Durability of Transcatheter Heart Valves: Standardized Definitions and Available Data
Abstract
:1. Introduction
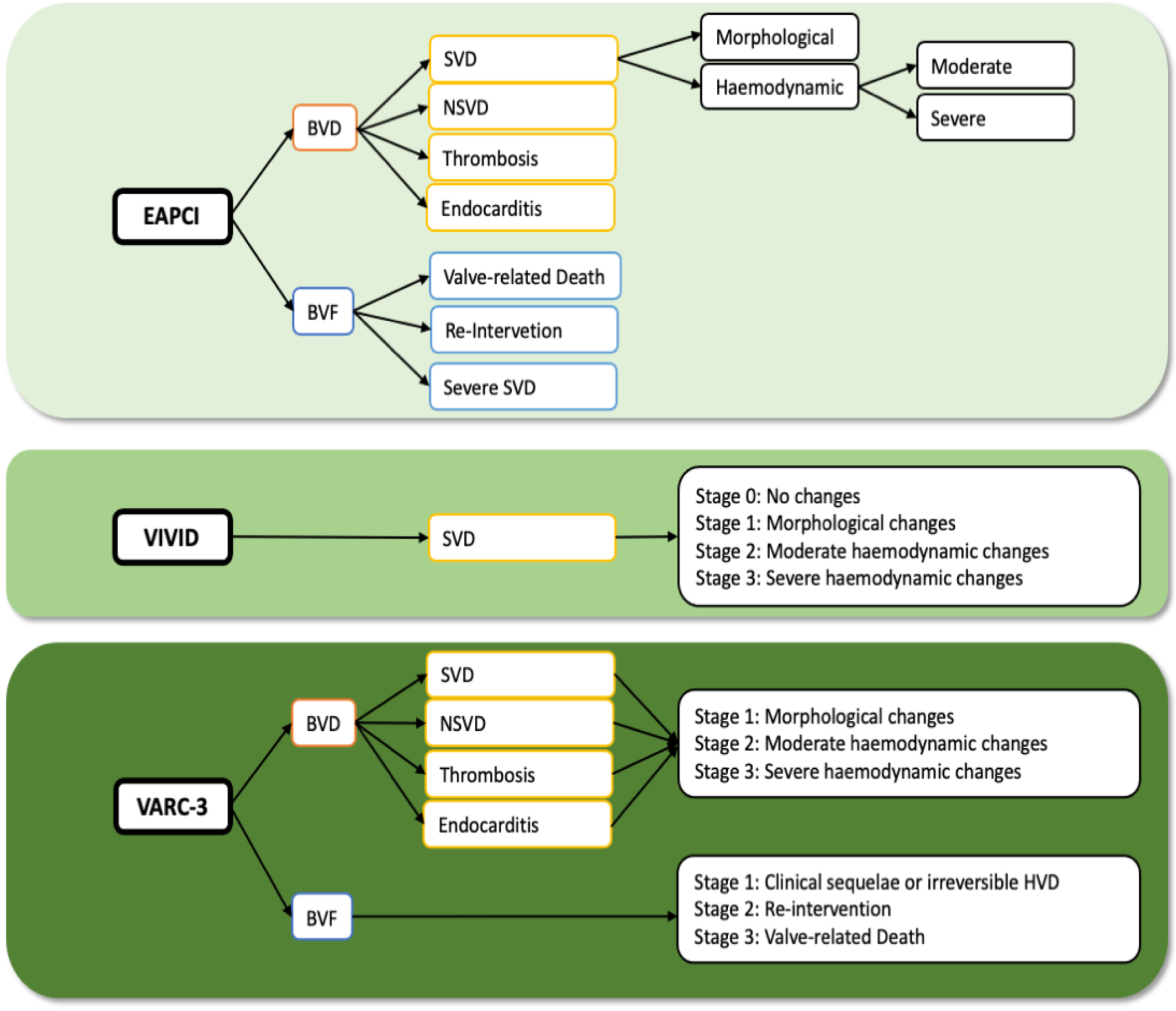
2. Definitions of Valve Durability
2.1. EAPCI Consensus Statement
2.2. VIVID Definition
2.3. VARC-3 Definition
3. Long-Term Durability of Transcatheter Heart Valves
3.1. 5-Year Outcomes after TAVR
3.2. 5–10-Year Outcomes after TAVR
3.3. ≥10-Year Outcomes after TAVR
| Author | FU (Years) | Sample (n) | Key Findings |
|---|---|---|---|
| Pibarot, P. [16] ** | 5 y | Sapien XT TAVR (n = 774) Sapien 3 TAVR (n = 891) SAVR (n = 664) | Sapien XT cohort vs. SAVR SVD (1.61% vs. 0.63%, p < 0.01) SVD related BVF (0.58% vs. 0.12%, p < 0.01) Sapien 3 vs. Sapien XT SVD (0.63% vs. 1.76%, p = 0.0001) SVD related BVF (0.21% vs. 0.65%, p = 0.03) Sapien 3 vs. SAVR: SVD (0.68% vs. 0.60%, p = 0.71) SVD related BVF (0.29% vs. 0.14%, p = 0.25) |
| Jørgesen, T.H. [22] *,** | 8 y | TAVR (145) SAVR (135) | BVD (TAVR 62.0% vs. SAVR 70.5%, p = 0.064) SVD (TAVR 8.8% vs. SAVR 15.7%, p = 0.068) NSVD (TAVR 43.9% vs. SAVR 60.7%, p = 0.0049) Thrombosis (0%) Endocarditis (TAVR 7.2% vs. SAVR 7.4%, p = 0.95) BVF (TAVR 8.7% vs. SAVR 10.5%, p = 0.61) |
| Aldalati, O. [20] *,** | 6.5 y | 269 TAVR 174 SAVR | Moderate SVD (TAVR 11.5% vs. SAVR 20.7%, p = 0.007) |
| Gleason, T.G. [17] * | 5 y | 391 (TAVR) 359 (SAVR) | Moderate SVD (TAVR 9.5% vs. SAVR 26.6%, p < 0.001) Severe SVD (TAVR 0.8% vs. SAVR 1.7%, p = 0.32) |
| Testa, L. [24] ** | 8 y | 990 TAVR | Moderate SVD (3.0%) Severe SVD (1.6%) Late BVF (2.5%) |
| Abdel-Wahab, M. [15] * | 5 y | BE TAVR (121) SE TAVR (120) | BVD (BE 22.5% vs. SE 20%, p = 0.91) SVD (BE 6.6% vs. SE 0%, p = 0.018) NSVD (BE 17.8% vs. SE 26.7%, p = 0.20) Thrombosis (BE 7.3% vs. SE 0.8%, p = 0.06) Endocarditis (BE 1.6% vs. SE 3.4%, p = 0.39) BVF (BE 4.1% vs. SE 3.4%, p = 0.63) |
| Sathananthan, J. [23] *,** | 10 y | 235 TAVR | SVD (6.5%) BVF (2.5%) |
| Murray, M.I. [25] * | 7 y | 103 TAVR | BVF (3.8%) Severe SVD (1.3%) Moderate SVD (8.9%) Thrombosis (1.3%) Endocarditis (1.3%) |
| Durand, E. [26] * | 7 y | 1403 TAVR | Moderate SVD (7.0%) Severe SVD (4.2%) BVF (1.9%) |
| Orvin, K. [27] *,** | 5 y | 450 TAVR | SVD (12.3%) BVF (0.6%) annualized incidence BVD (1.8%) annualized incidence |
| Panico, R.A. [28] * | 7 y | 278 TAVR | SVD 3.6% BVF 2.5% Thrombosis (0%) |
| Blackman, D.J. [21] *,** | 5.8 y | 241 TAVR | Severe SVD (<0.5%) Moderate SVD (8.7%) |
| Eltchaninoff, H. [18] * | 8 y | 378 TAVR | SVD (3.2%) Late BVF (0.58%) |
| Deutsch, M.-A. [29] * | 7 y | 300 TAVR | SVD (14.9%) BVF (n = 10) |
| Barbanti, M. [19] * | 8 y | 286 TAVR | Severe SVD (2.30%) Moderate SVD (5.87%) BVF (4.51%) Thrombosis (0%) |
| Didier, R. [14] * | 5 y | 4187 TAVR | Moderate SVD (13.3%) Severe SVD (2.5%) |
| Holy, E.W. [30] * | 8 y | 152 TAVR | BVF (4.5%) Severe/moderate SVD (0%) |
4. Misconceptions in Reporting Long-Term Results on TAVR Durability
4.1. Definitions
4.1.1. Structural Valve Deterioration
4.1.2. NSVD, Thrombosis and Endocarditis
4.2. Death as a Competing Risk
4.3. TAVR as a New and Quickly Developing Technique
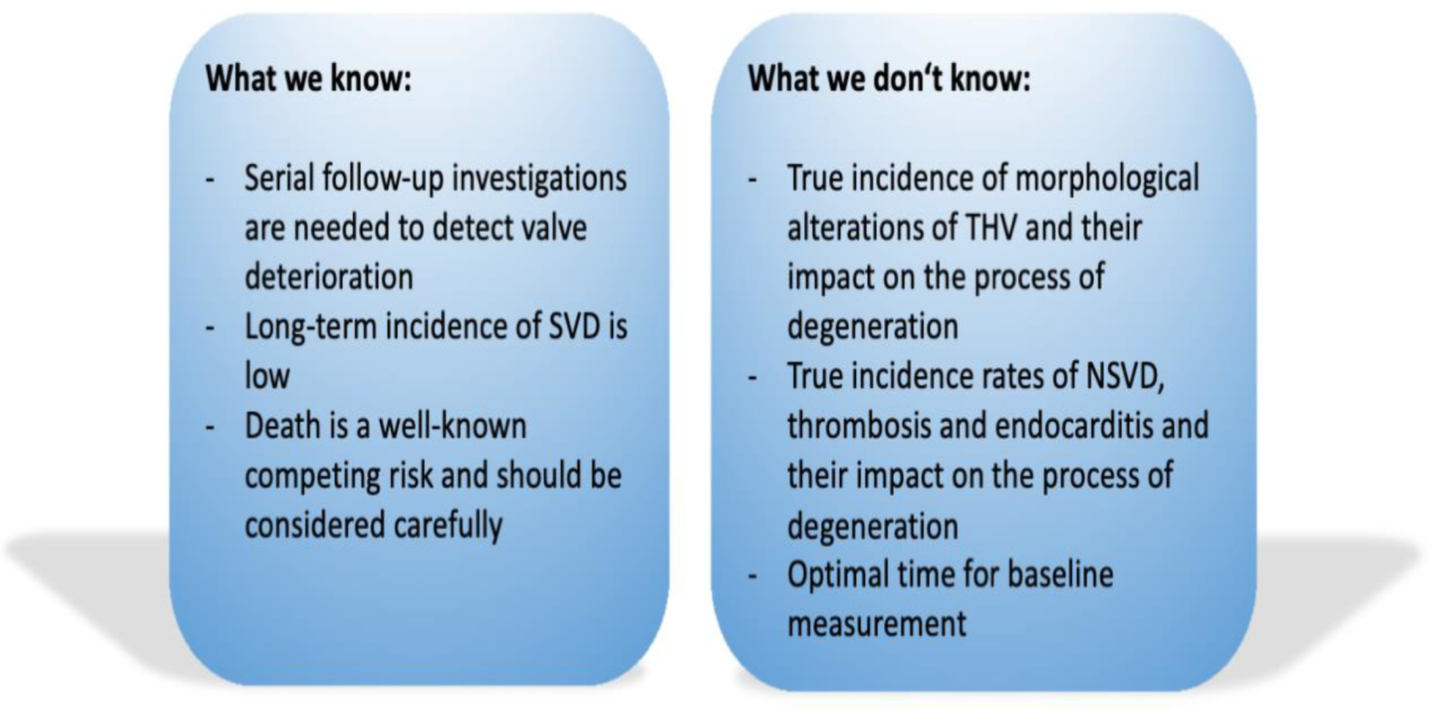
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zakkar, M.; Bryan, A.J.; Angelini, G.D. Aortic Stenosis: Diagnosis and Management. BMJ 2016, 355, i5425. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- VARC-3 WRITING COMMITTEE; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. Eur. Heart J. 2021, 42, ehaa799. [Google Scholar] [CrossRef]
- Dvir, D.; Bourguignon, T.; Otto, C.M.; Hahn, R.T.; Rosenhek, R.; Webb, J.G.; Treede, H.; Sarano, M.E.; Feldman, T.; Wijeysundera, H.C.; et al. Standardized Definition of Structural Valve Degeneration for Surgical and Transcatheter Bioprosthetic Aortic Valves. Circulation 2018, 137, 388–399. [Google Scholar] [CrossRef]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; Lancellotti, P.; Sondergaard, L.; Ludman, P.F.; Tamburino, C.; et al. Standardized Definitions of Structural Deterioration and Valve Failure in Assessing Long-Term Durability of Transcatheter and Surgical Aortic Bioprosthetic Valves: A Consensus Statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. J. Cardiothorac. Surg. 2017, 52, 408–417. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the Imaging Assessment of Prosthetic Heart Valves: A Report from the European Association of Cardiovascular Imaging Endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef] [Green Version]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.-A.; et al. Updated Standardized Endpoint Definitions for Transcatheter Aortic Valve Implantation: The Valve Academic Research Consortium-2 Consensus Document (VARC-2). Eur. J. Cardiovasc. Surg. 2012, 42, S45–S60. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.F.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Durack, D.T.; Lukes, A.S.; Bright, D.K. New Criteria for Diagnosis of Infective Endocarditis: Utilization of Specific Echocardiographic Findings. Duke Endocarditis Service. Am. J. Med. 1994, 96, 200–209. [Google Scholar] [CrossRef]
- Olaison, L.; Pettersson, G. Current Best Practices and Guidelines Indications for Surgical Intervention in Infective Endocarditis. Infect. Dis. Clin. N. Am. 2002, 16, 453–475. [Google Scholar] [CrossRef]
- Didier, R.; Eltchaninoff, H.; Donzeau-Gouge, P.; Chevreul, K.; Fajadet, J.; Leprince, P.; Leguerrier, A.; Lièvre, M.; Prat, A.; Teiger, E.; et al. Five-Year Clinical Outcome and Valve Durability After Transcatheter Aortic Valve Replacement in High-Risk Patients. Circulation 2018, 138, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; Landt, M.; Neumann, F.-J.; Massberg, S.; Frerker, C.; Kurz, T.; Kaur, J.; Toelg, R.; Sachse, S.; Jochheim, D.; et al. 5-Year Outcomes After TAVR With Balloon-Expandable Versus Self-Expanding Valves: Results From the CHOICE Randomized Clinical Trial. JACC Cardiovasc. Interv. 2020, 13, 1071–1082. [Google Scholar] [CrossRef]
- Pibarot, P.; Ternacle, J.; Jaber, W.A.; Salaun, E.; Dahou, A.; Asch, F.M.; Weissman, N.J.; Rodriguez, L.; Xu, K.; Annabi, M.-S.; et al. Structural Deterioration of Transcatheter Versus Surgical Aortic Valve Bioprostheses in the PARTNER-2 Trial. J. Am. Coll. Cardiol. 2020, 76, 1830–1843. [Google Scholar] [CrossRef]
- Gleason, T.G.; Reardon, M.J.; Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Lee, J.S.; Kleiman, N.S.; Chetcuti, S.; Hermiller, J.B.; Heiser, J.; et al. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J. Am. Coll. Cardiol. 2018, 72, 2687–2696. [Google Scholar] [CrossRef]
- Eltchaninoff, H.; Durand, E.; Avinée, G.; Tron, C.; Litzler, P.-Y.; Bauer, F.; Dacher, J.-N.; Werhlin, C.; Bouhzam, N.; Bettinger, N.; et al. Assessment of Structural Valve Deterioration of Transcatheter Aortic Bioprosthetic Balloon-Expandable Valves Using the New European Consensus Definition. EuroIntervention 2018, 14, e264–e271. [Google Scholar] [CrossRef]
- Barbanti, M.; Costa, G.; Zappulla, P.; Todaro, D.; Picci, A.; Rapisarda, G.; Di Simone, E.; Sicuso, R.; Buccheri, S.; Gulino, S.; et al. Incidence of Long-Term Structural Valve Dysfunction and Bioprosthetic Valve Failure After Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2018, 7, e008440. [Google Scholar] [CrossRef] [Green Version]
- Aldalati, O.; Kaura, A.; Khan, H.; Dworakowski, R.; Byrne, J.; Eskandari, M.; Deshpande, R.; Monaghan, M.; Wendler, O.; MacCarthy, P. Bioprosthetic Structural Valve Deterioration: How Do TAVR and SAVR Prostheses Compare? Int. J. Cardiol. 2018, 268, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Blackman, D.J.; Saraf, S.; MacCarthy, P.A.; Myat, A.; Anderson, S.G.; Malkin, C.J.; Cunnington, M.S.; Somers, K.; Brennan, P.; Manoharan, G.; et al. Long-Term Durability of Transcatheter Aortic Valve Prostheses. J. Am. Coll. Cardiol. 2019, 73, 537–545. [Google Scholar] [CrossRef]
- Jørgensen, T.H.; Thyregod, H.G.H.; Ihlemann, N.; Nissen, H.; Petursson, P.; Kjeldsen, B.J.; Steinbrüchel, D.A.; Olsen, P.S.; Søndergaard, L. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transscatheter vs. surgical aortic valve replacement. Eur. Heart J. 2021, 42, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Sathananthan, J.; Lauck, S.; Polderman, J.; Yu, M.; Stephenson, A.; Sathananthan, G.; Moss, R.; Cheung, A.; Ye, J.; Blanke, P.; et al. Ten Year Follow-up of High-Risk Patients Treated during the Early Experience with Transcatheter Aortic Valve Replacement. Catheter. Cardiovasc. Interv. 2021, 97, E431–E437. [Google Scholar] [CrossRef] [PubMed]
- Testa, L.; Latib, A.; Brambilla, N.; De Marco, F.; Fiorina, C.; Adamo, M.; Giannini, C.; Angelillis, M.; Barbanti, M.; Sgroi, C.; et al. Long-Term Clinical Outcome and Performance of Transcatheter Aortic Valve Replacement with a Self-Expandable Bioprosthesis. Eur. Heart J. 2020, 41, 1876–1886. [Google Scholar] [CrossRef]
- Murray, M.-I.; Hofmann, E.; De Rosa, R.; Mas-Peiro, S.; Seppelt, P.; Walther, T.; Zeiher, A.M.; Fichtlscherer, S.; Vasa-Nicotera, M. Hemodynamic Outcome and Valve Durability Beyond Five Years After Transcatheter Aortic Valve Replacement. J. Invasive Cardiol. 2020, 32, 82–87. [Google Scholar] [PubMed]
- Durand, E.; Sokoloff, A.; Urena-Alcazar, M.; Chevalier, B.; Chassaing, S.; Didier, R.; Tron, C.; Litzler, P.-Y.; Bouleti, C.; Himbert, D.; et al. Assessment of Long-Term Structural Deterioration of Transcatheter Aortic Bioprosthetic Valves Using the New European Definition. Circ. Cardiovasc. Interv. 2019, 12, e007597. [Google Scholar] [CrossRef] [PubMed]
- Orvin, K.; Zekry, S.B.; Morelli, O.; Barabash, I.M.; Segev, A.; Danenberg, H.; Assali, A.; Guetta, V.; Assa, H.V.; Zeniou, V.; et al. Long-Term Functional and Structural Durability of Bioprosthetic Valves Placed in the Aortic Valve Position via Percutaneous Rout in Israel. Am. J. Cardiol. 2019, 124, 1748–1756. [Google Scholar] [CrossRef]
- Panico, R.A.; Giannini, C.; De Carlo, M.; Angelillis, M.; Spontoni, P.; Pieroni, A.; Costa, G.; Bertini, P.; Guarracino, F.; Petronio, A.S. Long-Term Results and Durability of the CoreValve Transcatheter Aortic Bioprosthesis: Outcomes beyond Five Years. EuroIntervention 2019, 14, 1639–1647. [Google Scholar] [CrossRef]
- Deutsch, M.-A.; Erlebach, M.; Burri, M.; Hapfelmeier, A.; Witt, O.G.; Ziegelmueller, J.A.; Wottke, M.; Ruge, H.; Krane, M.; Piazza, N.; et al. Beyond the Five-Year Horizon: Long-Term Outcome of High-Risk and Inoperable Patients Undergoing TAVR with First-Generation Devices. EuroIntervention 2018, 14, 41–49. [Google Scholar] [CrossRef]
- Holy, E.W.; Kebernik, J.; Abdelghani, M.; Stämpfli, S.F.; Hellermann, J.; Allali, A.; El-Mawardy, M.; Sachse, S.; Lüscher, T.F.; Tanner, F.C.; et al. Long-Term Durability and Haemodynamic Performance of a Self-Expanding Transcatheter Heart Valve beyond Five Years after Implantation: A Prospective Observational Study Applying the Standardised Definitions of Structural Deterioration and Valve Failure. EuroIntervention 2018, 14, e390–e396. [Google Scholar] [CrossRef] [Green Version]
- Hahn, R.T.; Leipsic, J.; Douglas, P.S.; Jaber, W.A.; Weissman, N.J.; Pibarot, P.; Blanke, P.; Oh, J.K. Comprehensive Echocardiographic Assessment of Normal Transcatheter Valve Function. JACC Cardiovasc. Imaging 2019, 12, 25–34. [Google Scholar] [CrossRef]
- Midha, P.A.; Raghav, V.; Sharma, R.; Condado, J.F.; Okafor, I.U.; Rami, T.; Kumar, G.; Thourani, V.H.; Jilaihawi, H.; Babaliaros, V.; et al. The Fluid Mechanics of Transcatheter Heart Valve Leaflet Thrombosis in the Neosinus. Circulation 2017, 136, 1598–1609. [Google Scholar] [CrossRef]
- Généreux, P.; Head, S.J.; Hahn, R.; Daneault, B.; Kodali, S.; Williams, M.R.; van Mieghem, N.M.; Alu, M.C.; Serruys, P.W.; Kappetein, A.P.; et al. Paravalvular Leak after Transcatheter Aortic Valve Replacement: The New Achilles’ Heel? A Comprehensive Review of the Literature. J. Am. Coll. Cardiol. 2013, 61, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Doris, M.K.; Dweck, M.R. Is Bioprosthetic Leaflet Thrombosis a Trigger to Valve Degeneration? Heart 2018, 104, 792–793. [Google Scholar] [CrossRef]
- Flameng, W.; Herregods, M.-C.; Vercalsteren, M.; Herijgers, P.; Bogaerts, K.; Meuris, B. Prosthesis-Patient Mismatch Predicts Structural Valve Degeneration in Bioprosthetic Heart Valves. Circulation 2010, 121, 2123–2129. [Google Scholar] [CrossRef] [Green Version]
- Pibarot, P.; Dumesnil, J.G. Hemodynamic and Clinical Impact of Prosthesis-Patient Mismatch in the Aortic Valve Position and Its Prevention. J. Am. Coll. Cardiol. 2000, 36, 1131–1141. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richter, I.; Thiele, H.; Abdel-Wahab, M. Durability of Transcatheter Heart Valves: Standardized Definitions and Available Data. J. Clin. Med. 2021, 10, 4180. https://doi.org/10.3390/jcm10184180
Richter I, Thiele H, Abdel-Wahab M. Durability of Transcatheter Heart Valves: Standardized Definitions and Available Data. Journal of Clinical Medicine. 2021; 10(18):4180. https://doi.org/10.3390/jcm10184180
Chicago/Turabian StyleRichter, Ines, Holger Thiele, and Mohamed Abdel-Wahab. 2021. "Durability of Transcatheter Heart Valves: Standardized Definitions and Available Data" Journal of Clinical Medicine 10, no. 18: 4180. https://doi.org/10.3390/jcm10184180
APA StyleRichter, I., Thiele, H., & Abdel-Wahab, M. (2021). Durability of Transcatheter Heart Valves: Standardized Definitions and Available Data. Journal of Clinical Medicine, 10(18), 4180. https://doi.org/10.3390/jcm10184180






