Impact of SARS-CoV-2 Lockdown on Glycaemic Control: A Retrospective Observational Cohort Study in a Tertiary Setting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Extraction
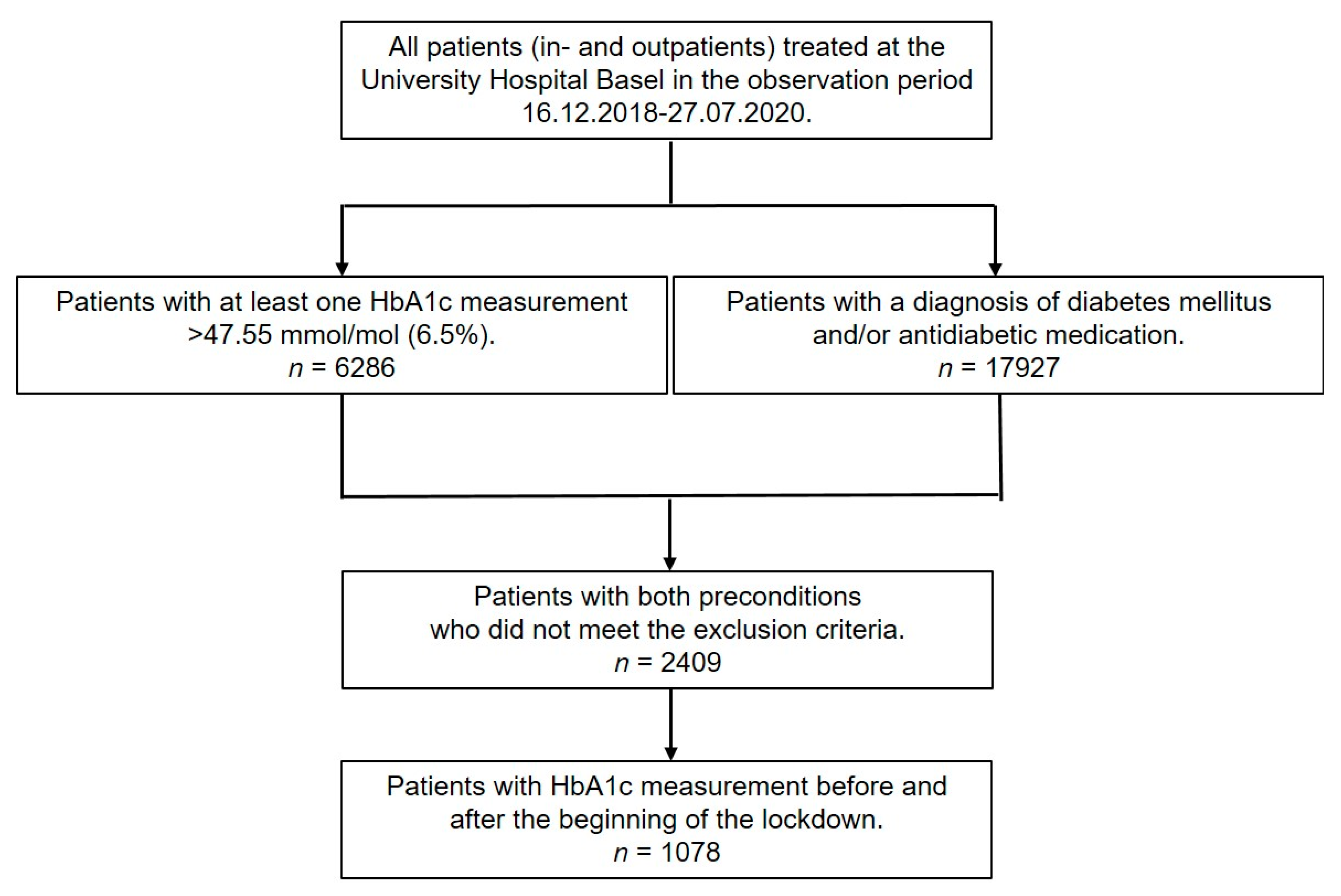
2.3. Participants
2.4. Variables
2.5. Definition of Comorbidities
2.6. Other Confounding Factors
2.7. Laboratory Methods
2.8. Biases
2.9. Study Size
2.10. Statistical Analysis
3. Results
3.1. Participants–Descriptive Data
3.2. Outcome Data
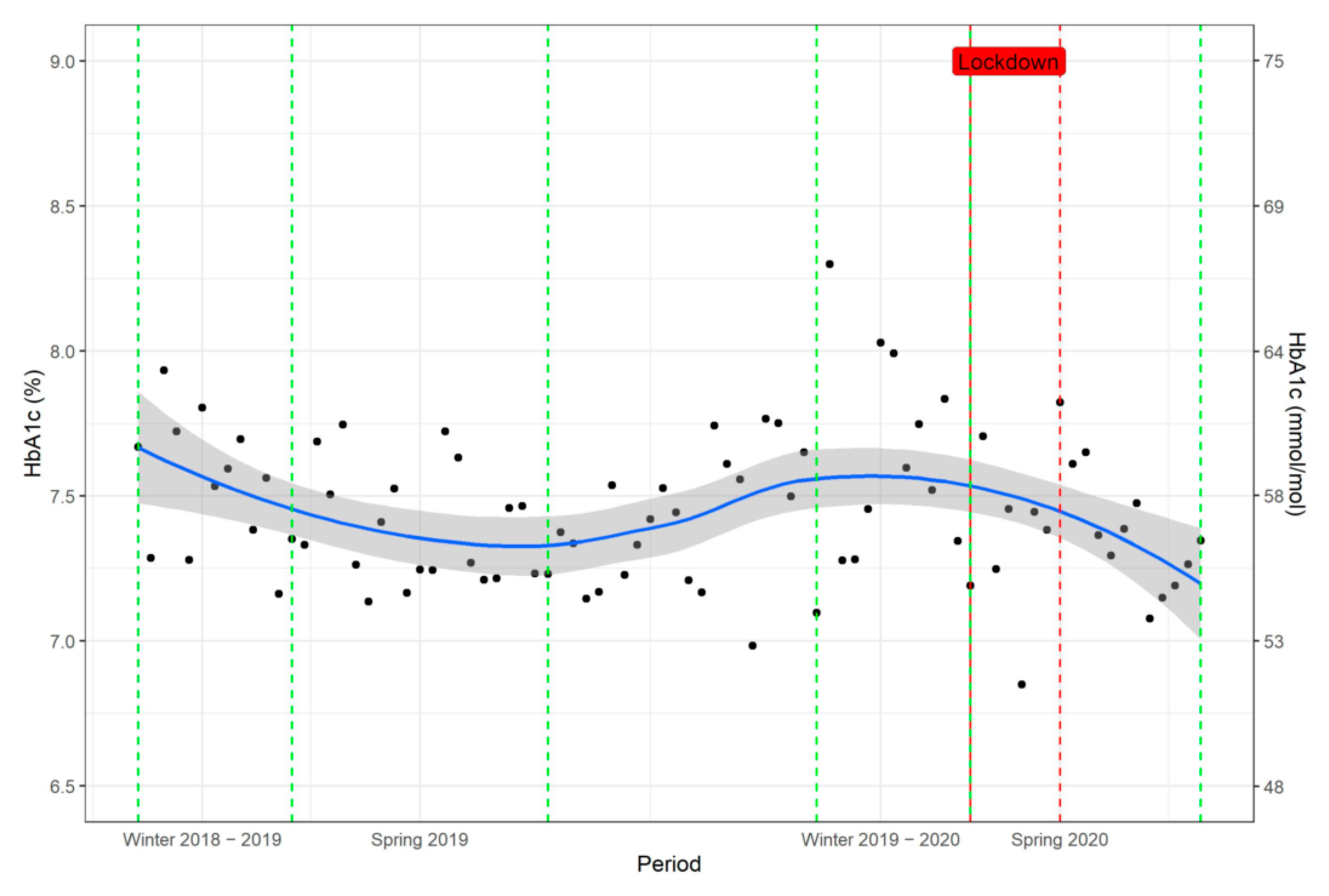
Glycaemic Control in the Longitudinal Follow-Up: Seasonal Changes
3.3. Main Results
Effect of Lockdown on Glycaemic Control
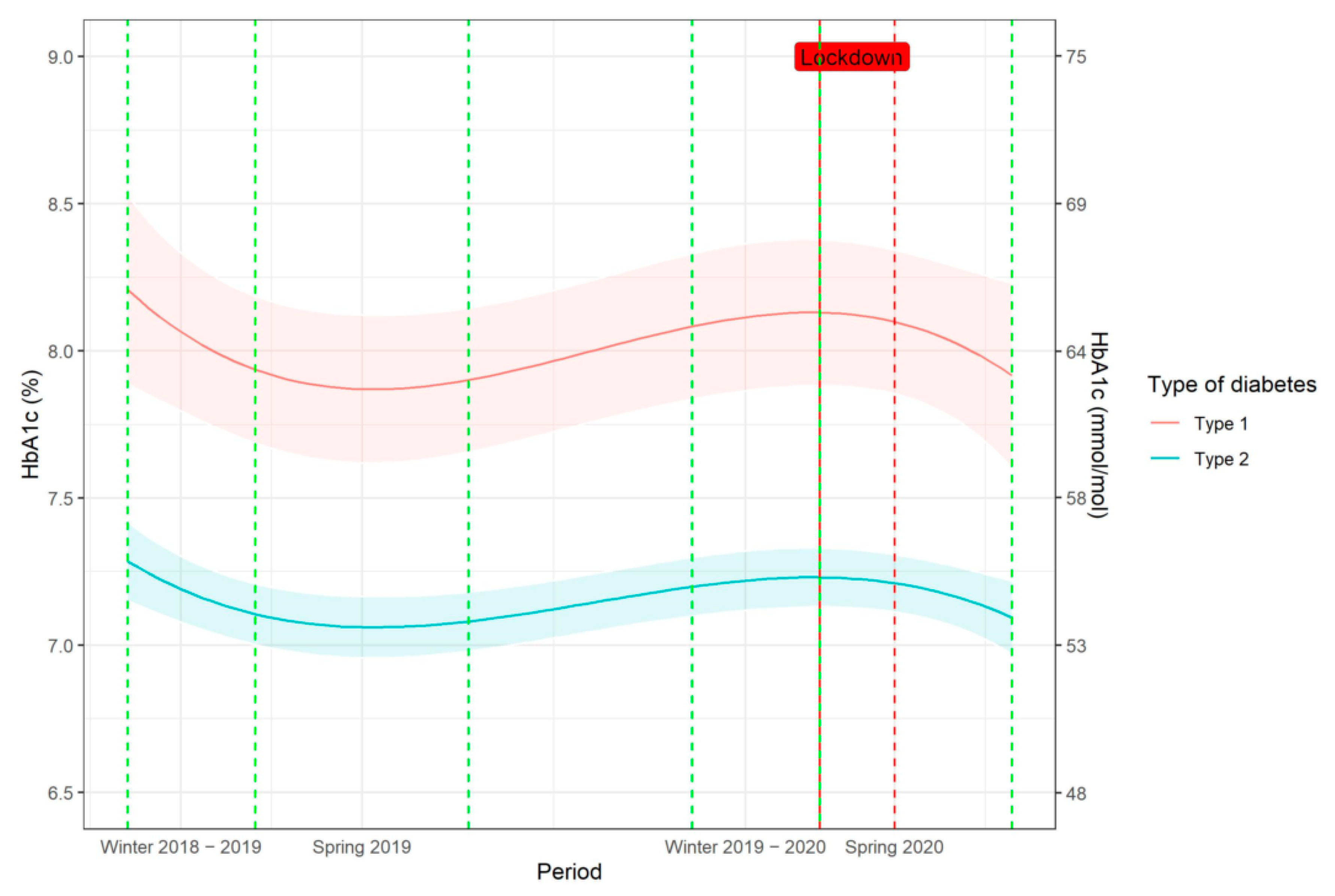
3.4. Additional Analyses
3.5. Effect of Seasonal Variations and Lockdown on Weight
3.6. Effect of Seasonal Variation and Lockdown on Outpatient Consultations and Hospitalisations
3.7. Impact of Confounding Factors on Glycaemic Control after the Lockdown
4. Discussion
4.1. Effect of Lockdown on Patients with Type 2 Diabetes
4.2. Seasonal Variation
4.3. Effects of Lockdown on Patients with Type 1 Diabetes
4.4. Risk Factors for Deterioration in Glycaemic Compensation after Lockdown
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allweiss, P. Diabetes and Disasters: Recent Studies and Resources for Preparedness. Curr. Diabetes Rep. 2019, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.A.; Smith, H.; Kuhadiya, N.; Leger, S.M.; Yau, C.L.; Reynolds, K.; Shi, L.; McDuffie, R.H.; Thethi, T.; John-Kalarickal, J. Impact of a Natural Disaster on Diabetes: Exacerbation of disparities and long-term consequences. Diabetes Care 2009, 32, 1632–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Batlle-Bayer, L.; Aldaco, R.; Bala, A.; Puig, R.; Laso, J.; Margallo, M.; Vázquez-Rowe, I.; Antó, J.M.; Fullana-I.-Palmer, P. Environmental and nutritional impacts of dietary changes in Spain during the COVID-19 lockdown. Sci. Total Environ. 2020, 748, 141410. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Camussi, E.; Piccinelli, C.; Senore, C.; Armaroli, P.; Ortale, A.; Garena, F.; Giordano, L. Did social isolation during the SARS-CoV-2 epidemic have an impact on the lifestyles of citizens? Epidemiol. Prev. 2020, 44, 353–362. [Google Scholar] [CrossRef]
- Kriaucioniene, V.; Bagdonaviciene, L.; Rodríguez-Pérez, C.; Petkeviciene, J. Associations between Changes in Health Behaviours and Body Weight during the COVID-19 Quarantine in Lithuania: The Lithuanian COVIDiet Study. Nutrients 2020, 12, 3119. [Google Scholar] [CrossRef]
- Robinson, E.; Boyland, E.; Chisholm, A.; Harrold, J.; Maloney, N.G.; Marty, L.; Mead, B.R.; Noonan, R.; Hardman, C.A. Obesity, eating behavior and physical activity during COVID-19 lockdown: A study of UK adults. Appetite 2020, 156, 104853. [Google Scholar] [CrossRef]
- Sidor, A.; Rzymski, P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef]
- Tison, G.H.; Avram, R.; Kuhar, P.; Abreau, S.; Marcus, G.M.; Pletcher, M.J.; Olgin, J.E. Worldwide Effect of COVID-19 on Physical Activity: A Descriptive Study. Ann. Intern. Med. 2020, 173, 767–770. [Google Scholar] [CrossRef]
- Vinkers, C.H.; van Amelsvoort, T.; Bisson, J.I.; Branchi, I.; Cryan, J.F.; Domschke, K.; Howes, O.D.; Manchia, M.; Pinto, L.; de Quervain, D.; et al. Stress resilience during the coronavirus pandemic. Eur. Neuropsychopharmacol. 2020, 35, 12–16. [Google Scholar] [CrossRef]
- Zhu, L.; She, Z.-G.; Cheng, X.; Qin, J.-J.; Zhang, X.-J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e3. [Google Scholar] [CrossRef]
- Biancalana, E.; Parolini, F.; Mengozzi, A.; Solini, A. Short-term impact of COVID-19 lockdown on metabolic control of patients with well-controlled type 2 diabetes: A single-centre observational study. Acta Diabetol. 2020, 58, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Bonora, B.M.; Boscari, F.; Avogaro, A.; Bruttomesso, D.; Fadini, G.P. Glycaemic Control Among People with Type 1 Diabetes During Lockdown for the SARS-CoV-2 Outbreak in Italy. Diabetes Ther. 2020, 11, 1369–1379. [Google Scholar] [CrossRef]
- Dover, A.R.; Ritchie, S.A.; McKnight, J.A.; Strachan, M.W.J.; Zammitt, N.N.; Wake, D.; Forbes, S.; Stimson, R.H.; Gibb, F.W. Assessment of the effect of the COVID-19 lockdown on glycaemic control in people with type 1 diabetes using flash glucose monitoring. Diabet. Med. 2020, 38, e14374. [Google Scholar] [CrossRef]
- Fernández, E.; Cortazar, A.; Bellido, V. Impact of COVID-19 lockdown on glycemic control in patients with type 1 diabetes. Diabetes Res. Clin. Pract. 2020, 166, 108348. [Google Scholar] [CrossRef] [PubMed]
- Karatas, S.; Yesim, T.; Beysel, S. Impact of lockdown COVID-19 on metabolic control in type 2 diabetes mellitus and healthy people. Prim. Care Diabetes 2021, 15, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Önmez, A.; Gamsızkan, Z.; Özdemir, Ş.; Kesikbaş, E.; Gökosmanoğlu, F.; Torun, S.; Cinemre, H. The effect of COVID-19 lockdown on glycemic control in patients with type 2 diabetes mellitus in Turkey. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1963–1966. [Google Scholar] [CrossRef]
- Rastogi, A.; Hiteshi, P.; Bhansali, A. Improved glycemic control amongst people with long-standing diabetes during COVID-19 lockdown: A prospective, observational, nested cohort study. Int. J. Diabetes Dev. Ctries. 2020, 40, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Sankar, P.; Ahmed, W.N.; Koshy, V.M.; Jacob, R.; Sasidharan, S. Effects of COVID-19 lockdown on type 2 diabetes, lifestyle and psychosocial health: A hospital-based cross-sectional survey from South India. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1815–1819. [Google Scholar] [CrossRef]
- Verma, A.; Rajput, R.; Verma, S.; Balania, V.K.; Jangra, B. Impact of lockdown in COVID 19 on glycemic control in patients with type 1 Diabetes Mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1213–1216. [Google Scholar] [CrossRef]
- D’Onofrio, L.; Pieralice, S.; Maddaloni, E.; Mignogna, C.; Sterpetti, S.; Coraggio, L.; Luordi, C.; Guarisco, G.; Leto, G.; Leonetti, F.; et al. Effects of the COVID -19 lockdown on glycaemic control in subjects with type 2 diabetes: The glycalock study. Diabetes Obes. Metab. 2021, 23, 1624–1630. [Google Scholar] [CrossRef]
- Beer-Borst, S.; Costanza, M.C.; Pechère-Bertschi, A.; Morabia, A. Twelve-year trends and correlates of dietary salt intakes for the general adult population of Geneva, Switzerland. Eur. J. Clin. Nutr. 2007, 63, 155–164. [Google Scholar] [CrossRef]
- Rolland, B.; Haesebaert, F.; Zante, E.; Benyamina, A.; Haesebaert, J.; Franck, N. Global Changes and Factors of Increase in Caloric/Salty Food Intake, Screen Use, and Substance Use During the Early COVID-19 Containment Phase in the General Population in France: Survey Study. JMIR Public Health Surveill. 2020, 6, e19630. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Lehtisalo, J.; Palmer, K.; Mangialasche, F.; Solomon, A.; Kivipelto, M.; Ngandu, T. Changes in Lifestyle, Behaviors, and Risk Factors for Cognitive Impairment in Older Persons During the First Wave of the Coronavirus Disease 2019 Pandemic in Finland: Results From the FINGER Study. Front. Psychiatry 2021, 12, 624125. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M. Bier-, Wein- und Schnapskonsum brechen ein: Die Schweiz geht nüchtern durch den Lockdown. Available online: https://nzzas.nzz.ch/wirtschaft/trockener-lockdown-schweizer-konsumieren-weniger-alkohol-ld.1558987 (accessed on 12 April 2021).
- Eidgenössisches Departement für Wirtschaft, Bildung und Forschung, Bundesamt für Landwirtschaft. Deutlicher Anstieg der Nachfrage nach Bio-Milchprodukten im Jahr. Available online: https://www.blw.admin.ch/blw/de/home/markt/marktbeobachtung/bio.html (accessed on 27 March 2021).
- Bundesamt für Statistik, Mobilitäts-Monitoring Covid. Available online: https://www.experimental.bfs.admin.ch/expstat/de/home/innovative-methoden/mobil.html (accessed on 27 March 2021).
- Cheval, B.; Sivaramakrishnan, H.; Maltagliati, S.; Fessler, L.; Forestier, C.; Sarrazin, P.; Orsholits, D.; Chalabaev, A.; Sander, D.; Ntoumanis, N.; et al. Relationships between changes in self-reported physical activity, sedentary behaviour and health during the coronavirus (COVID-19) pandemic in France and Switzerland. J. Sports Sci. 2020, 39, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, A.M.; Deforth, M.; Sjøgaard, G.; Johnston, V.; Volken, T.; Luomajoki, H.; Dratva, J.; Dressel, H.; Distler, O.; Melloh, M.; et al. No Evidence for a Decrease in Physical Activity Among Swiss Office Workers During COVID-19: A Longitudinal Study. Front. Psychol. 2021, 12, 620307. [Google Scholar] [CrossRef]
- Asplund, J. Seasonal Variation of HbA1c in Adult Diabetic Patients. Diabetes Care 1997, 20, 234. [Google Scholar] [CrossRef] [PubMed]
- Ferrie, C.D.; Sharpe, T.C.; Price, D.A.; Surtees, R.A. Seasonal variation of glycosylated haemoglobin. Arch. Dis. Child. 1987, 62, 959–960. [Google Scholar] [CrossRef] [Green Version]
- Gikas, A.; Sotiropoulos, A.; Pastromas, V.; Papazafiropoulou, A.; Apostolou, O.; Pappas, S. Seasonal variation in fasting glucose and HbA1c in patients with type 2 diabetes. Prim. Care Diabetes 2009, 3, 111–114. [Google Scholar] [CrossRef]
- Gómez-Huelgas, R.; Santamaria-Fernandez, S.; Guijarro-Merino, R.; Navajas-Luque, F.; Tinahones, F.J.; Román-Terán, C.M.S.; Picon, M.J.; Bernal-López, M.R. Seasonal variability of glycated hemoglobin in a diabetic population from southern Europe. J. Diabetes Complicat. 2013, 27, 618–620. [Google Scholar] [CrossRef]
- Ishii, H.; Suzuki, H.; Baba, T.; Nakamura, K.; Watanabe, T. Seasonal variation of glycemic control in type 2 diabetic patients. Diabetes Care 2001, 24, 1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.W. Seasonal Changes in Preprandial Glucose, A1C, and Blood Pressure in Diabetic Patients. Diabetes Care 2007, 30, 2501–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, G.A.; Edwards, O.M. Seasonal variation in glycated haemoglobin in diabetics. Ann. Clin. Biochem. Int. J. Lab. Med. 2001, 38, 59–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, M.T.R.E.P.; Lira, D.; Bacelar, C.; Oliveira, J.C.; de Carvalho, A.F.C. Seasonal variation of haemoglobin A1c in a Portuguese adult population. Arch. Endocrinol. Metab. 2015, 59, 231–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.-L.; Brimacombe, M.; Xie, M.; Rajan, M.; Wang, H.; Kolassa, J.; Crystal, S.; Chen, T.-C.; Pogach, L.; Safford, M. Seasonal Patterns in Monthly Hemoglobin A1c Values. Am. J. Epidemiol. 2005, 161, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raphael, A.; Friger, M.; Biderman, A. Seasonal variations in HbA1c among type 2 diabetes patients on a semi-arid climate between the years 2005–2015. Prim. Care Diabetes 2020, 15, 502–506. [Google Scholar] [CrossRef]
| Total N (%) | Diabetes Type 2 | Diabetes Type 1 | |
|---|---|---|---|
| N total | 1078 * | 925 | 145 |
| Anthropometric parameters | |||
| Age (years) at first HbA1c measurement | 59.05 (15.55) | 61.36 (14.07) | 45.19 (16.85) |
| Male gender n (%) | 688 (63.8) | 600 (64.9) | 82 (56.6) |
| Weight (kg), n = 589 | 85.90 (21.15) | 89.68 (21.16) | 71.81 (13.84) |
| BMI (kg/m2), n = 587 | 29.56 (6.63) | 30.95 (6.49) | 24.34 (3.63) |
| BP systolic (mm/Hg), n = 408 | 132.97 (18.00) | 133.49 (18.42) | 131.04 (15.87) |
| BP diastolic (mm Hg) n = 406 | 81.65 (10.59) | 82.23 (10.70) | 79.22 (9.62) |
| Comorbidities n (%) | n = 1032 | n = 893 | n = 131 |
| Hypertension | 734 (71.1) | 664 (74.4) | 67 (51.1) |
| Overweight | 187 (18.1) | 157 (17.6) | 28 (21.4) |
| Obesity | 393 (38.1) | 379 (42.4) | 13 (9.9) |
| Dyslipidaemia | 547 (53.0) | 500 (56.0) | 43 (32.8) |
| Obstructive sleep apnoea | 162 (15.7) | 157 (17.6) | 5 (3.8) |
| Coronary heart disease | 300 (29.1) | 281 (31.5) | 18 (13.7) |
| Chronic heart failure | 132 (12.8) | 132 (14.8) | 0 (0.0) |
| Stroke | 114 (11.0) | 101 (11.3) | 10 (7.6) |
| Periphery artery occlusive disease | 126 (12.2) | 114 (12.8) | 11 (8.4) |
| Chronic nephropathy | 735 (71.2) | 660 (73.9) | 68 (51.9) |
| Diabetic periphery neuropathy | 360 (34.9) | 289 (32.4) | 69 (52.7) |
| Diabetic retinopathy | 247 (23.9) | 189 (21.2) | 58 (44.3) |
| Gout | 86 (8.3) | 79 (8.8) | 7 (5.3) |
| Thyroid disease | 147 (14.2) | 125 (14.0) | 21 (16.0) |
| Depression | 125 (12.1) | 114 (12.8) | 11 (8.4) |
| Oncological disease | 238 (23.1) | 224 (25.1) | 12 (9.2) |
| COPD and asthma | 173 (16.8) | 164 (18.4) | 8 (6.1) |
| Smoking | 361 (35.0) | 318 (35.6) | 41 (31.3) |
| Inappropriate alcohol use | 289 (28.0) | 257 (28.8) | 29 (22.1) |
| Antidiabetic medication | n = 757 | n = 621 | n = 128 |
| Metformin | 406 (53.6) | 402 (64.7) | 4 (3.1) |
| SGLT2-Hemmer | 68 (9.0) | 68 (11.0) | 0 (0.0) |
| DPP-4-Inhibitoren | 221 (29.2) | 219 (35.3) | 0 (0.0) |
| Sulfonylureas | 41 (5.4) | 41 (6.6) | 0 (0.0) |
| other OAD | 11 (1.5) | 10 (1.6) | 0 (0.0) |
| At least one OAD | 484 (63.9) | 478 (77.0) | 4 (3.1) |
| 2 OAD preparations | 166 (21.9) | 165 (26.6) | 0 (0.0) |
| 3+ OAD preparations | 46 (6.1) | 46 (7.4) | 0 (0.0) |
| At least one Insulin | 486 (64.2) | 351 (56.5) | 128 (100) |
| Insulin with OAD | 250 (33.0) | 245 (39.5) | 4 (3.1) |
| At least one GLP-1 agonist | 226 (29.9) | 223 (35.9) | 2 (1.6) |
| GLP-1 agonist with any OAD | 150 (19.8) | 150 (24.2) | 0 (0.0) |
| Insulin + GLP-1 agonist | 138 (18.2) | 135 (21.7) | 2 (1.6) |
| OAD only | 183 (24.2) | 182 (29.1) | 0 (0.0) |
| Insulin only | 197 (26.0) | 70 (11.3) | 122 (95.3) |
| GLP-1 only | 37 (4.9) | 37 (6.0) | 0 (0.0) |
| Laboratory parameters mean (SD) | |||
| HbA1c (mmol/mol) | 55.63 (17.83) | 54.10 (17.39) | 64.81 (18.05) |
| (%) | 7.24 (1.63) | 7.10 (1.59) | 8.08 (1.65) |
| LDL (mmol/L) n = 766 | 2.05 (0.94) | 2.06 (0.95) | 2.03 (0.81) |
| GFR (mL/min) n = 1033 | 75.30 (30.93) | 72.10 (29.91) | 95.93 (28.57) |
| Total n (%) | Diabetes Type 2 | Diabetes Type 1 | |
|---|---|---|---|
| n total | 1078 * | 925 | 145 |
| Employment n = 888 † | |||
| High (professional and intermediate occupations) | 58 (6.5) | 46 (5.9) | 12 (11.5) |
| Medium (non-manual occupations | 71 (8.0) | 53 (6.8) | 17 (16.3) |
| Low (manual or lower occupations) | 370 (41.7) | 318 (40.9) | 49 (47.1) |
| Pensioner (including disability pension) | 337 (38.0) | 321 (41.3) | 16 (15.4) |
| Other | 52 (5.9) | 40 (5.1) | 10 (9.6) |
| Language | |||
| German | 936 (86.8) | 791 (85.5) | 138 (95.2) |
| Italian | 36 (3.3) | 34 (3.7) | 2 (1.4) |
| French | 23 (2.1) | 23 (2.5) | 0 (0.0) |
| Other | 83 (7.7) | 77 (8.3) | 5 (3.4) |
| Religion n = 623 | |||
| Catholic | 193 (31.0) | 160 (29.7) | 33 (40.7) |
| Protestant | 152 (24.4) | 126 (23.4) | 25 (30.9) |
| Moslem | 118 (18.9) | 113 (21.0) | 4 (4.9) |
| Other | 160 (25.7) | 140 (26.0) | 19 (23.5) |
| Insurance class | |||
| General | 1054 (97.8) | 901 (97.4) | 145 (100.0) |
| Private/half private | 24 (2.2) | 24 (2.6) | 0 (0.0) |
| Residence n = 1018 | |||
| Urban | 890 (87.4) | 770 (88.3) | 113 (81.9) |
| Rural | 54 (5.3) | 41 (4.7) | 13 (9.4) |
| Intermediate | 74 (7.3) | 61 (7.0) | 12 (8.7) |
| General practitioner | |||
| yes (%) | 875 (81.2) | 743 (80.3) | 124 (85.5) |
| Type of Diabetes | Spring–Winter 2018–2019 | Spring–Winter 2019–2020 | p |
|---|---|---|---|
| Absolute variations in HbA1c values | |||
| Type 2 | |||
| HbA1c% (mean (SD) | −0.13 (0.81) | −0.06 (1.25) | 0.350 |
| HbA1c mmol/mol (mean (SD) | −1.42 (8.85) | −0.66 (13.65) | |
| Type 1 | |||
| HbA1c% (mean (SD) | −0.27(0.70) | −0.25 (0.99) | 0.880 |
| HbA1c mmol/mol (mean (SD) | −2.95 (7.66) | −2.73 (10.83) | |
| Patients improved (%): relative HbA1c variation, delta < −5% | |||
| Type 2 | 30.3% | 29.0%, | 0.81 |
| Type 1 | 33.3% | 36.6% | 0.84 |
| Patient worsened (%): relative HbA1c variations, delta > 5% | |||
| Type 2 | 22.9% | 32.0% | 0.02 |
| Type 1 | 9.8% | 16.1% | 0.42 |
| Univariable Analysis | Multivariable Analysis N Patients = 305 | |||
|---|---|---|---|---|
| Patient Characteristic | Estimates [95% CI] | p-Value | Estimates (95% CI) | p-Value |
| Anthropometric parameters | ||||
| Age (years) at baseline | 0.0004 (−0.0004, 0.001) | 0.327 | ||
| Type of Diabetes = DM 2 | 0.03 (0.002, 0.07) | 0.039 | ||
| Gender = W | −0.01 (−0.03, 0.01) | 0.408 | ||
| Weight (kg) | 0.0004 (−0.0004, 0.001) | 0.309 | ||
| BMI (kg/m2) | 0.00116 (−0.001, 0.0035) | 0.336 | ||
| Blood pressure (mm/Hg) | ||||
| RR systolic | 0.000001 (−0.0009, 0.0009) | 0.998 | ||
| RR diastolic | −0.0015 (−0.0032, 0.00016) | 0.076 | ||
| Trend in the previous period (ref decreasing < 5%) | ||||
| Increasing > 5% | −0.073 (−0.099, −0.0474) | <0.001 | −0.08 (−0.11, −0.05) | <0.001 |
| Stable or dec.inc ≤ 5% | −0.004 (−0.041, 0.032) | 0.812 | −0.02 (−0.06, 0.02) | 0.414 |
| Comorbidities (ref = No) | ||||
| Hypertension | 0.00245 (−0.106, 0.111) | 0.965 | ||
| Overweight | −0.0403 (−0.0698, −0.0107) | 0.008 | ||
| Obesity | 0.00505 (−0.0199, 0.03) | 0.691 | ||
| Hyperlipidemia | 0.00668 (−0.0175, 0.0309) | 0.588 | ||
| Obstructive sleep apnea | −0.0137 (−0.0458, 0.0185) | 0.405 | ||
| Coronary heart disease | 0.00176 (−0.0243, 0.0278) | 0.894 | ||
| Chronic heart failure | 0.0167 (−0.021, 0.0544) | 0.386 | ||
| Stroke | 0.0349 (−0.00352, 0.0733) | 0.075 | ||
| Periphery artery occlusive disease | −0.0218 (−0.0578, 0.0143) | 0.236 | ||
| Chronic nephropathy | 0.0155 (−0.0112, 0.0422) | 0.254 | ||
| Periphery neuropathy | −0.00936 (−0.0339, 0.0152) | 0.454 | ||
| Diabetic retinopathy | −0.00628 (−0.0322, 0.0196) | 0.634 | ||
| Gout | 0.0229 (−0.023, 0.0687) | 0.328 | ||
| Hypothyroidism | 0.0236 (−0.0104, 0.0575) | 0.173 | 0.04 (0.01, 0.08) | 0.012 |
| Depression | −0.00737 (−0.0435, 0.0287) | 0.688 | ||
| Oncological disease | 0.0493 (0.0208, 0.0778) | 0.001 | ||
| COPD and asthma | 0.0236 (−0.00745, 0.0546) | 0.136 | ||
| Smoking | −0.00496 (−0.0298, 0.0198) | 0.694 | ||
| Inappropriate alcohol use | 0.022 (−0.00531, 0.0493) | 0.114 | 0.03 (0.00, 0.06) | 0.043 |
| Antidiabetic Medication | ||||
| OAD group | 0.012 (−0.0154, 0.0394) | 0.389 | ||
| Metformin | −0.00757 (−0.0341, 0.019) | 0.576 | ||
| SGLT2-Inhibitors | 0.0236 (−0.0207, 0.0679) | 0.295 | ||
| DPP-4- Inhibitors | 0.0375 (0.00831, 0.0666) | 0.012 | 0.03 (0.00, 0.06) | 0.030 |
| Sulfonylurea | −0.0486 (−0.104, 0.00705) | 0.087 | −0.06 (−0.14,0.01) | 0.095 |
| Other OAD | −0.056 (−0.169, 0.0573) | 0.332 | ||
| Insulin group | −0.0162 (−0.0446, 0.0121) | 0.261 | ||
| Insulin with OAD | 0.00464 (−0.0233, 0.0326) | 0.744 | ||
| GLP-1 agonist group | 0.0298 (0.000861, 0.0587) | 0.044 | ||
| GLP-1 agonist with OAD | 0.0246 (−0.008, 0.0572) | 0.139 | 0.04 (0.01,0.07) | 0.007 |
| Insulin + GLP-1 agonist | 0.0197 (−0.0138, 0.0532) | 0.248 | ||
| OAD only | 0.00245 (−0.0296, 0.0345) | 0.881 | ||
| Insulin only | −0.0244 (−0.0537, 0.0049) | 0.102 | ||
| GLP-1 only | 0.0567 (−0.018, 0.131) | 0.137 | ||
| Laboratory parameters | ||||
| LDL (mmol/L) | 0.0151 (0.00126, 0.0289) | 0.032 | ||
| Triglycerides (mmol/L) | −0.0139 (−0.039, 0.0112) | 0.267 | ||
| GFR (mL/min) | −0.000227 (−0.0006, 0.00014) | 0.226 | ||
| Albumin/creatinine ratio in urine | −0.00010 (−0.00026, 0.00006) | 0.22 | ||
| Socioeconomic characteristics | ||||
| Employment (ref = high occupation) | ||||
| Medium (non-manual occupations | 0.00774 (−0.0572, 0.0727) | 0.815 | ||
| Low (manual or lower occupations) | 0.0314 (−0.0175, 0.0803) | 0.207 | ||
| Pensioner (including invalidity-pension) | 0.0309 (−0.0184, 0.0803) | 0.218 | ||
| Other | −0.00955 (−0.0818, 0.0627) | 0.795 | ||
| Language (ref = DE) | ||||
| IT | −0.0267 (−0.093, 0.0395) | 0.428 | ||
| FR | 0.00956 (−0.0603, 0.0794) | 0.788 | ||
| Other | 0.0418 (0.0018, 0.0817) | 0.041 | ||
| Religion (ref = catholic) | ||||
| Evangelic | 0.0042 (−0.0413, 0.0497) | 0.856 | ||
| Muslim | −0.00657 (−0.0508, 0.0377) | 0.771 | ||
| Other | 0.0122 (−0.0305, 0.0549) | 0.574 | ||
| Insurance class | ||||
| Private/half private | 0.0596 (−0.0171, 0.136) | 0.127 | ||
| Residence (ref = Urban) | ||||
| Intermediate | −0.013058 (−0.064, 0.03825) | 0.617 | ||
| Rural | 0.008459 (−0.047, 0.06390) | 0.764 | ||
| Outpatient consultations | 0.00483 (0.0011, 0.00855) | 0.011 | ||
| Hospitalizations | 0.00686 (0.00139, 0.0123) | 0.014 | ||
| General practitioner | ||||
| No (ref = yes) | −0.012 (−0.0421, 0.018) | 0.432 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zechmann, S.; Hotz, L.; Di Gangi, S.; Baumgartl, K.; Plate, A.; Potlukova, E. Impact of SARS-CoV-2 Lockdown on Glycaemic Control: A Retrospective Observational Cohort Study in a Tertiary Setting. J. Clin. Med. 2021, 10, 4098. https://doi.org/10.3390/jcm10184098
Zechmann S, Hotz L, Di Gangi S, Baumgartl K, Plate A, Potlukova E. Impact of SARS-CoV-2 Lockdown on Glycaemic Control: A Retrospective Observational Cohort Study in a Tertiary Setting. Journal of Clinical Medicine. 2021; 10(18):4098. https://doi.org/10.3390/jcm10184098
Chicago/Turabian StyleZechmann, Stefan, Leana Hotz, Stefania Di Gangi, Klaus Baumgartl, Andreas Plate, and Eliska Potlukova. 2021. "Impact of SARS-CoV-2 Lockdown on Glycaemic Control: A Retrospective Observational Cohort Study in a Tertiary Setting" Journal of Clinical Medicine 10, no. 18: 4098. https://doi.org/10.3390/jcm10184098
APA StyleZechmann, S., Hotz, L., Di Gangi, S., Baumgartl, K., Plate, A., & Potlukova, E. (2021). Impact of SARS-CoV-2 Lockdown on Glycaemic Control: A Retrospective Observational Cohort Study in a Tertiary Setting. Journal of Clinical Medicine, 10(18), 4098. https://doi.org/10.3390/jcm10184098







