Outcomes in Critically Ill Patients Sedated with Intravenous Lormetazepam or Midazolam: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Data Sources
2.3. Patient Selection and Inclusion Criteria
2.4. Patient Grouping and Sedation Practice
2.5. Primary and Secondary Outcome Variables
2.6. Data Analysis and Statistics
3. Results
3.1. Patient Characteristics
3.2. Sedation Characteristics
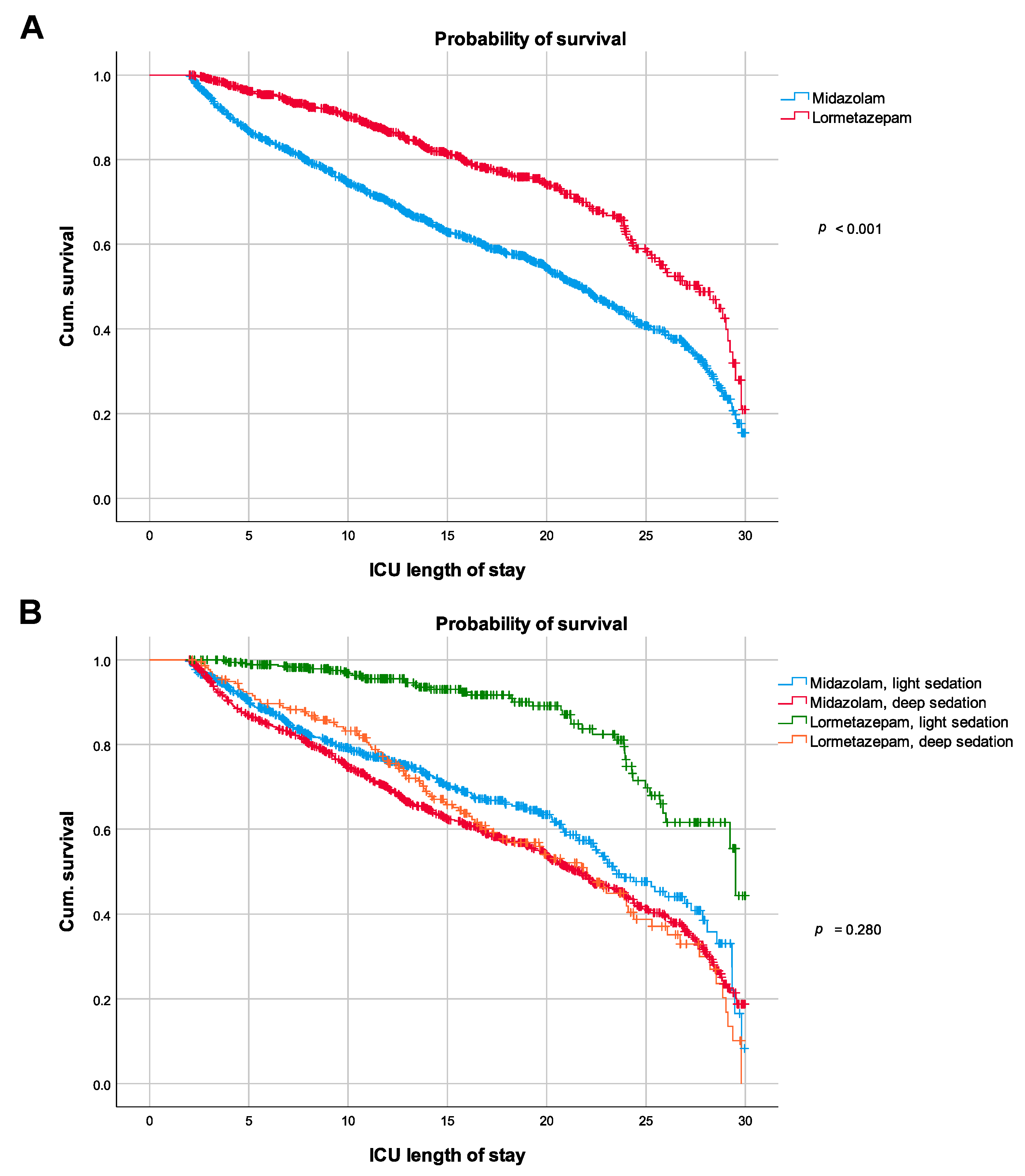
3.3. Primary Outcome Measure
3.4. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devlin, J.W.; Skrobik, Y.; Gelinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef] [Green Version]
- DAS-Taskforce; Baron, R.; Binder, A.; Biniek, R.; Braune, S.; Buerkle, H.; Dall, P.; Demirakca, S.; Eckardt, R.; Eggers, V.; et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015)—Short version. GMS Ger. Med. Sci. 2015, 13, Doc19. [Google Scholar] [CrossRef]
- Kollef, M.H.; Levy, N.T.; Ahrens, T.S.; Schaiff, R.; Prentice, D.; Sherman, G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest 1998, 114, 541–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzer, F.; Weiss, B.; Kumpf, O.; Treskatsch, S.; Spies, C.; Wernecke, K.D.; Krannich, A.; Kastrup, M. Early deep sedation is associated with decreased in-hospital and two-year follow-up survival. Crit. Care 2015, 19, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehabi, Y.; Chan, L.; Kadiman, S.; Alias, A.; Ismail, W.N.; Tan, M.A.; Khoo, T.M.; Ali, S.B.; Saman, M.A.; Shaltut, A.; et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: A prospective longitudinal multicentre cohort study. Intensive Care Med. 2013, 39, 910–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehabi, Y.; Bellomo, R.; Reade, M.C.; Bailey, M.; Bass, F.; Howe, B.; McArthur, C.; Seppelt, I.M.; Webb, S.; Weisbrodt, L.; et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am. J. Respir. Crit. Care Med. 2012, 186, 724–731. [Google Scholar] [CrossRef]
- Shehabi, Y.; Bellomo, R.; Kadiman, S.; Ti, L.K.; Howe, B.; Reade, M.C.; Khoo, T.M.; Alias, A.; Wong, Y.L.; Mukhopadhyay, A.; et al. Sedation intensity in the first 48 hours of mechanical ventilation and 180-day mortality: A multinational prospective longitudinal cohort study. Crit. Care Med. 2018, 46, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.P.; Pun, B.T.; Herr, D.L.; Maze, M.; Girard, T.D.; Miller, R.R.; Shintani, A.K.; Thompson, J.L.; Jackson, J.C.; Deppen, S.A.; et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA 2007, 298, 2644–2653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.; Franck, M.; Fischer, M.; Spies, C. Sedation and analgesia in German intensive care units: How is it done in reality? Results of a patient-based survey of analgesia and sedation. Intensive Care Med. 2006, 32, 1137–1142. [Google Scholar] [CrossRef]
- Payen, J.F.; Chanques, G.; Mantz, J.; Hercule, C.; Auriant, I.; Leguillou, J.L.; Binhas, M.; Genty, C.; Rolland, C.; Bosson, J.L. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: A prospective multicenter patient-based study. Anesthesiology 2007, 106, 687–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, T.M.; Ritz, R.; Haberthur, C.; Ha, H.R.; Hunkeler, W.; Sleight, A.J.; Scollo-Lavizzari, G.; Haefeli, W.E. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet 1995, 346, 145–147. [Google Scholar] [CrossRef]
- Balk, M.; Hentschke, H.; Rudolph, U.; Antkowiak, B.; Drexler, B. Differential depression of neuronal network activity by midazolam and its main metabolite 1-hydroxymidazolam in cultured neocortical slices. Sci. Rep. 2017, 7, 3503. [Google Scholar] [CrossRef] [Green Version]
- Carson, S.S.; Kress, J.P.; Rodgers, J.E.; Vinayak, A.; Campbell-Bright, S.; Levitt, J.; Bourdet, S.; Ivanova, A.; Henderson, A.G.; Pohlman, A.; et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit. Care Med. 2006, 34, 1326–1332. [Google Scholar] [CrossRef] [Green Version]
- Swart, E.L.; Zuideveld, K.P.; de Jongh, J.; Danhof, M.; Thijs, L.G.; Strack van Schijndel, R.M. Comparative population pharmacokinetics of lorazepam and midazolam during long-term continuous infusion in critically ill patients. Br. J. Clin. Pharmacol. 2004, 57, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Horowski, R. Dependence liability of lormetazepam: Are all benzodiazepines equal? The case of the new i.v. lormetazepam for anesthetic procedures. J. Neural Transm. 2020, 127, 1107–1115. [Google Scholar] [CrossRef]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef] [PubMed]
- Jakob, S.M.; Ruokonen, E.; Grounds, R.M.; Sarapohja, T.; Garratt, C.; Pocock, S.J.; Bratty, J.R.; Takala, J. For the Dexmedetomidine for Long-Term Sedation Investigators. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA 2012, 307, 1151–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandharipande, P.; Shintani, A.; Peterson, J.; Pun, B.T.; Wilkinson, G.R.; Dittus, R.S.; Bernard, G.R.; Ely, E.W. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 2006, 104, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Spies, C.; Shehabi, Y. Ten tips for ICU sedation. Intensive Care Med. 2018, 44, 1141–1143. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Shehabi, Y.; Walsh, T.S.; Pandharipande, P.P.; Ball, J.A.; Spronk, P.; Longrois, D.; Strøm, T.; Conti, G.; Funk, G.-C.; et al. Comfort and patient-centred care without excessive sedation: The eCASH concept. Intensive Care Med. 2016, 42, 962–971. [Google Scholar] [CrossRef] [Green Version]
- Young, C.C.; Prielipp, R.C. Benzodiazepines in the intensive care unit. Crit. Care Clin. 2001, 17, 843–862. [Google Scholar] [CrossRef]
- Simon, B.T.; Scallan, E.M.; Odette, O.; Ebner, L.S.; Cerullo, M.N.; Follette, C.; Cox, S.K.; Doherty, T.J.; Lizarraga, I. Pharmacokinetics and pharmacodynamics of midazolam following intravenous and intramuscular administration to sheep. Am. J. Vet. Res. 2017, 78, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.D.; Rodarte, A.; Blumer, J.L.; Khoo, K.C.; Akbari, B.; Pou, S.; Kearns, G.L. The single-dose pharmacokinetics of midazolam and its primary metabolite in pediatric patients after oral and intravenous administration. J. Clin. Pharmacol. 2001, 41, 1359–1369. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristic | Lormetazepam (n = 1208) | Midazolam (n = 2106) | p |
|---|---|---|---|
| Age, years a | 61.2 (16.1) | 61.6 (15.9) | 0.49 |
| Female sex, n (%) | 406 (34%) | 700 (33%) | 0.83 |
| BMI, kg/m2 a | 27.0 (7.1) | 27.3 (6.6) | 0.41 |
| APACHE II a | 19.8 (9.6) | 23.6 (9.8) | <0.001 |
| Charlson’s comorbidity index a | 5.6 (3.4) | 5.8 (3.1) | 0.3 |
| Preexisting delirium, n (%) | 903 (75%) | 765 (36%) | <0.001 |
| Reason of admission, n (%) | |||
| Elective surgery | 335 (28%) | 365 (17%) | <0.001 |
| Emergency surgery | 294 (24%) | 479 (23%) | |
| Medical | 465 (38%) | 933 (44%) | |
| No data | 114 (9%) | 329 (16%) |
| Characteristic | Lormetazepam (n = 1208) | Midazolam (n = 2106) | p |
|---|---|---|---|
| Sedation index for the first 48 h after ICU admission a | 1.7 (1.5) | 4.10 (1.0) | <0.001 |
| Sedation index ≥ 1.5 in first 48 h, n (% of non-missing) | 355 (40.4%) d | 1506 (95.9%) d | <0.001 |
| Sedation index < 1.5 in first 48 h, n (% of non-missing) | 523 (59.6%) d | 64 (4.1%) d | |
| RASS b | 0 (−1; 0.5) | −4 (−5; −3) | <0.001 |
| Total rate of delirium, n (%) | 837 (69%) | 677 (32%) | <0.001 |
| Any bolus administration c, n (%) | 1010 (84%) | 518 (25%) | <0.001 |
| Any continuous administration c, n (%) | 433 (36%) | 1873 (89%) | <0.001 |
| Sedation Index by Sedative * | |||||
|---|---|---|---|---|---|
| Midazolam | Lormetazepam | ||||
| n | Mean | n | Mean | ||
| Year | 2006 | 114 | 3.412 | 0 | |
| 2007 | 119 | 3.654 | 0 | ||
| 2008 | 173 | 3.799 | 3 | 0.889 | |
| 2009 | 227 | 4.027 | 10 | 0.542 | |
| 2010 | 290 | 4.221 | 6 | 0.125 | |
| 2011 | 285 | 4.190 | 19 | 0.591 | |
| 2012 | 265 | 4.279 | 44 | 0.668 | |
| 2013 | 158 | 4.292 | 60 | 1.458 | |
| 2014 | 145 | 4.077 | 103 | 1.878 | |
| 2015 | 92 | 3.884 | 190 | 1.624 | |
| 2016 | 66 | 4.374 | 278 | 1.491 | |
| 2017 | 61 | 4.443 | 264 | 1.863 | |
| 2018 | 111 | 4.067 | 231 | 1.848 | |
| Variable | Lormetazepam (n = 1208) | Midazolam (n = 2106) | p |
|---|---|---|---|
| Hospital mortality, n (%) | 276 (23%) | 883 (42%) | <0.001 |
| Duration of mechanical ventilation, hours a | 520.7 (712.1) | 606.9 (633.9) | 0.004 |
| ICU length of stay, days a | 24 (23.1) | 31.5 (28.7) | <0.001 |
| Hospital length of stay, days a | 40.6 (36.8) | 49.7 (43.1) | <0.001 |
| Variable | Odds Ratio (95% Confidence Interval) for In-Hospital Mortality | p |
|---|---|---|
| Use of midazolam, yes/no | 2.04 (1.71–2.45) | <0.001 |
| APACHE II | 1.03 (1.02–1.04) | <0.001 |
| Age, years | 1.01 (1.01–1.02) | <0.001 |
| Gender, female | 1.15 (0.97–1.37) | 0.11 |
| Cause of admission, medical | 1.23 (0.99–1.53) | 0.06 |
| Cause of admission, emergency surgery | 0.89 (0.69–1.14) | 0.35 |
| Variable | Hazard Ratio (95% Confidence Interval) for In-Hospital Mortality | p |
|---|---|---|
| Model 1 | ||
| Use of midazolam, yes/no | 1.75 (1.46–2.09) | <0.001 |
| Age, years | 1.01 (1.01–1.02) | <0.001 |
| Gender, female | 1.07 (0.91–1.25) | 0.43 |
| APACHE II | 1.03 (1.02–1.04) | <0.001 |
| Emergency surgery, yes/no | 0.77 (0.61–0.98) | 0.04 |
| Model 2 | ||
| Use of midazolam, yes/no | 1.04 (0.83–1.31) | 0.97 |
| Sedation index ≥ 1.5 in the first 48 h, yes/no | 3.14 (2.23–4.43) | <0.001 |
| Age, years | 1.01 (1.01–1.02) | <0.001 |
| Gender, female | 1.04 (0.87–1.26) | 0.46 |
| APACHE II | 1.03 (1.02–1.04) | <0.001 |
| Emergency surgery, yes/no | 0.72 (0.54–0.94) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, B.; Hilfrich, D.; Vorderwülbecke, G.; Heinrich, M.; Grunow, J.J.; Paul, N.; Kruppa, J.; Neuner, B.; Drexler, B.; Balzer, F.; et al. Outcomes in Critically Ill Patients Sedated with Intravenous Lormetazepam or Midazolam: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 4091. https://doi.org/10.3390/jcm10184091
Weiss B, Hilfrich D, Vorderwülbecke G, Heinrich M, Grunow JJ, Paul N, Kruppa J, Neuner B, Drexler B, Balzer F, et al. Outcomes in Critically Ill Patients Sedated with Intravenous Lormetazepam or Midazolam: A Retrospective Cohort Study. Journal of Clinical Medicine. 2021; 10(18):4091. https://doi.org/10.3390/jcm10184091
Chicago/Turabian StyleWeiss, Björn, David Hilfrich, Gerald Vorderwülbecke, Maria Heinrich, Julius J. Grunow, Nicolas Paul, Jochen Kruppa, Bruno Neuner, Berthold Drexler, Felix Balzer, and et al. 2021. "Outcomes in Critically Ill Patients Sedated with Intravenous Lormetazepam or Midazolam: A Retrospective Cohort Study" Journal of Clinical Medicine 10, no. 18: 4091. https://doi.org/10.3390/jcm10184091
APA StyleWeiss, B., Hilfrich, D., Vorderwülbecke, G., Heinrich, M., Grunow, J. J., Paul, N., Kruppa, J., Neuner, B., Drexler, B., Balzer, F., & Spies, C. D. (2021). Outcomes in Critically Ill Patients Sedated with Intravenous Lormetazepam or Midazolam: A Retrospective Cohort Study. Journal of Clinical Medicine, 10(18), 4091. https://doi.org/10.3390/jcm10184091






