In Silico Analysis to Explore Lineage-Independent and -Dependent Transcriptional Programs Associated with the Process of Endothelial and Neural Differentiation of Human Induced Pluripotent Stem Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Grouping of Genes That Exhibit Significant Differential Expression
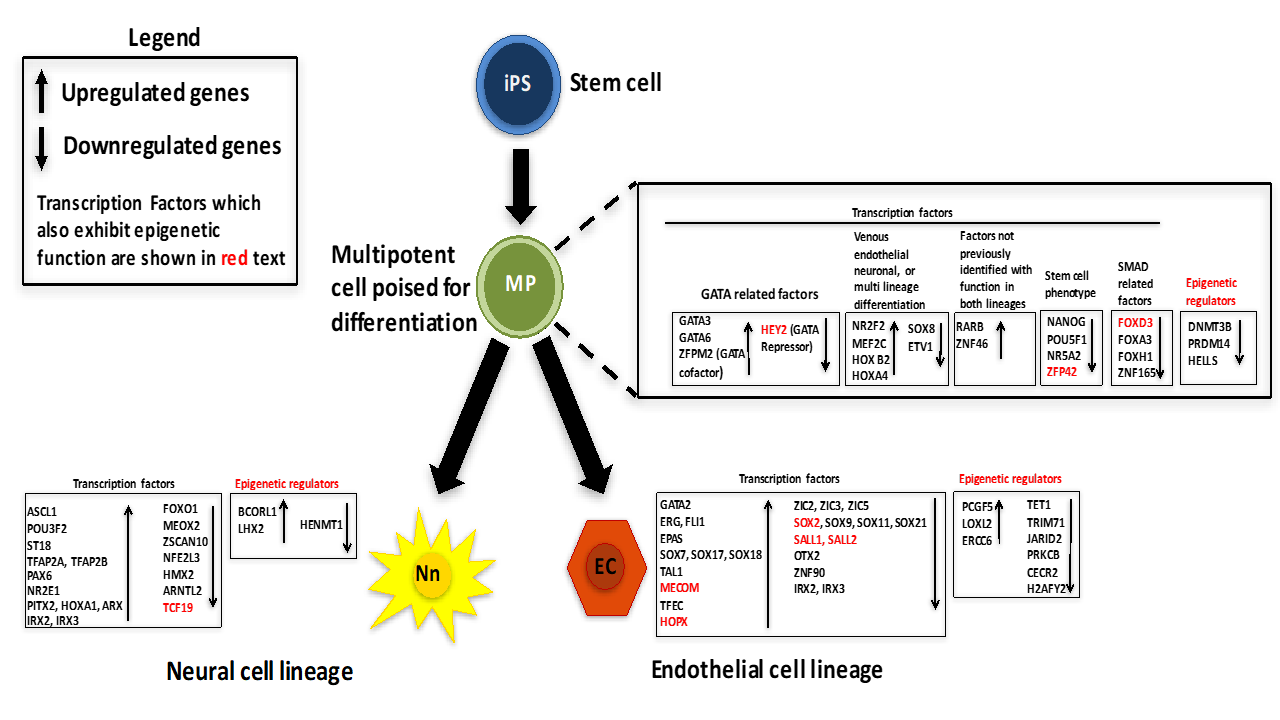
2.2. Characterization of Transcription Factors That Participate in a General Differentiation Process
2.3. Transcription Factors That Participate in Establishment of Endothelial Cell Phenotype
2.3.1. Transcription Factors That Are Upregulated in Endothelial Cell Lineage
2.3.2. Transcription Factors That Are Downregulated in Endothelial Cell Lineage
2.4. Transcription Factors That Participate in Establishment of Neural Cell Phenotype
2.4.1. Transcription Factors That Are Upregulated in Neural Cell Lineage
2.4.2. Transcription Factors That Are Downregulated in Neural Cell Lineage
2.5. Transcription Factors That Play Opposing Roles in Establishment of Endothelial and Neural Cell Phenotype
2.6. Epigenetic Regulators of Endothelial and Neural Cell Differentiation
2.6.1. Characterization of Epigenetic Factors That Participate in General Differentiation Process
2.6.2. Epigenetic Factors That Participate in Establishment of Endothelial Cell Phenotype
2.6.3. Epigenetic Factors That Participate in Establishment of Neuronal Cell Phenotype
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marcelo, K.L.; Goldie, L.C.; Hirschi, K.K. Regulation of endothelial cell differentiation and specification. Circ. Res. 2013, 112, 1272–1287. [Google Scholar] [CrossRef] [Green Version]
- De Val, S.; Black, B.L. Transcriptional control of endothelial cell development. Dev. Cell. 2009, 16, 180–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Kim, T.M.; Malik, A.B. Transcriptional regulation of endothelial cell and vascular development. Circ. Res. 2013, 112, 1380–1400. [Google Scholar] [CrossRef]
- Dejana, E.; Hirschi, K.K.; Simons, M. The molecular basis of endothelial cell plasticity. Nat. Commun. 2017, 8, 14361. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Hirschi, K.K. Endothelial Cell Development and Its Application to Regenerative Medicine. Circ. Res. 2019, 125, 489–501. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, J.Y.; Park, C. The ETS Factor, ETV2: A Master Regulator for Vascular Endothelial Cell Development. Mol. Cells 2015, 38, 1029–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, S.; Li, H.; Liu, J. Dynamic signaling for neural stem cell fate determination. Cell Adh. Migr. 2009, 3, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flitsch, L.J.; Laupman, K.E.; Brustle, O. Transcription Factor-Based Fate Specification and Forward Programming for Neural Regeneration. Front. Cell Neurosci. 2020, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Kyba, M. What is a Master Regulator? J. Stem Cell Res. Ther. 2013, 3, 114. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.L.; Rebay, I. Master regulators in development: Views from the Drosophila retinal determination and mammalian pluripotency gene networks. Dev. Biol. 2017, 421, 93–107. [Google Scholar] [CrossRef]
- De Val, S.; Chi, N.C.; Meadows, S.M.; Minovitsky, S.; Anderson, J.P.; Harris, I.S.; Ehlers, M.L.; Agarwal, P.; Visel, A.; Xu, S.M.; et al. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell 2008, 135, 1053–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, J.B.; McCutcheon, S.R.; Dube, S.; Barrera, A.; Klann, T.S.; Rice, G.A.; Adkar, S.S.; Soderling, S.H.; Reddy, T.E.; Gersbach, C.A. Master Regulators and Cofactors of Human Neuronal Cell Fate Specification Identified by CRISPR Gene Activation Screens. Cell Rep. 2020, 33, 108460. [Google Scholar] [CrossRef]
- Kahroba, H.; Ramezani, B.; Maadi, H.; Sadeghi, M.R.; Jaberie, H.; Ramezani, F. The role of Nrf2 in neural stem/progenitors cells: From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res. Rev. 2021, 65, 101211. [Google Scholar] [CrossRef]
- Minami, T.; Muramatsu, M.; Kume, T. Organ/Tissue-Specific Vascular Endothelial Cell Heterogeneity in Health and Disease. Biol. Pharm. Bull. 2019, 42, 1609–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, E.E.; Chenoweth, J.G.; Shin, J.H.; Collado-Torres, L.; Kim, S.K.; Micali, N.; Wang, Y.; Colantuoni, C.; Straub, R.E.; Hoeppner, D.J.; et al. Dissecting transcriptomic signatures of neuronal differentiation and maturation using iPSCs. Nat. Commun. 2020, 11, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belt, H.; Koponen, J.K.; Kekarainen, T.; Puttonen, K.A.; Makinen, P.I.; Niskanen, H.; Oja, J.; Wirth, G.; Koistinaho, J.; Kaikkonen, M.U.; et al. Temporal Dynamics of Gene Expression During Endothelial Cell Differentiation From Human iPS Cells: A Comparison Study of Signalling Factors and Small Molecules. Front. Cardiovasc. Med. 2018, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Haile, Y.; Nakhaei-Nejad, M.; Boakye, P.A.; Baker, G.; Smith, P.A.; Murray, A.G.; Giuliani, F.; Jahroudi, N. Reprogramming of HUVECs into induced pluripotent stem cells (HiPSCs), generation and characterization of HiPSC-derived neurons and astrocytes. PLoS ONE 2015, 10, e0119617. [Google Scholar] [CrossRef] [Green Version]
- Nakhaei-Nejad, M.; Farhan, M.; Mojiri, A.; Jabbari, H.; Murray, A.G.; Jahroudi, N. Regulation of von Willebrand Factor Gene in Endothelial Cells That Are Programmed to Pluripotency and Differentiated Back to Endothelial Cells. Stem Cells 2019, 37, 542–554. [Google Scholar] [CrossRef]
- Harris, M.A.; Clark, J.; Ireland, A.; Lomax, J.; Ashburner, M.; Foulger, R.; Eilbeck, K.; Lewis, S.; Marshall, B.; Mungall, C.; et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004, 32, D258–D261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, M.; Turner, J.; Fox, A.; Chisholm, O.; Crossley, M.; Chong, B. hFOG-2, a novel zinc finger protein, binds the co-repressor mCtBP2 and modulates GATA-mediated activation. J. Biol. Chem. 1999, 274, 23491–23498. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.R.; McKinsey, T.A.; Xu, H.; Wang, D.Z.; Richardson, J.A.; Olson, E.N. FOG-2, a heart- and brain-enriched cofactor for GATA transcription factors. Mol. Cell Biol. 1999, 19, 4495–4502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolle, P. Developmental expression of retinoic acid receptors (RARs). Nucl. Recept. Signal. 2009, 7, e006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polvani, S.; Pepe, S.; Milani, S.; Galli, A. COUP-TFII in Health and Disease. Cells 2019, 9, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenne, M.; Maconochie, M.K.; Neun, R.; Pattyn, A.; Chambon, P.; Krumlauf, R.; Rijli, F.M. Hoxa2 and Hoxb2 control dorsoventral patterns of neuronal development in the rostral hindbrain. Neuron 1999, 22, 677–691. [Google Scholar] [CrossRef] [Green Version]
- Potthoff, M.J.; Olson, E.N. MEF2: A central regulator of diverse developmental programs. Development 2007, 134, 4131–4140. [Google Scholar] [CrossRef] [Green Version]
- Seifert, A.; Werheid, D.F.; Knapp, S.M.; Tobiasch, E. Role of Hox genes in stem cell differentiation. World J. Stem Cells 2015, 7, 583–595. [Google Scholar] [CrossRef]
- Fu, K.; Nakano, H.; Morselli, M.; Chen, T.; Pappoe, H.; Nakano, A.; Pellegrini, M. A temporal transcriptome and methylome in human embryonic stem cell-derived cardiomyocytes identifies novel regulators of early cardiac development. Epigenetics 2018, 13, 1013–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.C.; Wong, W.K.; Feng, B. Decoding the Pluripotency Network: The Emergence of New Transcription Factors. Biomedicines 2013, 1, 49–78. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.D.; Kim, H.; Ekram, M.B.; Yu, S.; Faulk, C.; Kim, J. Rex1/Zfp42 as an epigenetic regulator for genomic imprinting. Hum. Mol. Genet. 2011, 20, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Lukoseviciute, M.; Gavriouchkina, D.; Williams, R.M.; Hochgreb-Hagele, T.; Senanayake, U.; Chong-Morrison, V.; Thongjuea, S.; Repapi, E.; Mead, A.; Sauka-Spengler, T. From Pioneer to Repressor: Bimodal foxd3 Activity Dynamically Remodels Neural Crest Regulatory Landscape In Vivo. Dev. Cell 2018, 47, 608–628 e606. [Google Scholar] [CrossRef] [Green Version]
- Weider, M.; Wegner, M. SoxE factors: Transcriptional regulators of neural differentiation and nervous system development. Semin. Cell Dev. Biol. 2017, 63, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, K.; Udono, M.; Ueno, K.; Ohkawa, Y.; Nagasaki, M.; Sekiya, S.; Suzuki, A. The Dynamics of Transcriptional Activation by Hepatic Reprogramming Factors. Mol. Cell 2020, 79, 660–676 e668. [Google Scholar] [CrossRef]
- Shekhar, A.; Lin, X.; Lin, B.; Liu, F.Y.; Zhang, J.; Khodadadi-Jamayran, A.; Tsirigos, A.; Bu, L.; Fishman, G.I.; Park, D.S. ETV1 activates a rapid conduction transcriptional program in rodent and human cardiomyocytes. Sci. Rep. 2018, 8, 9944. [Google Scholar] [CrossRef]
- Fischer, A.; Klattig, J.; Kneitz, B.; Diez, H.; Maier, M.; Holtmann, B.; Englert, C.; Gessler, M. Hey basic helix-loop-helix transcription factors are repressors of GATA4 and GATA6 and restrict expression of the GATA target gene ANF in fetal hearts. Mol. Cell Biol. 2005, 25, 8960–8970. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.; Heisig, J.; Kneitz, S.; Wolf, E.; Eilers, M.; Gessler, M. Mechanisms of epigenetic and cell-type specific regulation of Hey target genes in ES cells and cardiomyocytes. J. Mol. Cell Cardiol. 2015, 79, 79–88. [Google Scholar] [CrossRef]
- Gibbs, Z.A.; Reza, L.C.; Cheng, C.C.; Westcott, J.M.; McGlynn, K.; Whitehurst, A.W. The testis protein ZNF165 is a SMAD3 cofactor that coordinates oncogenic TGFbeta signaling in triple-negative breast cancer. Elife 2020, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Attisano, L.; Silvestri, C.; Izzi, L.; Labbe, E. The transcriptional role of Smads and FAST (FoxH1) in TGFbeta and activin signalling. Mol. Cell Endocrinol. 2001, 180, 3–11. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, W. The Role of SMAD2/3 in Human Embryonic Stem Cells. Front. Cell Dev. Biol. 2020, 8, 653. [Google Scholar] [CrossRef]
- Hyslop, L.; Stojkovic, M.; Armstrong, L.; Walter, T.; Stojkovic, P.; Przyborski, S.; Herbert, M.; Murdoch, A.; Strachan, T.; Lako, M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells 2005, 23, 1035–1043. [Google Scholar] [CrossRef]
- Saha, S.K.; Jeong, Y.; Cho, S.; Cho, S.G. Systematic expression alteration analysis of master reprogramming factor OCT4 and its three pseudogenes in human cancer and their prognostic outcomes. Sci. Rep. 2018, 8, 14806. [Google Scholar] [CrossRef]
- Poursani, E.M.; Mohammad Soltani, B.; Mowla, S.J. Differential Expression of OCT4 Pseudogenes in Pluripotent and Tumor Cell Lines. Cell J. 2016, 18, 28–36. [Google Scholar] [CrossRef]
- Gronostajski, R.M. Roles of the NFI/CTF gene family in transcription and development. Gene 2000, 249, 31–45. [Google Scholar] [CrossRef]
- Nagai, N.; Ohguchi, H.; Nakaki, R.; Matsumura, Y.; Kanki, Y.; Sakai, J.; Aburatani, H.; Minami, T. Downregulation of ERG and FLI1 expression in endothelial cells triggers endothelial-to-mesenchymal transition. PLoS Genet. 2018, 14, e1007826. [Google Scholar] [CrossRef]
- Lazrak, M.; Deleuze, V.; Noel, D.; Haouzi, D.; Chalhoub, E.; Dohet, C.; Robbins, I.; Mathieu, D. The bHLH TAL-1/SCL regulates endothelial cell migration and morphogenesis. J. Cell Sci. 2004, 117, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; McKnight, S.L.; Russell, D.W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997, 11, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayadi, A.; Jeyakani, J.; Seet, S.H.; Wei, C.L.; Bourque, G.; Bard, F.A.; Jenkins, N.A.; Copeland, N.G.; Bard-Chapeau, E.A. Functional features of EVI1 and EVI1Delta324 isoforms of MECOM gene in genome-wide transcription regulation and oncogenicity. Oncogene 2016, 35, 2311–2321. [Google Scholar] [CrossRef] [PubMed]
- Ivanochko, D.; Halabelian, L.; Henderson, E.; Savitsky, P.; Jain, H.; Marcon, E.; Duan, S.; Hutchinson, A.; Seitova, A.; Barsyte-Lovejoy, D.; et al. Direct interaction between the PRDM3 and PRDM16 tumor suppressors and the NuRD chromatin remodeling complex. Nucleic Acids Res. 2019, 47, 1225–1238. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Senyuk, V.; Sitailo, S.; Chi, Y.; Nucifora, G. Interaction of EVI1 with cAMP-responsive element-binding protein-binding protein (CBP) and p300/CBP-associated factor (P/CAF) results in reversible acetylation of EVI1 and in co-localization in nuclear speckles. J. Biol. Chem. 2001, 276, 44936–44943. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, I.; Margueron, R.; Shukeir, N.; Eisold, M.; Fritzsch, C.; Richter, F.M.; Mittler, G.; Genoud, C.; Goyama, S.; Kurokawa, M.; et al. Prdm3 and Prdm16 are H3K9me1 methyltransferases required for mammalian heterochromatin integrity. Cell 2012, 150, 948–960. [Google Scholar] [CrossRef] [Green Version]
- Geraud, C.; Schledzewski, K.; Demory, A.; Klein, D.; Kaus, M.; Peyre, F.; Sticht, C.; Evdokimov, K.; Lu, S.; Schmieder, A.; et al. Liver sinusoidal endothelium: A microenvironment-dependent differentiation program in rat including the novel junctional protein liver endothelial differentiation-associated protein-1. Hepatology 2010, 52, 313–326. [Google Scholar] [CrossRef]
- Mahony, C.B.; Fish, R.J.; Pasche, C.; Bertrand, J.Y. tfec controls the hematopoietic stem cell vascular niche during zebrafish embryogenesis. Blood 2016, 128, 1336–1345. [Google Scholar] [CrossRef] [Green Version]
- Palpant, N.J.; Wang, Y.; Hadland, B.; Zaunbrecher, R.J.; Redd, M.; Jones, D.; Pabon, L.; Jain, R.; Epstein, J.; Ruzzo, W.L.; et al. Chromatin and Transcriptional Analysis of Mesoderm Progenitor Cells Identifies HOPX as a Regulator of Primitive Hematopoiesis. Cell Rep. 2017, 20, 1597–1608. [Google Scholar] [CrossRef] [Green Version]
- Mariotto, A.; Pavlova, O.; Park, H.S.; Huber, M.; Hohl, D. HOPX: The Unusual Homeodomain-Containing Protein. J. Investig. Dermatol. 2016, 136, 905–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, A.; Hochedlinger, K. The sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013, 12, 15–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamachi, Y.; Kondoh, H. Sox proteins: Regulators of cell fate specification and differentiation. Development 2013, 140, 4129–4144. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Williams, J.; Smallwood, P.M.; Nathans, J. Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina. PLoS ONE 2015, 10, e0143650. [Google Scholar] [CrossRef] [Green Version]
- Diamand, K.E.M.; Barratt, K.S.; Arkell, R.M. Overview of Rodent Zic Genes. Adv. Exp. Med. Biol. 2018, 1046, 179–207. [Google Scholar] [CrossRef]
- de Celis, J.F.; Barrio, R. Regulation and function of Spalt proteins during animal development. Int. J. Dev. Biol. 2009, 53, 1385–1398. [Google Scholar] [CrossRef] [Green Version]
- Hoch, R.V.; Lindtner, S.; Price, J.D.; Rubenstein, J.L. OTX2 Transcription Factor Controls Regional Patterning within the Medial Ganglionic Eminence and Regional Identity of the Septum. Cell Rep. 2015, 12, 482–494. [Google Scholar] [CrossRef] [Green Version]
- Cohen, D.R.; Cheng, C.W.; Cheng, S.H.; Hui, C.C. Expression of two novel mouse Iroquois homeobox genes during neurogenesis. Mech. Dev. 2000, 91, 317–321. [Google Scholar] [CrossRef]
- Wapinski, O.L.; Vierbuchen, T.; Qu, K.; Lee, Q.Y.; Chanda, S.; Fuentes, D.R.; Giresi, P.G.; Ng, Y.H.; Marro, S.; Neff, N.F.; et al. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell 2013, 155, 621–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Sudhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Marro, S.; Pang, Z.P.; Yang, N.; Tsai, M.C.; Qu, K.; Chang, H.Y.; Sudhof, T.C.; Wernig, M. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell 2011, 9, 374–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamizu, K.; Sharov, A.A.; Piao, Y.; Amano, M.; Yu, H.; Nishiyama, A.; Dudekula, D.B.; Schlessinger, D.; Ko, M.S. Generation and gene expression profiling of 48 transcription-factor-inducible mouse embryonic stem cell lines. Sci. Rep. 2016, 6, 25667. [Google Scholar] [CrossRef]
- Matsushita, F.; Kameyama, T.; Kadokawa, Y.; Marunouchi, T. Spatiotemporal expression pattern of Myt/NZF family zinc finger transcription factors during mouse nervous system development. Dev. Dyn. 2014, 243, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Colasante, G.; Rubio, A.; Massimino, L.; Broccoli, V. Direct Neuronal Reprogramming Reveals Unknown Functions for Known Transcription Factors. Front. Neurosci. 2019, 13, 283. [Google Scholar] [CrossRef]
- Lee, J.; Taylor, C.A.; Barnes, K.M.; Shen, A.; Stewart, E.V.; Chen, A.; Xiang, Y.K.; Bao, Z.; Shen, K. A Myt1 family transcription factor defines neuronal fate by repressing non-neuronal genes. Elife 2019, 8, e46703. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, M.; Simoes-Costa, M. Heterodimerization of TFAP2 pioneer factors drives epigenomic remodeling during neural crest specification. Genome Res. 2020, 30, 35–48. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, L.; Han, Y.; Wu, F.; Yang, W.S.; Zhang, Z.; Huo, T.; Zhu, Y.; Yu, C.; Kim, H.; et al. Acetylation of histone H3K27 signals the transcriptional elongation for estrogen receptor alpha. Commun. Biol. 2020, 3, 165. [Google Scholar] [CrossRef] [Green Version]
- Manuel, M.N.; Mi, D.; Mason, J.O.; Price, D.J. Regulation of cerebral cortical neurogenesis by the Pax6 transcription factor. Front. Cell Neurosci. 2015, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- O'Loghlen, A.; Martin, N.; Krusche, B.; Pemberton, H.; Alonso, M.M.; Chandler, H.; Brookes, S.; Parrinello, S.; Peters, G.; Gil, J. The nuclear receptor NR2E1/TLX controls senescence. Oncogene 2015, 34, 4069–4077. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.M.; Skidmore, J.M.; Philips, S.T.; Vieira, C.; Gage, P.J.; Condie, B.G.; Raphael, Y.; Martinez, S.; Camper, S.A. PITX2 is required for normal development of neurons in the mouse subthalamic nucleus and midbrain. Dev. Biol. 2004, 267, 93–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Ceballos, E.; Gudas, L.J. Hoxa1 is required for the retinoic acid-induced differentiation of embryonic stem cells into neurons. J. Neurosci. Res. 2008, 86, 2809–2819. [Google Scholar] [CrossRef]
- Arendt, D.; Bertucci, P.Y.; Achim, K.; Musser, J.M. Evolution of neuronal types and families. Curr. Opin. Neurobiol. 2019, 56, 144–152. [Google Scholar] [CrossRef]
- Dharaneeswaran, H.; Abid, M.R.; Yuan, L.; Dupuis, D.; Beeler, D.; Spokes, K.C.; Janes, L.; Sciuto, T.; Kang, P.M.; Jaminet, S.S.; et al. FOXO1-mediated activation of Akt plays a critical role in vascular homeostasis. Circ. Res. 2014, 115, 238–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.Y.; Hwang, I.; Muller, F.L.; Paik, J.H. Functional regulation of FoxO1 in neural stem cell differentiation. Cell Death Differ. 2015, 22, 2034–2045. [Google Scholar] [CrossRef] [Green Version]
- Santo, E.E.; Paik, J. FOXO in Neural Cells and Diseases of the Nervous System. Curr. Top. Dev. Biol. 2018, 127, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Lehtinen, M.K.; Merlo, P.; Villen, J.; Gygi, S.; Bonni, A. Regulation of neuronal cell death by MST1-FOXO1 signaling. J. Biol. Chem. 2009, 284, 11285–11292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paik, J.H.; Kollipara, R.; Chu, G.; Ji, H.; Xiao, Y.; Ding, Z.; Miao, L.; Tothova, Z.; Horner, J.W.; Carrasco, D.R.; et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 2007, 128, 309–323. [Google Scholar] [CrossRef] [Green Version]
- Gorski, D.H.; Walsh, K. Control of vascular cell differentiation by homeobox transcription factors. Trends Cardiovasc. Med. 2003, 13, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Banda, M.; Speyer, C.L.; Smith, J.S.; Rabson, A.B.; Gorski, D.H. Regulation of the expression and activity of the antiangiogenic homeobox gene GAX/MEOX2 by ZEB2 and microRNA-221. Mol. Cell Biol. 2010, 30, 3902–3913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skamagki, M.; Correia, C.; Yeung, P.; Baslan, T.; Beck, S.; Zhang, C.; Ross, C.A.; Dang, L.; Liu, Z.; Giunta, S.; et al. ZSCAN10 expression corrects the genomic instability of iPSCs from aged donors. Nat. Cell Biol. 2017, 19, 1037–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevillard, G.; Blank, V. NFE2L3 (NRF3): The Cinderella of the Cap'n'Collar transcription factors. Cell Mol. Life Sci. 2011, 68, 3337–3348. [Google Scholar] [CrossRef]
- Wang, W.; Grimmer, J.F.; Van De Water, T.R.; Lufkin, T. Hmx2 and Hmx3 homeobox genes direct development of the murine inner ear and hypothalamus and can be functionally replaced by Drosophila Hmx. Dev. Cell 2004, 7, 439–453. [Google Scholar] [CrossRef] [Green Version]
- Olkkonen, J.; Kouri, V.P.; Kuusela, E.; Ainola, M.; Nordstrom, D.; Eklund, K.K.; Mandelin, J. DEC2 Blocks the Effect of the ARNTL2/NPAS2 Dimer on the Expression of PER3 and DBP. J. Circadian. Rhythm. 2017, 15, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebailly, B.; Boitard, C.; Rogner, U.C. Circadian rhythm-related genes: Implication in autoimmunity and type 1 diabetes. Diabetes Obes. Metab. 2015, 17 (Suppl. S1), 134–138. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Linnemann, A.K.; Fontaine, D.A.; Whillock, A.L.; Harris, T.W.; Schleis, G.J.; Truchan, N.A.; Marty-Santos, L.; Lavine, J.A.; Cleaver, O.; et al. Tcf19 is a novel islet factor necessary for proliferation and survival in the INS-1 beta-cell line. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E600–E610. [Google Scholar] [CrossRef]
- Sen, S.; Sanyal, S.; Srivastava, D.K.; Dasgupta, D.; Roy, S.; Das, C. Transcription factor 19 interacts with histone 3 lysine 4 trimethylation and controls gluconeogenesis via the nucleosome-remodeling-deacetylase complex. J. Biol. Chem. 2017, 292, 20362–20378. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Nishihara, S.; Kamimura, M.; Shiraishi, T.; Otoguro, T.; Uehara, M.; Maeda, Y.; Ogura, K.; Lumsden, A.; Ogura, T. The prepattern transcription factor Irx2, a target of the FGF8/MAP kinase cascade, is involved in cerebellum formation. Nat. Neurosci. 2004, 7, 605–612. [Google Scholar] [CrossRef]
- Hu, W.; Xin, Y.; Zhang, L.; Hu, J.; Sun, Y.; Zhao, Y. Iroquois Homeodomain transcription factors in ventricular conduction system and arrhythmia. Int. J. Med. Sci. 2018, 15, 808–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarlett, K.; Pattabiraman, V.; Barnett, P.; Liu, D.; Anderson, L.M. The proangiogenic effect of iroquois homeobox transcription factor Irx3 in human microvascular endothelial cells. J. Biol. Chem. 2015, 290, 6303–6315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbagh, M.F.; Heng, J.S.; Luo, C.; Castanon, R.G.; Nery, J.R.; Rattner, A.; Goff, L.A.; Ecker, J.R.; Nathans, J. Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. Elife 2018, 7, e36187. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Harrell, J.C.; Perou, C.M.; Dudley, A.C. Identification of a stable molecular signature in mammary tumor endothelial cells that persists in vitro. Angiogenesis 2014, 17, 511–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, M.; Shima, N.; Kimoto, M.; Ebihara, N.; Murakami, A.; Yamagami, S. Markers for distinguishing cultured human corneal endothelial cells from corneal stromal myofibroblasts. Curr. Eye Res. 2015, 40, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity (Edinb) 2010, 105, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Gagliardi, M.; Strazzullo, M.; Matarazzo, M.R. DNMT3B Functions: Novel Insights From Human Disease. Front. Cell Dev. Biol. 2018, 6, 140. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohenauer, T.; Moore, A.W. The Prdm family: Expanding roles in stem cells and development. Development 2012, 139, 2267–2282. [Google Scholar] [CrossRef] [Green Version]
- Sybirna, A.; Tang, W.W.C.; Pierson Smela, M.; Dietmann, S.; Gruhn, W.H.; Brosh, R.; Surani, M.A. A critical role of PRDM14 in human primordial germ cell fate revealed by inducible degrons. Nat. Commun. 2020, 11, 1282. [Google Scholar] [CrossRef]
- Seki, Y. PRDM14 Is a Unique Epigenetic Regulator Stabilizing Transcriptional Networks for Pluripotency. Front. Cell Dev. Biol. 2018, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Zocchi, L.; Mehta, A.; Wu, S.C.; Wu, J.; Gu, Y.; Wang, J.; Suh, S.; Spitale, R.C.; Benavente, C.A. Chromatin remodeling protein HELLS is critical for retinoblastoma tumor initiation and progression. Oncogenesis 2020, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myant, K.; Stancheva, I. LSH cooperates with DNA methyltransferases to repress transcription. Mol. Cell Biol. 2008, 28, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Zhou, X.; Zhou, J.; Gong, S.; Hu, G.; Li, J.; Huang, K.; Lai, P.; Shi, G.; Hutchins, A.P.; et al. PCGF5 is required for neural differentiation of embryonic stem cells. Nat. Commun. 2018, 9, 1463. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Huang, Y.; Zhang, J.; Liu, M.; Ji, H.; Wang, C.; Cao, N.; Li, C.; Xia, Y.; Jiang, Q.; et al. Polycomb group RING finger proteins 3/5 activate transcription via an interaction with the pluripotency factor Tex10 in embryonic stem cells. J. Biol. Chem. 2017, 292, 21527–21537. [Google Scholar] [CrossRef] [Green Version]
- Cebria-Costa, J.P.; Pascual-Reguant, L.; Gonzalez-Perez, A.; Serra-Bardenys, G.; Querol, J.; Cosin, M.; Verde, G.; Cigliano, R.A.; Sanseverino, W.; Segura-Bayona, S.; et al. LOXL2-mediated H3K4 oxidation reduces chromatin accessibility in triple-negative breast cancer cells. Oncogene 2020, 39, 79–121. [Google Scholar] [CrossRef] [Green Version]
- Gokbuget, D.; Blelloch, R. Epigenetic control of transcriptional regulation in pluripotency and early differentiation. Development 2019, 146, dev164772. [Google Scholar] [CrossRef] [Green Version]
- Iturbide, A.; Pascual-Reguant, L.; Fargas, L.; Cebria, J.P.; Alsina, B.; Garcia de Herreros, A.; Peiro, S. LOXL2 Oxidizes Methylated TAF10 and Controls TFIID-Dependent Genes during Neural Progenitor Differentiation. Mol. Cell 2015, 58, 755–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bignon, M.; Pichol-Thievend, C.; Hardouin, J.; Malbouyres, M.; Brechot, N.; Nasciutti, L.; Barret, A.; Teillon, J.; Guillon, E.; Etienne, E.; et al. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood 2011, 118, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Beerens, N.; Hoeijmakers, J.H.; Kanaar, R.; Vermeulen, W.; Wyman, C. The CSB protein actively wraps DNA. J. Biol. Chem. 2005, 280, 4722–4729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, E.W.; Croteau, D.L.; Bohr, V.A. Heterochromatin: An epigenetic point of view in aging. Exp. Mol. Med. 2020, 52, 1466–1474. [Google Scholar] [CrossRef]
- Wei, J.W.; Huang, K.; Yang, C.; Kang, C.S. Non-coding RNAs as regulators in epigenetics (Review). Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Tan, F.E.; Yeo, G.W. Blurred Boundaries: The RNA Binding Protein Lin28A Is Also an Epigenetic Regulator. Mol. Cell 2016, 61, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Hafner, M.; Max, K.E.; Bandaru, P.; Morozov, P.; Gerstberger, S.; Brown, M.; Molina, H.; Tuschl, T. Identification of mRNAs bound and regulated by human LIN28 proteins and molecular requirements for RNA recognition. RNA 2013, 19, 613–626. [Google Scholar] [CrossRef] [Green Version]
- Loedige, I.; Gaidatzis, D.; Sack, R.; Meister, G.; Filipowicz, W. The mammalian TRIM-NHL protein TRIM71/LIN-41 is a repressor of mRNA function. Nucleic Acids Res. 2013, 41, 518–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.; Yao, B.; Shin, J.; Lin, L.; Kim, N.; Song, Q.; Liu, S.; Su, Y.; Guo, J.U.; Huang, L.; et al. Lin28A Binds Active Promoters and Recruits Tet1 to Regulate Gene Expression. Mol. Cell 2016, 61, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Mitschka, S.; Ulas, T.; Goller, T.; Schneider, K.; Egert, A.; Mertens, J.; Brustle, O.; Schorle, H.; Beyer, M.; Klee, K.; et al. Co-existence of intact stemness and priming of neural differentiation programs in mES cells lacking Trim71. Sci. Rep. 2015, 5, 11126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas, E.; Rybak-Wolf, A.; Rohde, A.M.; Nguyen, D.T.; Wulczyn, F.G. Lin41/Trim71 is essential for mouse development and specifically expressed in postnatal ependymal cells of the brain. Front. Cell Dev. Biol. 2015, 3, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Fernandez, L.A.; Jux, B.; Bille, M.; Port, Y.; Schneider, K.; Geyer, M.; Mayer, G.; Kolanus, W. The mRNA repressor TRIM71 cooperates with Nonsense-Mediated Decay factors to destabilize the mRNA of CDKN1A/p21. Nucleic Acids Res. 2019, 47, 11861–11879. [Google Scholar] [CrossRef]
- Pastor, W.A.; Aravind, L.; Rao, A. TETonic shift: Biological roles of TET proteins in DNA demethylation and transcription. Nat. Rev. Mol. Cell Biol. 2013, 14, 341–356. [Google Scholar] [CrossRef] [Green Version]
- Cakouros, D.; Hemming, S.; Gronthos, K.; Liu, R.; Zannettino, A.; Shi, S.; Gronthos, S. Specific functions of TET1 and TET2 in regulating mesenchymal cell lineage determination. Epigenetics Chromatin. 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Pasini, D.; Cloos, P.A.; Walfridsson, J.; Olsson, L.; Bukowski, J.P.; Johansen, J.V.; Bak, M.; Tommerup, N.; Rappsilber, J.; Helin, K. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature 2010, 464, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsova, V.L.; Tchekanov, A.; Li, X.; Yan, X.; Tsigelny, I.F. Polycomb repressive 2 complex-Molecular mechanisms of function. Protein Sci. 2019, 28, 1387–1399. [Google Scholar] [CrossRef]
- Herz, H.M.; Shilatifard, A. The JARID2-PRC2 duality. Genes Dev. 2010, 24, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Metzger, E.; Imhof, A.; Patel, D.; Kahl, P.; Hoffmeyer, K.; Friedrichs, N.; Muller, J.M.; Greschik, H.; Kirfel, J.; Ji, S.; et al. Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature 2010, 464, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Banting, G.S.; Barak, O.; Ames, T.M.; Burnham, A.C.; Kardel, M.D.; Cooch, N.S.; Davidson, C.E.; Godbout, R.; McDermid, H.E.; Shiekhattar, R. CECR2, a protein involved in neurulation, forms a novel chromatin remodeling complex with SNF2L. Hum. Mol. Genet 2005, 14, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, L.R.; Picketts, D.J. The role of ISWI chromatin remodeling complexes in brain development and neurodevelopmental disorders. Mol. Cell Neurosci. 2018, 87, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Biterge, B.; Schneider, R. Histone variants: Key players of chromatin. Cell Tissue Res. 2014, 356, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Soragni, E.; Chou, C.J.; Rusche, J.R.; Gottesfeld, J.M. Mechanism of Action of 2-Aminobenzamide HDAC Inhibitors in Reversing Gene Silencing in Friedreich's Ataxia. Front. Neurol. 2015, 6, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagan, J.K.; Arnold, J.; Hanchard, K.J.; Kumar, R.; Bruno, T.; Jones, M.J.; Richard, D.J.; Forrest, A.; Spurdle, A.; Verdin, E.; et al. A novel corepressor, BCoR-L1, represses transcription through an interaction with CtBP. J. Biol. Chem. 2007, 282, 15248–15257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaito, S.; Iwama, A. Pathogenic Impacts of Dysregulated Polycomb Repressive Complex Function in Hematological Malignancies. Int. J. Mol. Sci. 2020, 22, 74. [Google Scholar] [CrossRef]
- Chou, S.J.; Tole, S. Lhx2, an evolutionarily conserved, multifunctional regulator of forebrain development. Brain Res. 2019, 1705, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, B.; Khatri, Z.; Maheshwari, U.; Gupta, R.; Roy, B.; Pradhan, S.J.; Karmodiya, K.; Padmanabhan, H.; Shetty, A.S.; Balaji, C.; et al. LHX2 Interacts with the NuRD Complex and Regulates Cortical Neuron Subtype Determinants Fezf2 and Sox11. J. Neurosci. 2017, 37, 194–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotnikova, A.; Baranauske, S.; Osipenko, A.; Klimasauskas, S.; Vilkaitis, G. Mechanistic insights into small RNA recognition and modification by the HEN1 methyltransferase. Biochem. J. 2013, 453, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Hempfling, A.L.; Lim, S.L.; Adelson, D.L.; Evans, J.; O'Connor, A.E.; Qu, Z.P.; Kliesch, S.; Weidner, W.; O'Bryan, M.K.; Bergmann, M. Expression patterns of HENMT1 and PIWIL1 in human testis: Implications for transposon expression. Reproduction 2017, 154, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Xiao, R. An emerging role of chromatin-interacting RNA-binding proteins in transcription regulation. Essays Biochem. 2020, 64, 907–918. [Google Scholar] [CrossRef]
- Diaz-Munoz, M.D.; Turner, M. Uncovering the Role of RNA-Binding Proteins in Gene Expression in the Immune System. Front. Immunol. 2018, 9, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembele, D. Analysis of high-throughput biological data using their rank values. Stat. Methods Med. Res. 2019, 28, 2276–2291. [Google Scholar] [CrossRef]
- Dembele, D.; Kastner, P. Fold change rank ordering statistics: A new method for detecting differentially expressed genes. BMC Bioinform. 2014, 15, 14. [Google Scholar] [CrossRef] [Green Version]
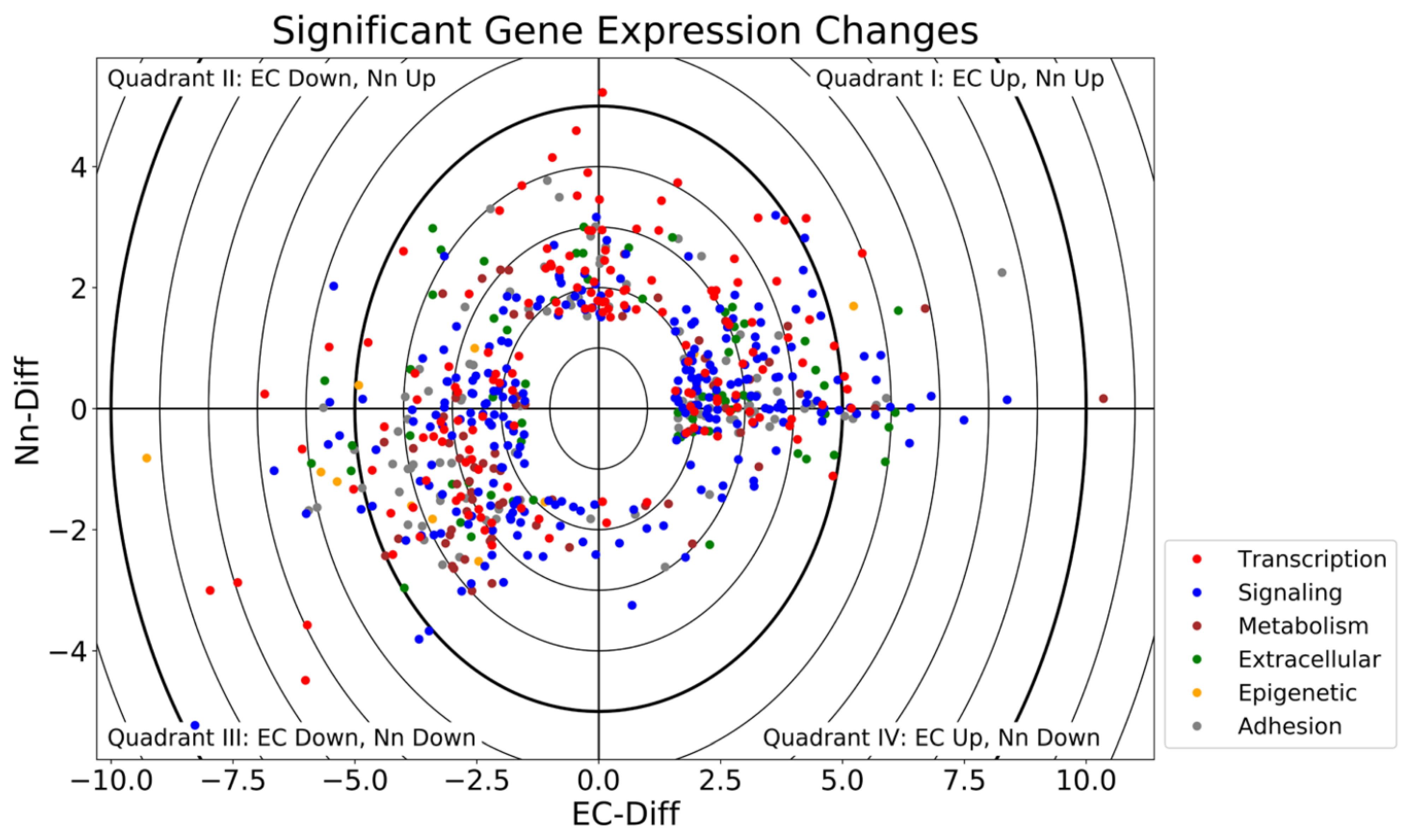
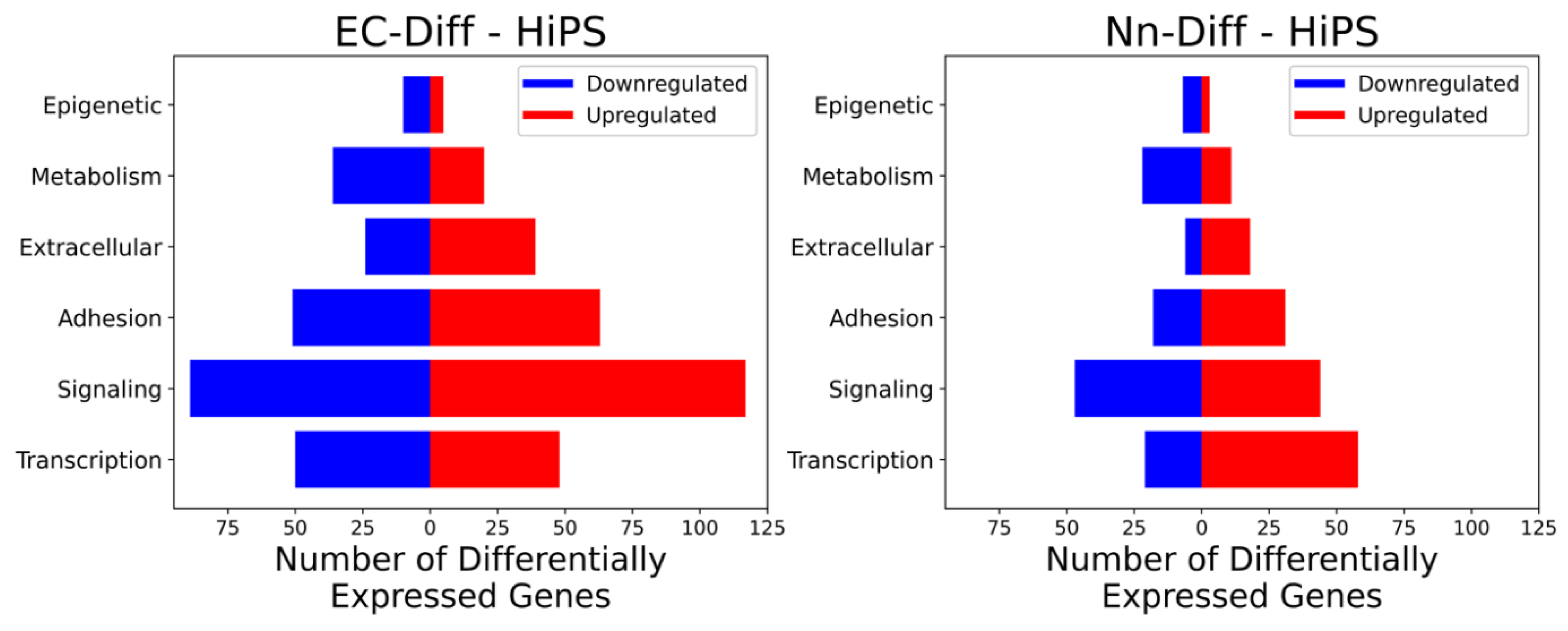
| Gene | EC-Fold Change | Nn-Fold Change | |
|---|---|---|---|
| 1 | GATA3 | 51.058 | 22.919 |
| 2 | GATA6 | 42.389 | 5.923 |
| 3 | ZFPM2 | 33.551 | 10.32 |
| 4 | RARB | 23.55 | 15.564 |
| 5 | NR2F2 | 17.982 | 9.556 |
| 6 | HOXB2 | 9.633 | 8.887 |
| 7 | MEF2C | 7.634 | 4.935 |
| 8 | HOXA4 | 7.239 | 4.241 |
| 9 | ZNF436 | 6.887 | 5.562 |
| Gene | EC-Fold Change | Nn-Fold Change | |
|---|---|---|---|
| 1 | POU5F1 | −334.824 | −9.624 |
| 2 | POU5F1P3 | −169.194 | −7.315 |
| 3 | NANOG | −64.627 | −22.43 |
| 4 | ZFP42 | −62.901 | −11.91 |
| 5 | NANOG///NANOGP1 | −19.196 | −3.314 |
| 6 | FOXD3 | −18.649 | −5.317 |
| 7 | NR5A2 | −16.392 | −13.733 |
| 8 | SOX8 | −13.993 | −3.103 |
| 9 | FOXA3 | −12.691 | −4.342 |
| 10 | HEY2 | −7.758 | −4.113 |
| 11 | ZNF165 | −7.582 | −2.87 |
| 12 | ETV1 | −6.688 | −6.025 |
| 13 | FOXH1 | −6.352 | −3.391 |
| Gene | Fold Change | |
|---|---|---|
| 1 | EPAS1/// LOC100652809 | 117.891 |
| 2 | SOX17 | 91.658 |
| 3 | SOX7 | 74.419 |
| 4 | MECOM | 66.16 |
| 5 | ERG | 53.912 |
| 6 | TFEC | 50.453 |
| 7 | FLI1 | 37.099 |
| 8 | HOPX | 36.998 |
| 9 | SOX18 | 36.295 |
| 10 | TAL1 | 34.351 |
| 11 | ELK3 | 30.421 |
| 12 | HOXB7 | 28.461 |
| 13 | HHEX | 27.973 |
| 14 | GATA2 | 23.984 |
| 15 | HCLS1 | 23.087 |
| 16 | HOXA5 | 20.052 |
| 17 | TBX18 | 19.162 |
| 18 | HOXB3 | 18.896 |
| 19 | BNC1 | 12.005 |
| 20 | ZEB1 | 11.675 |
| 21 | KLF9 | 10.852 |
| 22 | HOXD1 | 10.312 |
| 23 | NFIB | 10.1 |
| 24 | HLX | 9.854 |
| 25 | NPAS2 | 9.726 |
| 26 | FOSL2 | 9.72 |
| 27 | ELF4 | 8.969 |
| 28 | KLF2 | 8.559 |
| 29 | FOXC1 | 7.728 |
| 30 | HOXA11 | 7.292 |
| 31 | HOXA10-HOXA9///HOXA9 ///MIR196B | 7.169 |
| 32 | ATF6 | 6.881 |
| 33 | FOXF1 | 6.471 |
| 34 | ZNF521 | 6.404 |
| 35 | ZBTB38 | 5.751 |
| 36 | IRF6 | 5.398 |
| 37 | MMP14 | 5.361 |
| 38 | ETS1 | 5.245 |
| 39 | STAT6 | 5.227 |
| Gene | Fold Change | |
|---|---|---|
| 1 | ZIC2 | −506.971 |
| 2 | SOX2 | −243.085 |
| 3 | ZIC5 | −170.82 |
| 4 | ZIC3 | −143.398 |
| 5 | OTX2 | −120.52 |
| 6 | SOX11 | −79.857 |
| 7 | SALL1 | −46.361 |
| 8 | SOX21 | −46.13 |
| 9 | SALL2 | −42.04 |
| 10 | ZNF90 | −38.389 |
| 11 | ZNF423 | −36.361 |
| 12 | SOX9 | −27.58 |
| 13 | BCL11A | −23.86 |
| 14 | MYCN | −23.094 |
| 15 | TFAP2C | −20.154 |
| 16 | PBX1 | −16.377 |
| 17 | MKX | −13.612 |
| 18 | ZNF154 | −13.29 |
| 19 | HESX1 | −12.089 |
| 20 | EBF1 | −11.098 |
| 21 | CUX2 | −10.449 |
| 22 | ESRRG | −10.06 |
| 23 | HES6 | −9.416 |
| 24 | E2F5 | −9.012 |
| 25 | MYB | −8.481 |
| 26 | POU3F1 | −8.471 |
| 27 | ZNF649 | −8.279 |
| 28 | SCAND3 | −7.674 |
| 29 | TRERF1 | −7.328 |
| 30 | DLX1 | −7.172 |
| 31 | ZNF93 | −7.085 |
| 32 | TAF4B | −6.925 |
| 33 | ZNF398 | −6.401 |
| 34 | CITED1 | −5.991 |
| 35 | ZFP37 | −5.643 |
| Gene | EC-Fold Change | Nn-Fold Change | |
|---|---|---|---|
| 1 | IRX3 | −21.279 | 6.33 |
| 2 | IRX2 | −10.615 | 6.497 |
| Gene | Fold Change | |
|---|---|---|
| 1 | TFAP2B | 113.974 |
| 2 | TFAP2A | 62.803 |
| 3 | PAX6 | 51.035 |
| 4 | POU3F2 | 24.153 |
| 5 | NR2E1 | 22.86 |
| 6 | ST18 | 19.396 |
| 7 | ASCL1 | 17.971 |
| 8 | PITX2 | 17.764 |
| 9 | HOXA1 | 16.684 |
| 10 | ARX | 14.939 |
| 11 | MAF | 13.489 |
| 12 | NR2F1 | 13.313 |
| 13 | FOXG1 | 12.871 |
| 14 | MEIS1 | 11.929 |
| 15 | DLX5 | 11.139 |
| 16 | ZEB2 | 11.09 |
| 17 | TWIST1 | 9.671 |
| 18 | IRX5 | 9.371 |
| 19 | DMRT2 | 9.171 |
| 20 | WNT5A | 8.17 |
| 21 | POU4F1 | 7.833 |
| 22 | NEUROD1 | 7.794 |
| 23 | MSX2 | 7.759 |
| 24 | DLX6 | 7.711 |
| 25 | SIX3 | 7.682 |
| 26 | MEIS2 | 7.505 |
| 27 | ONECUT2 | 6.997 |
| 28 | ZFHX4 | 6.941 |
| 29 | HOXC6 | 6.668 |
| 30 | MAFB | 6.001 |
| 31 | TSHZ2 | 5.903 |
| 32 | CBFA2T3 | 5.476 |
| 33 | HOXC4 | 5.247 |
| 34 | CSRNP3 | 4.986 |
| 35 | ISL1 | 4.49 |
| 36 | HOXC8 | 4.382 |
| 37 | ZHX1 | 3.944 |
| 38 | EMX2 | 3.913 |
| 39 | ALX1 | 3.862 |
| 40 | RUNX1T1 | 3.694 |
| 41 | NEUROG1 | 3.44 |
| 42 | SNAI2 | 3.352 |
| 43 | LHX2 | 3.162 |
| 44 | GRHL3 | 3.14 |
| 45 | HOXD3 | 3.139 |
| 46 | OTP | 3.021 |
| 47 | LHX9 | 2.848 |
| Gene | Fold Change | |
|---|---|---|
| 1 | FOXO1 | −6.445 |
| 2 | NFE2L3 | −4.486 |
| 3 | ZSCAN10 | −3.712 |
| 4 | MEOX2 | −3.541 |
| 5 | HMX2 | −3.477 |
| 6 | TCF19 | −3.131 |
| 7 | ARNTL2 | −3.127 |
| Gene | EC-Fold Change | Nn-Fold Change | |
|---|---|---|---|
| 1 | DNMT3B | −44.269 | −5.983 |
| 2 | PRDM14 | −14.327 | −3.042 |
| 3 | HELLS | −5.666 | −9.624 |
| Gene | Fold Change | |
|---|---|---|
| 1 | PCGF5 | 9.831 |
| 2 | LOXL2 | 7.979 |
| 3 | ERCC6 | 5.444 |
| Gene | Fold Change | |
|---|---|---|
| 1 | LIN28A | −616.831 |
| 2 | LIN28B | −254.621 |
| 3 | TET1 | −62.007 |
| 4 | TRIM71 | −51.797 |
| 5 | JARID2 | −12.625 |
| 6 | PRKCB | −8.155 |
| 7 | CECR2 | −6.632 |
| 8 | H2AFY2 | −5.827 |
| Gene | Fold Change | |
|---|---|---|
| 1 | BCORL1 | 3.425 |
| 2 | LHX2 | 3.162 |
| Gene | Fold Change | |
|---|---|---|
| 1 | HENMT1 | −2.912 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakhaei-Nejad, M.; Trinity, L.; Jabbari, H.; Pasdar, M.; Jahroudi, N. In Silico Analysis to Explore Lineage-Independent and -Dependent Transcriptional Programs Associated with the Process of Endothelial and Neural Differentiation of Human Induced Pluripotent Stem Cells. J. Clin. Med. 2021, 10, 4161. https://doi.org/10.3390/jcm10184161
Nakhaei-Nejad M, Trinity L, Jabbari H, Pasdar M, Jahroudi N. In Silico Analysis to Explore Lineage-Independent and -Dependent Transcriptional Programs Associated with the Process of Endothelial and Neural Differentiation of Human Induced Pluripotent Stem Cells. Journal of Clinical Medicine. 2021; 10(18):4161. https://doi.org/10.3390/jcm10184161
Chicago/Turabian StyleNakhaei-Nejad, Maryam, Luke Trinity, Hosna Jabbari, Manijeh Pasdar, and Nadia Jahroudi. 2021. "In Silico Analysis to Explore Lineage-Independent and -Dependent Transcriptional Programs Associated with the Process of Endothelial and Neural Differentiation of Human Induced Pluripotent Stem Cells" Journal of Clinical Medicine 10, no. 18: 4161. https://doi.org/10.3390/jcm10184161
APA StyleNakhaei-Nejad, M., Trinity, L., Jabbari, H., Pasdar, M., & Jahroudi, N. (2021). In Silico Analysis to Explore Lineage-Independent and -Dependent Transcriptional Programs Associated with the Process of Endothelial and Neural Differentiation of Human Induced Pluripotent Stem Cells. Journal of Clinical Medicine, 10(18), 4161. https://doi.org/10.3390/jcm10184161







