Von Willebrand factor and angiogenesis: Basic and applied issues. J. Thromb. Haemost. 2017, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Randi, A.M.; Smith, K.E.; Castaman, G. von Willebrand factor regulation of blood vessel formation. Blood 2018, 132, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, C.; Lenting, P.J.; Denis, C.V. von Willebrand factor and inflammation. J. Thromb. Haemost. 2017, 15, 1285–1294. [Google Scholar] [CrossRef]
- Patmore, S.; Dhami, S.P.S.; O’Sullivan, J.M. Von Willebrand factor and cancer; metastasis and coagulopathies. J. Thromb. Haemost. 2020, 18, 2444–2456. [Google Scholar] [CrossRef] [PubMed]
- Bladbjerg, E.M.; de Maat, M.P.; Christensen, K.; Bathum, L.; Jespersen, J.; Hjelmborg, J. Genetic influence on thrombotic risk markers in the elderly--a Danish twin study. J. Thromb. Haemost. 2006, 4, 599–607. [Google Scholar] [CrossRef]
- Souto, J.C.; Almasy, L.; Soria, J.M.; Buil, A.; Stone, W.; Lathrop, M.; Blangero, J.; Fontcuberta, J. Genome-wide linkage analysis of von Willebrand factor plasma levels: Results from the GAIT project. Thromb. Haemost. 2003, 89, 468–474. [Google Scholar] [PubMed]
- Gill, J.C.; Endres-Brooks, J.; Bauer, P.J.; Marks, W.J., Jr.; Montgomery, R.R. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood 1987, 69, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Gallinaro, L.; Cattini, M.G.; Sztukowska, M.; Padrini, R.; Sartorello, F.; Pontara, E.; Bertomoro, A.; Daidone, V.; Pagnan, A.; Casonato, A. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood 2008, 111, 3540–3545. [Google Scholar] [CrossRef]
- Murray, G.P.; Post, S.R.; Post, G.R. ABO blood group is a determinant of von Willebrand factor protein levels in human pulmonary endothelial cells. J. Clin. Pathol. 2020, 73, 347–349. [Google Scholar] [CrossRef]
- Higasa, S.; Tokugawa, T.; Sawada, A. Diagnosis and management of von Willebrand disease. Rinsho Ketsueki 2018, 59, 2222–2232. [Google Scholar] [CrossRef]
- James, P.D.; Goodeve, A.C. von Willebrand disease. Genet. Med. 2011, 13, 365–376. [Google Scholar] [CrossRef]
- Sadler, J.E.; Mannucci, P.M.; Berntorp, E.; Bochkov, N.; Boulyjenkov, V.; Ginsburg, D.; Meyer, D.; Peake, I.; Rodeghiero, F.; Srivastava, A. Impact, diagnosis and treatment of von Willebrand disease. Thromb. Haemost. 2000, 84, 160–174. [Google Scholar] [CrossRef]
- Sadler, J.E.; Budde, U.; Eikenboom, J.C.; Favaloro, E.J.; Hill, F.G.; Holmberg, L.; Ingerslev, J.; Lee, C.A.; Lillicrap, D.; Mannucci, P.M.; et al. Update on the pathophysiology and classification of von Willebrand disease: A report of the Subcommittee on von Willebrand Factor. J. Thromb. Haemost. 2006, 4, 2103–2114. [Google Scholar] [CrossRef]
- Othman, M. Platelet-type von Willebrand disease: A rare, often misdiagnosed and underdiagnosed bleeding disorder. Semin. Thromb. Hemost. 2011, 37, 464–469. [Google Scholar] [CrossRef]
- Yadegari, H.; Oldenburg, J. The Current Understanding of Molecular Pathogenesis of Quantitative von Willebrand Disease, Types 1 and 3. Hamostaseologie 2020, 40, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, M. Thrombosis and von Willebrand Factor. Adv. Exp. Med. Biol. 2017, 906, 285–306. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Barbour, T.; Johnson, S.; Cohney, S.; Hughes, P. Thrombotic microangiopathy and associated renal disor-ders. Nephrol. Dial. Transplant. 2012, 27, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Sadler, J.E. Pathophysiology of thrombotic thrombocytopenic purpura. Blood 2017, 130, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Furlan, M.; Robles, R.; Solenthaler, M.; Lämmle, B. Acquired deficiency of von Willebrand factor-cleaving protease in a patient with thrombotic thrombocytopenic purpura. Blood 1998, 91, 2839–2846. [Google Scholar] [CrossRef]
- Yavuz, B.B.; Dede, D.S.; Yavuz, B.; Cankurtaran, M.; Halil, M.; Ulger, Z.; Cankurtaran, E.S.; Aytemir, K.; Kabakci, G.; Haznedaroglu, I.C.; et al. Potential biomarkers for vascular damage in Alzheimer’s disease: Thrombomodulin and von Willebrand factor. J. Nutr. Health Aging 2010, 14, 439–441. [Google Scholar] [CrossRef]
- Mari, D.; Parnetti, L.; Coppola, R.; Bottasso, B.; Reboldi, G.P.; Senin, U.; Mannucci, P.M. Hemostasis abnormalities in patients with vascular dementia and Alzheimer’s disease. Thromb. Haemost. 1996, 75, 216–218. [Google Scholar] [CrossRef]
- De Oliveira, C.O.; Reimer, A.G.; Da Rocha, A.B.; Grivicich, I.; Schneider, R.F.; Roisenberg, I.; Regner, A.; Simon, D. Plasma von Willebrand factor levels correlate with clinical outcome of severe traumatic brain injury. J. Neurotrauma 2007, 24, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- El Basset Abo El Ezz, A.A.; Abd El Hafez, M.A.; El Amrousy, D.M.; El Momen Suliman, G.A. The predictive value of Von Willebrand factor antigen plasma levels in children with acute lung injury. Pediatr. Pulmonol. 2017, 52, 91–97. [Google Scholar] [CrossRef] [PubMed]
- van der Vorm, L.N.; Visser, R.; Huskens, D.; Veninga, A.; Adams, D.L.; Remijn, J.A.; Hemker, H.C.; Rensma, P.L.; van Horssen, R.; de Laat, B. Circulating active von Willebrand factor levels are increased in chronic kidney disease and end-stage renal disease. Clin. Kidney J. 2020, 13, 72–74. [Google Scholar] [CrossRef]
- Ito, Y.; Sorensen, K.K.; Bethea, N.W.; Svistounov, D.; McCuskey, M.K.; Smedsrod, B.H.; McCuskey, R.S. Age-related changes in the hepatic microcirculation in mice. Exp. Gerontol. 2007, 42, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Hilmer, S.N.; Cogger, V.C.; Fraser, R.; McLean, A.J.; Sullivan, D.; Le Couteur, D.G. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology 2005, 42, 1349–1354. [Google Scholar] [CrossRef]
- Cogger, V.C.; Warren, A.; Fraser, R.; Ngu, M.; McLean, A.J.; Le Couteur, D.G. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Exp. Gerontol. 2003, 38, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Coppola, R.; Mari, D.; Lattuada, A.; Franceschi, C. Von Willebrand factor in Italian centenarians. Haematologica 2003, 88, 39–43. [Google Scholar]
- Albánez, S.; Ogiwara, K.; Michels, A.; Hopman, W.; Grabell, J.; James, P.; Lillicrap, D. Aging and ABO blood type influence von Willebrand factor and factor VIII levels through interrelated mechanisms. J. Thromb. Haemost. 2016, 14, 953–963. [Google Scholar] [CrossRef]
- Biguzzi, E.; Castelli, F.; Lijfering, W.M.; Cannegieter, S.C.; Eikenboom, J.; Rosendaal, F.R.; van Hylckama Vlieg, A. Rise of levels of von Willebrand factor and factor VIII with age: Role of genetic and acquired risk factors. Thromb. Res. 2021, 197, 172–178. [Google Scholar] [CrossRef]
- Miesbach, W.; Alesci, S.; Krekeler, S.; Seifried, E. Age-dependent increase of FVIII:C in mild haemophilia A. Haemophilia 2009, 15, 1022–1026. [Google Scholar] [CrossRef]
- Rejtő, J.; Königsbrügge, O.; Grilz, E.; Hofer, S.; Mauracher, L.M.; Gabler, C.; Schuster, G.; Feistritzer, C.; Sunder-Plaßmann, R.; Quehenberger, P.; et al. Influence of blood group, von Willebrand factor levels, and age on factor VIII levels in non-severe haemophilia A. J. Thromb. Haemost. 2020, 18, 1081–1086. [Google Scholar] [CrossRef]
- Kokame, K.; Sakata, T.; Kokubo, Y.; Miyata, T. von Willebrand factor-to-ADAMTS13 ratio increases with age in a Japanese population. J. Thromb. Haemost. 2011, 9, 1426–1428. [Google Scholar] [CrossRef]
- Al-Awadhi, A.M.; Al-Sharrah, S.K.; Jadaon, M.M.; Al-Sayegh, F. Investigating the influence of age, gender and ABO blood group on ADAMTS-13 antigen and activity levels in healthy Arabs. Blood Transfus. 2014, 12, 138–140. [Google Scholar] [CrossRef]
- Davies, J.A.; Hathaway, L.S.; Collins, P.W.; Bowen, D.J. von Willebrand factor: Demographics of plasma protein level in a large blood donor cohort from South Wales in the United Kingdom. Haemophilia 2012, 18, e79–e81. [Google Scholar] [CrossRef] [PubMed]
- Kadir, R.A.; Economides, D.L.; Sabin, C.A.; Owens, D.; Lee, C.A. Variations in coagulation factors in women: Effects of age, ethnicity, menstrual cycle and combined oral contraceptive. Thromb. Haemost. 1999, 82, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Blombäck, M.; Konkle, B.A.; Manco-Johnson, M.J.; Bremme, K.; Hellgren, M.; Kaaja, R. Preanalytical conditions that affect coagulation testing, including hormonal status and therapy. J. Thromb. Haemost. 2007, 5, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Chapin, J. Von Willebrand disease in the elderly: Clinical perspectives. Clin. Interv. Aging 2018, 13, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- van Loon, J.E.; Kavousi, M.; Leebeek, F.W.; Felix, J.F.; Hofman, A.; Witteman, J.C.; de Maat, M.P. von Willebrand factor plasma levels, genetic variations and coronary heart disease in an older population. J. Thromb. Haemost. 2012, 10, 1262–1269. [Google Scholar] [CrossRef]
- Borchiellini, A.; Fijnvandraat, K.; ten Cate, J.W.; Pajkrt, D.; van Deventer, S.J.; Pasterkamp, G.; Meijer-Huizinga, F.; Zwart-Huinink, L.; Voorberg, J.; van Mourik, J.A. Quantitative analysis of von Willebrand factor propeptide release in vivo: Effect of experimental endotoxemia and administration of 1-deamino-8-D-arginine vasopressin in humans. Blood 1996, 88, 2951–2958. [Google Scholar] [CrossRef]
- Habe, K.; Wada, H.; Ito-Habe, N.; Hatada, T.; Matsumoto, T.; Ohishi, K.; Maruyama, K.; Imai, H.; Mizutani, H.; Nobori, T. Plasma ADAMTS13, von Willebrand factor (VWF) and VWF propeptide profiles in patients with DIC and related diseases. Thromb. Res. 2012, 129, 598–602. [Google Scholar] [CrossRef]
- Conroy, A.L.; Phiri, H.; Hawkes, M.; Glover, S.; Mallewa, M.; Seydel, K.B.; Taylor, T.E.; Molyneux, M.E.; Kain, K.C. Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: A retrospective case-control study. PLoS ONE 2010, 5, e15291. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.M.; Skrzynski, C.; Nesslinger, M.; Skipka, G.; Muller, K.M. Correlation of age with in vivo expression of endothelial markers. Exp. Gerontol. 2002, 37, 713–719. [Google Scholar] [CrossRef]
- Mohebali, D.; Kaplan, D.; Carlisle, M.; Supiano, M.A.; Rondina, M.T. Alterations in platelet function during aging: Clinical correlations with thromboinflammatory disease in older adults. J. Am. Geriatr. Soc. 2014, 62, 529–535. [Google Scholar] [CrossRef]
- Zhou, X.J.; Vaziri, N.D.; Zhang, J.; Wang, H.W.; Wang, X.Q. Association of renal injury with nitric oxide deficiency in aged SHR: Prevention by hypertension control with AT1 blockade. Kidney Int. 2002, 62, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Pie, J.E.; Baek, S.Y.; Kim, H.P.; Ryu, S.D.; Chung, W.G.; Cha, Y.N.; Park, C.S. Age-related decline of inducible nitric oxide synthase gene expression in primary cultured rat hepatocytes. Mol. Cells 2002, 13, 399–406. [Google Scholar] [PubMed]
- Torregrossa, A.C.; Aranke, M.; Bryan, N.S. Nitric oxide and geriatrics: Implications in diagnostics and treatment of the elderly. J. Geriatr. Cardiol. 2011, 8, 230–242. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S. Low VWF: Insights into pathogenesis, diagnosis, and clinical management. Blood Adv. 2020, 4, 3191–3199. [Google Scholar] [CrossRef]
- Seaman, C.D.; Ragni, M.V. The Association of Aging With Von Willebrand Factor Levels and Bleeding Risk in Type 1 Von Willebrand Disease. Clin. Appl. Thromb. Hemost. 2018, 24, 434–438. [Google Scholar] [CrossRef]
- Seaman, C.D.; Ragni, M.V. The Effect of Age on von Willebrand Factor and Bleeding Symptoms in von Willebrand Disease. Thromb. Haemost. 2020, 120, 1159–1165. [Google Scholar] [CrossRef]
- Abou-Ismail, M.Y.; Ogunbayo, G.O.; Secic, M.; Kouides, P.A. Outgrowing the laboratory diagnosis of type 1 von Willebrand disease: A two decade study. Am. J. Hematol. 2018, 93, 232–237. [Google Scholar] [CrossRef]
- Rydz, N.; Grabell, J.; Lillicrap, D.; James, P.D. Changes in von Willebrand factor level and von Willebrand activity with age in type 1 von Willebrand disease. Haemophilia 2015, 21, 636–641. [Google Scholar] [CrossRef]
- Sanders, Y.V.; Giezenaar, M.A.; Laros-van Gorkom, B.A.; Meijer, K.; van der Bom, J.G.; Cnossen, M.H.; Nijziel, M.R.; Ypma, P.F.; Fijnvandraat, K.; Eikenboom, J.; et al. von Willebrand disease and aging: An evolving phenotype. J. Thromb. Haemost. 2014, 12, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Vischer, U.M. von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J. Thromb. Haemost. 2006, 4, 1186–1193. [Google Scholar] [CrossRef]
- Birch, K.A.; Pober, J.S.; Zavoico, G.B.; Means, A.R.; Ewenstein, B.M. Calcium/calmodulin transduces thrombin-stimulated secretion: Studies in intact and minimally permeabilized human umbilical vein endothelial cells. J. Cell Biol. 1992, 118, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Matsushita, K.; Yamakuchi, M.; Morrell, C.N.; Cao, W.; Lowenstein, C.J. Ceramide triggers Weibel-Palade body exocytosis. Circ. Res. 2004, 95, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Morrell, C.N.; Lowenstein, C.J. Sphingosine 1-phosphate activates Weibel-Palade body exocytosis. Proc. Natl. Acad. Sci. USA 2004, 101, 11483–11487. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Yamakuchi, M.; Morrell, C.N.; Ozaki, M.; O’Rourke, B.; Irani, K.; Lowenstein, C.J. Vascular endothelial growth factor regulation of Weibel-Palade-body exocytosis. Blood 2005, 105, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Vischer, U.M.; de Moerloose, P. von Willebrand factor: From cell biology to the clinical management of von Willebrand’s disease. Crit. Rev. Oncol. Hematol. 1999, 30, 93–109. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef]
- Branchford, B.R.; Carpenter, S.L. The Role of Inflammation in Venous Thromboembolism. Front. Pediatr. 2018, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Sanders, J.L.; Kizer, J.R.; Boudreau, R.M.; Odden, M.C.; Zeki Al Hazzouri, A.; Arnold, A.M. Trajectories of function and biomarkers with age: The CHS All Stars Study. Int. J. Epidemiol. 2016, 45, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Gano, L.B.; Eskurza, I.; Silver, A.E.; Gates, P.E.; Jablonski, K.; Seals, D.R. Vascular endothelial dysfunction with aging: Endothelin-1 and endothelial nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H425–H432. [Google Scholar] [CrossRef]
- Matsushita, K.; Morrell, C.N.; Cambien, B.; Yang, S.X.; Yamakuchi, M.; Bao, C.; Hara, M.R.; Quick, R.A.; Cao, W.; O’Rourke, B.; et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 2003, 115, 139–150. [Google Scholar] [CrossRef]
- Nakayama, T.; Sato, W.; Yoshimura, A.; Zhang, L.; Kosugi, T.; Campbell-Thompson, M.; Kojima, H.; Croker, B.P.; Nakagawa, T. Endothelial von Willebrand factor release due to eNOS deficiency predisposes to thrombotic microangiopathy in mouse aging kidney. Am. J. Pathol. 2010, 176, 2198–2208. [Google Scholar] [CrossRef]
- Afanas’ev, I. Superoxide and nitric oxide in senescence and aging. Front. Biosci. 2009, 14, 3899–3912. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leitner, G.C.; Schmetterer, L.; Kapiotis, S.; Jilma, B. Effects of endothelin-1 and phenylephrine on plasma levels of von Willebrand factor and protein S. Thromb. Res. 2010, 125, e5–e8. [Google Scholar] [CrossRef] [PubMed]
- Somanath, P.R.; Podrez, E.A.; Chen, J.; Ma, Y.; Marchant, K.; Antoch, M.; Byzova, T.V. Deficiency in core circadian protein Bmal1 is associated with a prothrombotic and vascular phenotype. J. Cell Physiol. 2011, 226, 132–140. [Google Scholar] [CrossRef]
- Hemmeryckx, B.; Van Hove, C.E.; Fransen, P.; Emmerechts, J.; Kauskot, A.; Bult, H.; Lijnen, H.R.; Hoylaerts, M.F. Progression of the prothrombotic state in aging Bmal1-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2552–2559. [Google Scholar] [CrossRef]
- Duncan, M.J.; Prochot, J.R.; Cook, D.H.; Tyler Smith, J.; Franklin, K.M. Influence of aging on Bmal1 and Per2 expression in extra-SCN oscillators in hamster brain. Brain Res. 2013, 1491, 44–53. [Google Scholar] [CrossRef]
- Dmitrieva, N.I.; Burg, M.B. Secretion of von Willebrand factor by endothelial cells links sodium to hypercoagulability and thrombosis. Proc. Natl. Acad. Sci. USA 2014, 111, 6485–6490. [Google Scholar] [CrossRef] [PubMed]
- McKinley, M.J.; Denton, D.A.; Thomas, C.J.; Woods, R.L.; Mathai, M.L. Differential effects of aging on fluid intake in response to hypovolemia, hypertonicity, and hormonal stimuli in Munich Wistar rats. Proc. Natl. Acad. Sci. USA 2006, 103, 3450–3455. [Google Scholar] [CrossRef] [PubMed]
- Begg, D.P. Disturbances of thirst and fluid balance associated with aging. Physiol. Behav. 2017, 178, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Miescher, E.; Fortney, S.M. Responses to dehydration and rehydration during heat exposure in young and older men. Am. J. Physiol. 1989, 257, R1050–R1056. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.M.; Sussman, I.I.; Nagel, R.L. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood 1994, 83, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Carallo, C.; Tripolino, C.; De Franceschi, M.S.; Irace, C.; Xu, X.Y.; Gnasso, A. Carotid endothelial shear stress reduction with aging is associated with plaque development in twelve years. Atherosclerosis 2016, 251, 63–69. [Google Scholar] [CrossRef] [PubMed]
- van den Dries, L.W.; Gruters, R.A.; Hövels–van der Borden, S.B.; Kruip, M.J.; de Maat, M.P.; van Gorp, E.; van der Ende, M.E. von Willebrand Factor is elevated in HIV patients with a history of thrombosis. Front. Microbiol. 2015, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- O’regan, N.; Gegenbauer, K.; O’Sullivan, J.M.; Maleki, S.; Brophy, T.M.; Dalton, N.; Chion, A.; Fallon, P.G.; Grau, G.E.; Budde, U. A novel role for von Willebrand factor in the pathogenesis of experimental cerebral malaria. Blood J. Am. Soc. Hematol. 2016, 127, 1192–1201. [Google Scholar] [CrossRef]
- Chen, H.; Ning, Z.; Qiu, Y.; Liao, Y.; Chang, H.; Ai, Y.; Wei, Y.; Deng, Y.; Shen, Y. Elevated levels of von Willebrand factor and high mobility group box 1 (HMGB1) are associated with disease severity and clinical outcome of scrub typhus. Int. J. Infect. Dis. 2017, 61, 114–120. [Google Scholar] [CrossRef]
- Djamiatun, K.; Van der Ven, A.J.; de Groot, P.G.; Faradz, S.M.; Hapsari, D.; Dolmans, W.M.; Sebastian, S.; Fijnheer, R.; de Mast, Q. Severe dengue is associated with consumption of von Willebrand factor and its cleaving enzyme ADAMTS-13. PLoS Negl. Trop. Dis. 2012, 6, e1628. [Google Scholar] [CrossRef][Green Version]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef]
- Robba, C.; Battaglini, D.; Pelosi, P.; Rocco, P.R. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020, 14, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Bazzan, M.; Montaruli, B.; Sciascia, S.; Cosseddu, D.; Norbiato, C.; Roccatello, D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patients. Intern. Emerg. Med. 2020, 15, 861–863. [Google Scholar] [CrossRef]
- Mei, Z.W.; van Wijk, X.M.; Pham, H.P.; Marin, M.J. Role of von Willebrand Factor in COVID-19 Associated Coagulopathy. J. Appl. Lab. Med. 2021, 6, 1305–1315. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Gandet, F.F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Klok, F.; Kruip, M.; Van der Meer, N.; Arbous, M.; Gommers, D.; Kant, K.; Kaptein, F.; van Paassen, J.; Stals, M.; Huisman, M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Klok, F.; Kruip, M.; Van der Meer, N.; Arbous, M.; Gommers, D.; Kant, K.; Kaptein, F.; van Paassen, J.; Stals, M.; Huisman, M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, S.; Coppens, M.; van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.; Bouman, C.C.; Beenen, L.F.; Kootte, R.S.; Heijmans, J. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef]
- Poissy, J.; Goutay, J.; Caplan, M.; Parmentier, E.; Duburcq, T.; Lassalle, F.; Jeanpierre, E.; Rauch, A.; Labreuche, J.; Susen, S. Pulmonary embolism in patients with COVID-19: Awareness of an increased prevalence. Circulation 2020, 142, 184–186. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Liu, P.P.; Blet, A.; Smyth, D.; Li, H. The science underlying COVID-19: Implications for the cardiovascular system. Circulation 2020, 142, 68–78. [Google Scholar] [CrossRef]
- Becker, R.C.; Sexton, T.; Smyth, S.; International, C.-T.B.C.I. COVID-19 and biomarkers of thrombosis: Focus on von Willebrand factor and extracellular vesicles. J. Thromb. Thrombolysis 2021, 1–10. [Google Scholar] [CrossRef]
- Chandel, A.; Patolia, S.; Looby, M.; Bade, N.; Khangoora, V.; King, C.S. Association of D-dimer and Fibrinogen with Hypercoagulability in COVID-19 Requiring Extracorporeal Membrane Oxygenation. J. Intensive Care Med. 2021, 36, 689–695. [Google Scholar] [CrossRef]
- Küçükceran, K.; Ayranci, M.K.; Girişgin, A.S.; Koçak, S. Predictive value of D-dimer/albumin ratio and fibrinogen/albumin ratio for in-hospital mortality in patients with COVID-19. Int. J. Clin. Pract. 2021, 75, e14263. [Google Scholar] [CrossRef]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Bashir, D.A.; Da, Q.; Pradhan, S.; Sekhar, N.; Valladolid, C.; Lam, F.; Guffey, D.; Goldman, J.; Desai, M.S.; Cruz, M.A. Secretion of von Willebrand Factor and Suppression of ADAMTS-13 Activity by Markedly High Concentration of Ferritin. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029621992128. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.E.; Fogarty, H.; Karampini, E.; Lavin, M.; Schneppenheim, S.; Dittmer, R.; Morrin, H.; Glavey, S.; Ni Cheallaigh, C.; Bergin, C. ADAMTS13 regulation of VWF multimer distribution in severe COVID-19. J. Thromb. Haemost. 2021. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.V.; Arachchillage, D.J.; Ridge, C.A.; Bianchi, P.; Doyle, J.F.; Garfield, B.; Ledot, S.; Morgan, C.; Passariello, M.; Price, S. Pulmonary angiopathy in severe COVID-19: Physiologic, imaging, and hematologic observations. Am. J. Respir. Crit. Care Med. 2020, 202, 690–699. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of coronavirus disease 2019. Crit. Care Med. 2020, 27, 1451–1454. [Google Scholar] [CrossRef]
- Tiwari, N.R.; Phatak, S.; Sharma, V.R.; Agarwal, S.K. COVID-19 and thrombotic microangiopathies. Thromb. Res. 2021, 202, 191–198. [Google Scholar] [CrossRef]
- Mancini, I.; Baronciani, L.; Artoni, A.; Colpani, P.; Biganzoli, M.; Cozzi, G.; Novembrino, C.; Boscolo Anzoletti, M.; De Zan, V.; Pagliari, M.T. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J. Thrombo. Haemost. 2021, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Doevelaar, A.A.; Bachmann, M.; Hölzer, B.; Seibert, F.S.; Rohn, B.J.; Bauer, F.; Witzke, O.; Dittmer, U.; Bachmann, M.; Yilmaz, S. von Willebrand factor multimer formation contributes to immunothrombosis in coronavirus disease. Crit. Care Med. 2021, 49, e512–e520. [Google Scholar] [CrossRef]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020, 18, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Benoit, S.W.; de Oliveira, M.H.S.; Lippi, G.; Favaloro, E.J.; Benoit, J.L. ADAMTS13 activity to von Willebrand factor antigen ratio predicts acute kidney injury in patients with COVID-19: Evidence of SARS-CoV-2 induced secondary thrombotic microangiopathy. Int. J. Lab. Hematol. 2020. [Google Scholar]
- Morici, N.; Bottiroli, M.; Fumagalli, R.; Marini, C.; Cattaneo, M. Role of von Willebrand factor and ADAMTS-13 in the pathogenesis of thrombi in SARS-CoV-2 infection: Time to rethink. Thromb. Haemost. 2020, 120, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Ledderose, S.; Schulz, H. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Z.; Long, Q.; Huang, J.; Hong, T.; Liu, W.; Lin, J. Insights into immunothrombosis: The interplay among Neutrophil Extracellular Trap, von Willebrand factor, and ADAMTS13. Front. Immunol. 2020, 11, 610696. [Google Scholar] [CrossRef]
- Aksenova, A.Y. Von Willebrand factor and endothelial damage: A possible association with COVID-19. Ecol. Genet. 2020, 18, 135–138. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.V.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef]
- Amraei, R.; Yin, W.; Napoleon, M.A.; Suder, E.L.; Berrigan, J.; Zhao, Q.; Olejnik, J.; Chandler, K.B.; Xia, C.; Feldman, J.; et al. CD209L/L-SIGN and CD209/DC-SIGN Act as Receptors for SARS-CoV-2. ACS Cent. Sci. 2021, 7, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Silva, R.A.; Da Silva, D.G.; Montecucco, F.; Mach, F.; Stergiopulos, N.; da Silva, R.F.; Santos, R.A. The angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas receptor axis: A potential target for treating thrombotic diseases. Thromb. Haemost. 2012, 108, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Del Pozo, C.H.; Prosper, F. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905-913.e7. [Google Scholar] [CrossRef]
- Samavati, L.; Uhal, B.D. ACE2, much more than just a receptor for SARS-CoV-2. Front. Cell. Infect. Microbiol. 2020, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Cugno, M.; Meroni, P.L.; Gualtierotti, R.; Griffini, S.; Grovetti, E.; Torri, A.; Lonati, P.; Grossi, C.; Borghi, M.O.; Novembrino, C. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J. Autoimmun. 2021, 116, 102560. [Google Scholar] [CrossRef]
- Gragnano, F.; Sperlongano, S.; Golia, E.; Natale, F.; Bianchi, R.; Crisci, M.; Fimiani, F.; Pariggiano, I.; Diana, V.; Carbone, A. The role of von Willebrand factor in vascular inflammation: From pathogenesis to targeted therapy. Mediat. Inflamm. 2017, 2017, 5620314. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.A.; Colley, L.; Agbaedeng, T.A.; Ellison-Hughes, G.M.; Ross, M.D. Vascular manifestations of COVID-19–thromboembolism and microvascular dysfunction. Front. Cardiovasc. Med. 2020, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Sandersjöö, A.; Bellander, B.-M. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb. Res. 2020, 194, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; You, B.; Chen, S.-P.; Liao, J.K.; Sun, J. Tumor necrosis factor-α downregulates endothelial nitric oxide synthase mRNA stability via translation elongation factor 1-α 1. Cir. Res. 2008, 103, 591–597. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, B.; Su, Z.; Liu, Y.; Shelite, T.R.; Chang, Q.; Wang, P.; Bukreyev, A.; Soong, L.; Jin, Y. EPAC regulates von Willebrand factor secretion from endothelial cells in a PI3K/eNOS-dependent manner during inflammation. Bio. Rxiv. 2020. [Google Scholar]
- Fang, W.; Jiang, J.; Su, L.; Shu, T.; Liu, H.; Lai, S.; Ghiladi, R.A.; Wang, J. The role of NO in COVID-19 and potential therapeutic strategies. Free. Radic. Biol. Med. 2021, 163, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, B.; Yazici, A. Could the decrease in the endothelial nitric oxide (NO) production and NO bioavailability be the crucial cause of COVID-19 related deaths? Med. Hypotheses 2020, 144, 109970. [Google Scholar] [CrossRef]
- Bernardo, A.; Ball, C.; Nolasco, L.; Moake, J.F.; Dong, J.-f. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell–derived ultralarge von Willebrand factor multimers under flow. Blood 2004, 104, 100–106. [Google Scholar] [CrossRef]
- Huisman, A.; Beun, R.; Sikma, M.; Westerink, J.; Kusadasi, N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS-CoV-2. Int. J. Lab. Hematol. 2020, 42, e211–e212. [Google Scholar] [CrossRef]
- Rauch, A.; Labreuche, J.; Lassalle, F.; Goutay, J.; Caplan, M.; Charbonnier, L.; Rohn, A.; Jeanpierre, E.; Dupont, A.; Duhamel, A. Coagulation biomarkers are independent predictors of increased oxygen requirements in COVID-19. J. Thromb. Haemost. 2020, 18, 2942–2953. [Google Scholar] [CrossRef]
- Ladikou, E.E.; Sivaloganathan, H.; Milne, K.M.; Arter, W.E.; Ramasamy, R.; Saad, R.; Stoneham, S.M.; Philips, B.; Eziefula, A.C.; Chevassut, T. Von Willebrand factor (vWF): Marker of endothelial damage and thrombotic risk in COVID-19? Clin. Med. (Lond.) 2020, 20, e178–e182. [Google Scholar] [CrossRef]
- Ward, S.E.; Curley, G.F.; Lavin, M.; Fogarty, H.; Karampini, E.; McEvoy, N.L.; Clarke, J.; Boylan, M.; Alalqam, R.; Worrall, A.P.; et al. Von Willebrand factor propeptide in severe coronavirus disease 2019 (COVID-19): Evidence of acute and sustained endothelial cell activation. Br. J. Haematol. 2021, 192, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Chocron, R.; Gendron, N.; Bory, O.; Beauvais, A.; Peron, N.; Khider, L.; Guerin, C.L.; Goudot, G.; Levasseur, F.; et al. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis 2021. [Google Scholar] [CrossRef]
- Delrue, M.; Siguret, V.; Neuwirth, M.; Joly, B.; Beranger, N.; Sene, D.; Chousterman, B.G.; Voicu, S.; Bonnin, P.; Megarbane, B.; et al. von Willebrand factor/ADAMTS13 axis and venous thromboembolism in moderate-to-severe COVID-19 patients. Br. J. Haematol. 2021, 192, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Taus, F.; Salvagno, G.; Canè, S.; Fava, C.; Mazzaferri, F.; Carrara, E.; Petrova, V.; Barouni, R.M.; Dima, F.; Dalbeni, A.; et al. Platelets Promote Thromboinflammation in SARS-CoV-2 Pneumonia. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2975–2989. [Google Scholar] [CrossRef] [PubMed]
- Marco, A.; Marco, P. Von Willebrand factor and ADAMTS13 activity as clinical severity markers in patients with COVID-19. J. Thromb. Thrombolysis 2021, 1–7. [Google Scholar] [CrossRef]
- De Cristofaro, R.; Liuzzo, G.; Sacco, M.; Lancellotti, S.; Pedicino, D.; Andreotti, F. Marked von Willebrand factor and factor VIII elevations in severe acute respiratory syndrome coronavirus-2-positive, but not severe acute respiratory syndrome coronavirus-2-negative, pneumonia: A case—Control study. Blood Coagul. Fibrinolysis 2021, 32, 285–289. [Google Scholar] [CrossRef]
- Blasi, A.; von Meijenfeldt, F.A.; Adelmeijer, J.; Calvo, A.; Ibanez, C.; Perdomo, J.; Reverter, J.C.; Lisman, T. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J. Thromb. Haemost. 2020, 18, 2646–2653. [Google Scholar] [CrossRef]
- Ruberto, F.; Chistolini, A.; Curreli, M.; Frati, G.; Marullo, A.G.M.; Biondi-Zoccai, G.; Mancone, M.; Sciarretta, S.; Miraldi, F.; Alessandri, F.; et al. Von Willebrand factor with increased binding capacity is associated with reduced platelet aggregation but enhanced agglutination in COVID-19 patients: Another COVID-19 paradox? J. Thromb. Thrombolysis 2021, 52, 105–110. [Google Scholar] [CrossRef]
- Sweeney, J.M.; Barouqa, M.; Krause, G.J.; Gonzalez-Lugo, J.D.; Rahman, S.; Gil, M.R. Evidence for secondary thrombotic microangiopathy in COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Rodríguez Rodríguez, M.; Castro Quismondo, N.; Zafra Torres, D.; Gil Alos, D.; Ayala, R.; Martinez-Lopez, J. Increased von Willebrand factor antigen and low ADAMTS13 activity are related to poor prognosis in COVID-19 patients. Int. J. Lab. Hematol. 2021, 43, O152–O155. [Google Scholar] [CrossRef]
- Hardy, M.; Michaux, I.; Lessire, S.; Douxfils, J.; Dogne, J.M.; Bareille, M.; Horlait, G.; Bulpa, P.; Chapelle, C.; Laporte, S.; et al. Prothrombotic hemostasis disturbances in patients with severe COVID-19: Individual daily data. Data Brief 2020, 33, 106519. [Google Scholar] [CrossRef]
- Masi, P.; Hekimian, G.; Lejeune, M.; Chommeloux, J.; Desnos, C.; Pineton De Chambrun, M.; Martin-Toutain, I.; Nieszkowska, A.; Lebreton, G.; Brechot, N.; et al. Systemic Inflammatory Response Syndrome Is a Major Contributor to COVID-19-Associated Coagulopathy: Insights From a Prospective, Single-Center Cohort Study. Circulation 2020, 142, 611–614. [Google Scholar] [CrossRef]
- von Meijenfeldt, F.A.; Havervall, S.; Adelmeijer, J.; Lundström, A.; Rudberg, A.S.; Magnusson, M.; Mackman, N.; Thalin, C.; Lisman, T. Prothrombotic changes in patients with COVID-19 are associated with disease severity and mortality. Res. Pract. Thromb. Haemost. 2021, 5, 132–141. [Google Scholar] [CrossRef]
- De Jongh, R.; Ninivaggi, M.; Mesotten, D.; Bai, C.; Marcus, B.; Huskens, D.; Stragier, H.; Miszta, A.; Verbruggen, J.; de Laat-Kremers, R.M.W.; et al. Vascular activation is a strong predictor of mortality in coronavirus disease 2019 patients on the ICU. Blood Coagul. Fibrinolysis 2021, 32, 290–293. [Google Scholar] [CrossRef]
- Pascreau, T.; Zia-Chahabi, S.; Zuber, B.; Tcherakian, C.; Farfour, E.; Vasse, M. ADAMTS 13 deficiency is associated with abnormal distribution of von Willebrand factor multimers in patients with COVID-19. Thrombosis Res. 2021, 204, 138–140. [Google Scholar] [CrossRef]
- Pinsky, D.J.; Naka, Y.; Liao, H.; Oz, M.C.; Wagner, D.D.; Mayadas, T.N.; Johnson, R.C.; Hynes, R.O.; Heath, M.; Lawson, C.A. Hypoxia-induced exocytosis of endothelial cell Weibel-Palade bodies. A mechanism for rapid neutrophil recruitment after cardiac preservation. J. Clin. Investig. 1996, 97, 493–500. [Google Scholar] [CrossRef]
- Mojiri, A.; Nakhaii-Nejad, M.; Phan, W.-L.; Kulak, S.; Radziwon-Balicka, A.; Jurasz, P.; Michelakis, E.; Jahroudi, N. Hypoxia results in upregulation and de novo activation of von Willebrand factor expression in lung endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Alavi, P.; Lorenzana Carrillo, M.A.; Nakhaei-Nejad, M.; Sergi, C.M.; Thebaud, B.; Aird, W.C.; Jahroudi, N. Endothelial cells of different organs exhibit heterogeneity in von Willebrand factor expression in response to hypoxia. Atherosclerosis 2019, 282, 1–10. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, T.; Ma, L.; Zhang, H.; Wang, H.; Wei, W.; Pei, H.; Hao, L. The impact of ABO blood group on COVID-19 infection risk and mortality: A systematic review and meta-analysis. Blood Rev. 2020, 48, 100785. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Diaz, E.; Llopis, J.; Parra, R.; Roig, I.; Ferrer, G.; Grifols, J.; Millán, A.; Ene, G.; Ramiro, L.; Maglio, L. Relationship between the ABO blood group and COVID-19 susceptibility, severity and mortality in two cohorts of patients. Blood Transfus. 2021, 19, 54. [Google Scholar] [PubMed]
- Kageyama, S.; Yamamoto, H.; Nakazawa, H.; Matsushita, J.; Kouyama, T.; Gonsho, A.; Ikeda, Y.; Yoshimoto, R. Pharmacokinetics and pharmacodynamics of AJW200, a humanized monoclonal antibody to von Willebrand factor, in monkeys. Arter. Thromb. Vasc. Biol. 2002, 22, 187–192. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.F.; De Maeyer, B.; Deckmyn, H.; Vanhoorelbeke, K. Von Willebrand factor: Drug and drug target. Former. Curr. Drug Targets-Cardiovasc. Hematol. Disord. 2009, 9, 9–20. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.F.; Stoll, G.; Wagner, D.D.; Kleinschnitz, C. von Willebrand factor: An emerging target in stroke therapy. Stroke 2012, 43, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knöbl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F.; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Knoebl, P.; Cataland, S.; Peyvandi, F.; Coppo, P.; Scully, M.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Minkue Mi Edou, J.; et al. Efficacy and safety of open-label caplacizumab in patients with exacerbations of acquired thrombotic thrombocytopenic purpura in the HERCULES study. J. Thromb. Haemost. 2020, 18, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Carrim, N.; Neves, M.A.; McKeown, T.; Stratton, T.W.; Coelho, R.M.; Lei, X.; Chen, P.; Xu, J.; Dai, X.; et al. Platelets and platelet adhesion molecules: Novel mechanisms of thrombosis and anti-thrombotic therapies. Thromb. J. 2016, 14, 29. [Google Scholar] [CrossRef]
- Zheng, L.; Mao, Y.; Abdelgawwad, M.S.; Kocher, N.K.; Li, M.; Dai, X.; Li, B.; Zheng, X.L. Therapeutic efficacy of the platelet glycoprotein Ib antagonist anfibatide in murine models of thrombotic thrombocytopenic purpura. Blood Adv. 2016, 1, 75–83. [Google Scholar] [CrossRef]
- Plaimauer, B.; Kremer Hovinga, J.A.; Juno, C.; Wolfsegger, M.J.; Skalicky, S.; Schmidt, M.; Grillberger, L.; Hasslacher, M.; Knöbl, P.; Ehrlich, H.; et al. Recombinant ADAMTS13 normalizes von Willebrand factor-cleaving activity in plasma of acquired TTP patients by overriding inhibitory antibodies. J. Thromb. Haemost. 2011, 9, 936–944. [Google Scholar] [CrossRef]
- Chen, J.; Reheman, A.; Gushiken, F.C.; Nolasco, L.; Fu, X.; Moake, J.L.; Ni, H.; López, J.A. N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J. Clin. Investig. 2011, 121, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Rottenstreich, A.; Hochberg-Klein, S.; Rund, D.; Kalish, Y. The role of N-acetylcysteine in the treatment of thrombotic thrombocytopenic purpura. J. Thromb. Thrombolysis 2016, 41, 678–683. [Google Scholar] [CrossRef]
- Sakai, K.; Someya, T.; Harada, K.; Yagi, H.; Matsui, T.; Matsumoto, M. Novel aptamer to von Willebrand factor A1 domain (TAGX-0004) shows total inhibition of thrombus formation superior to ARC1779 and comparable to caplacizumab. Haematologica 2020, 105, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, K.D.; Buchtele, N.; Schoergenhofer, C.; Derhaschnig, U.; Gelbenegger, G.; Brostjan, C.; Zhu, S.; Gilbert, J.C.; Jilma, B. The aptamer BT200 effectively inhibits von Willebrand factor (VWF) dependent platelet function after stimulated VWF release by desmopressin or endotoxin. Sci. Rep. 2020, 10, 11180. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).