Collagen XII Deficiency Increases the Risk of Anterior Cruciate Ligament Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
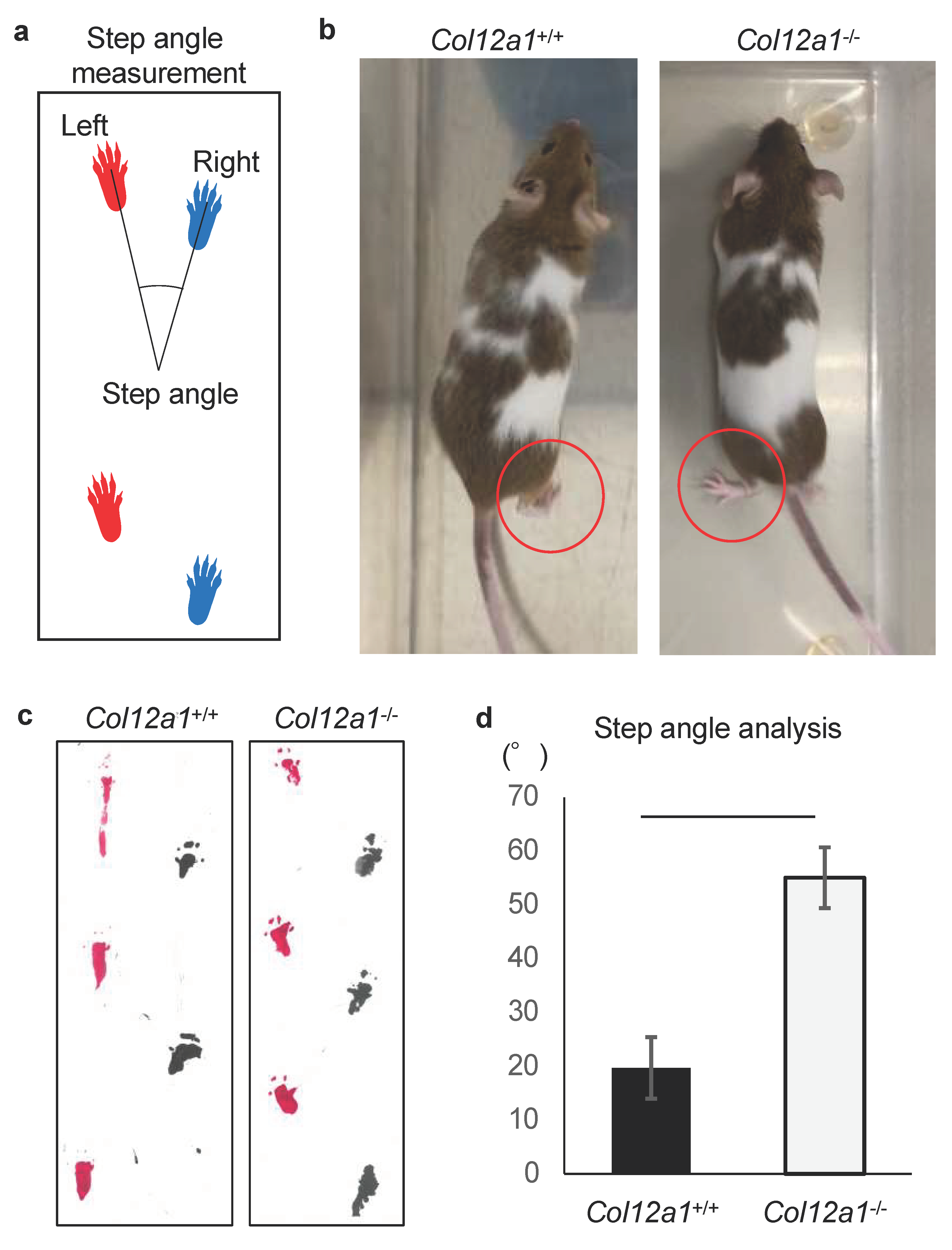
2.2. Gait Analysis
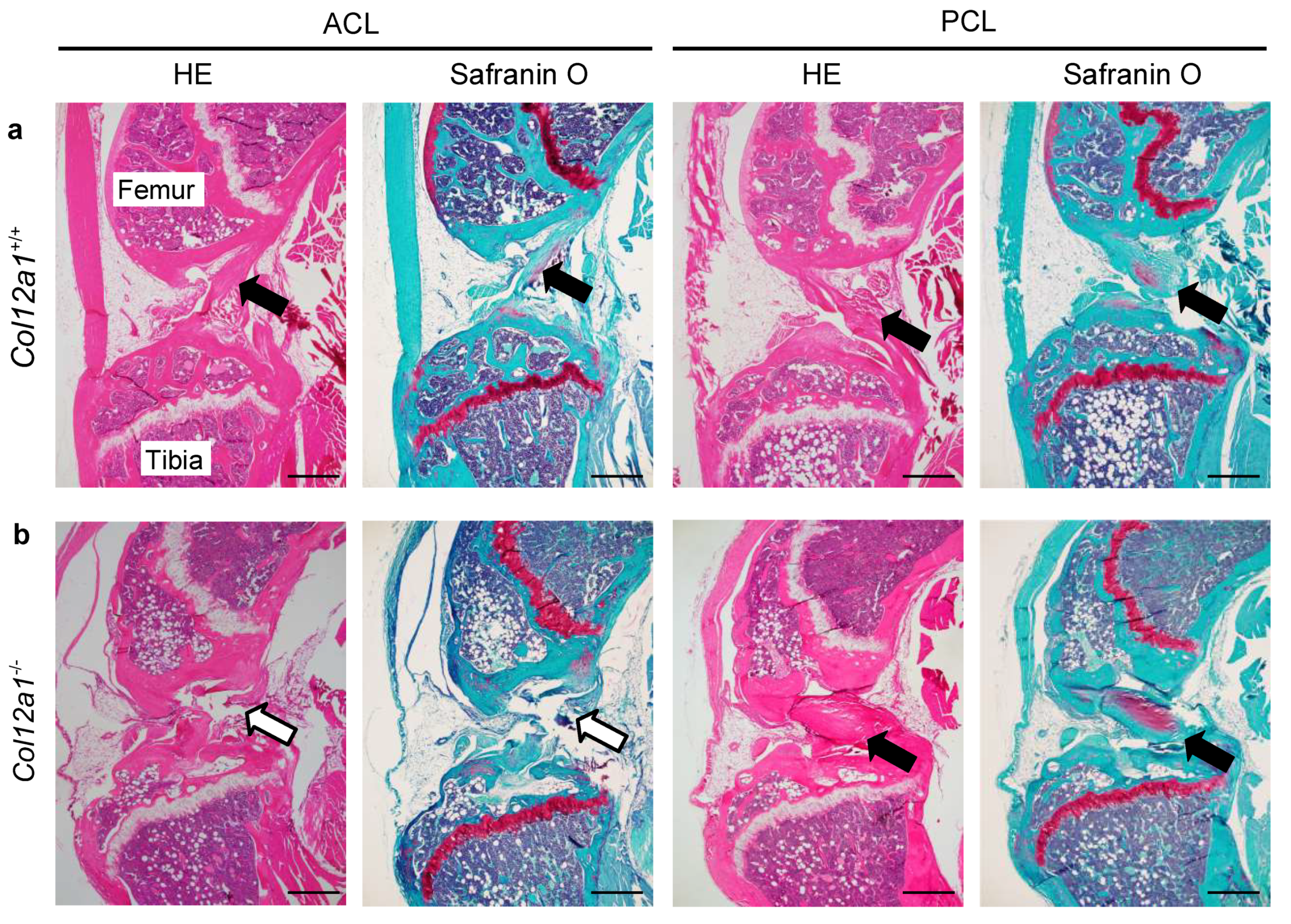
2.3. Histological Analysis
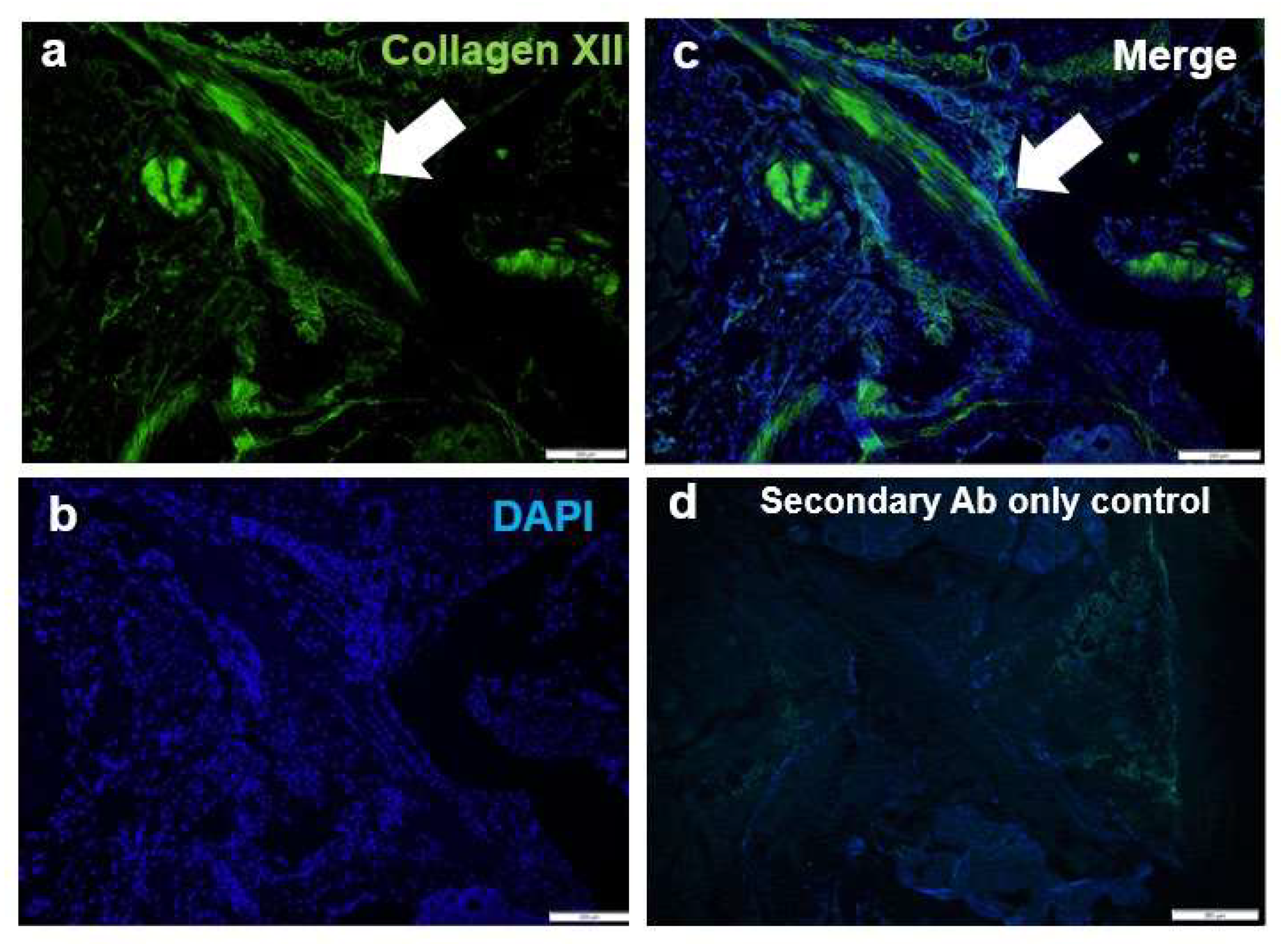
2.4. Immunofluorescence Analysis
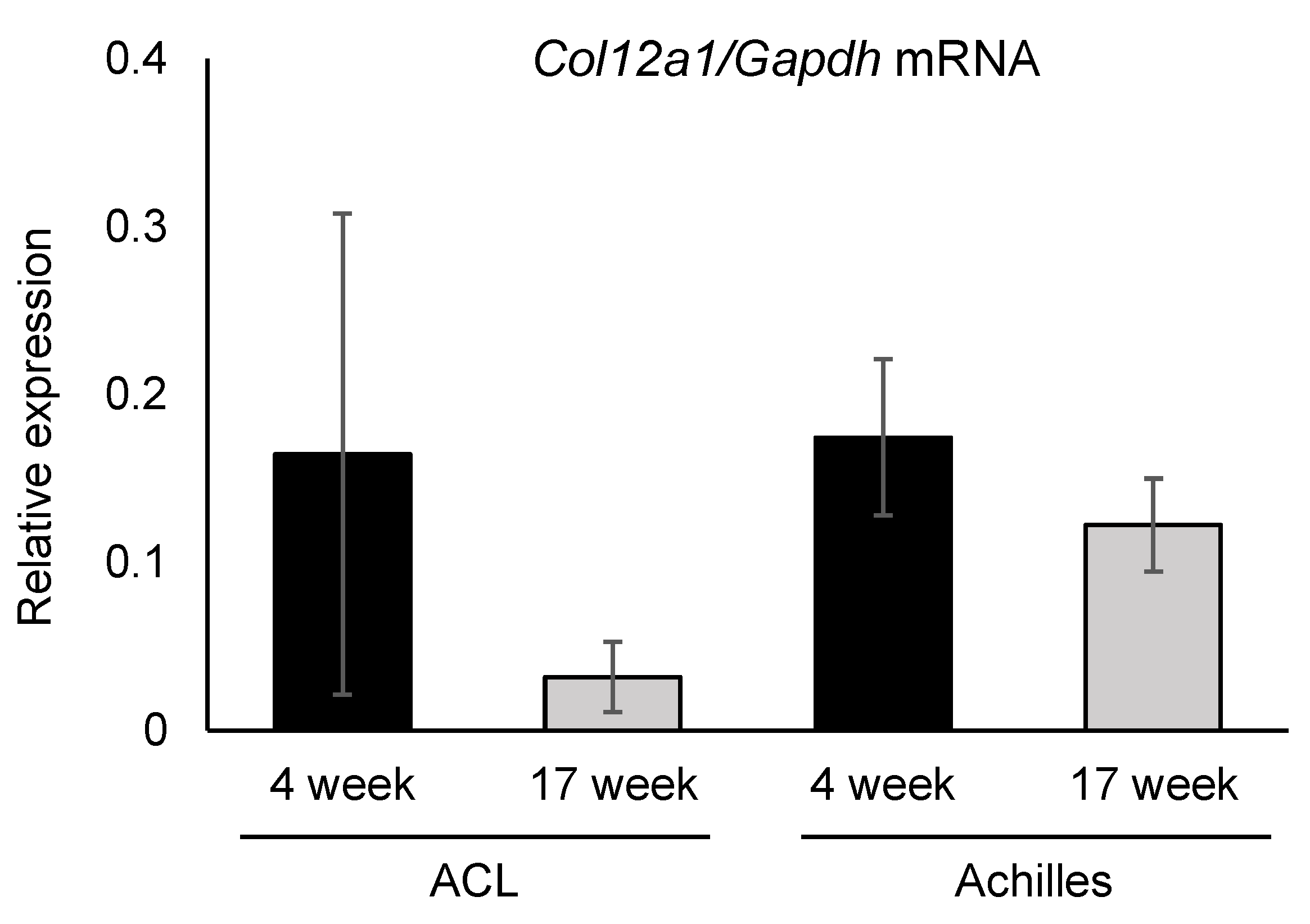
2.5. Real-Time RT-PCR Analysis
2.6. Statistical Analysis
3. Results
3.1. Col12a1 Deficient Mice Demonstrate Knee Deformity with an Abnormal Gait
3.2. Collagen XII Deficiency Causes ACL Discontinuity
3.3. Collagen XII Is a Component for the ACL
3.4. Collagen XII Expression Tends to Decrease in Knee Ligaments and the Achilles Tendon with Aging
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gobbi, A.; Francisco, R. Factors affecting return to sports after anterior cruciate ligament reconstruction with patellar tendon and hamstring graft: A prospective clinical investigation. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, R.J.; Rivera-Vega, A.; Miranda, G.; Micheo, W. Anterior cruciate ligament injury: Identification of risk factors and prevention strategies. Curr. Sports Med. Rep. 2014, 13, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Posthumus, M.; September, A.V.; O’Cuinneagain, D.; Van Der Merwe, W.; Schwellnus, M.P.; Collins, M. The association between the COL12A1 gene and anterior cruciate ligament ruptures. Br. J. Sports Med. 2010, 44, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.H.; Yoo, J.H.; Kim, K. Il bone contusion and associated meniscal and medial collateral ligament injury in patients with anterior cruciate ligament rupture. J. Bone Jt. Surg.-Ser. A 2011, 93, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Øiestad, B.E.; Engebretsen, L.; Storheim, K.; Risberg, M.A. Knee osteoarthritis after anterior cruciate ligament injury: A systematic review. Am. J. Sports Med. 2009, 37, 1434–1443. [Google Scholar] [CrossRef]
- Meuffels, D.E.; Favejee, M.M.; Vissers, M.M.; Heijboer, M.P.; Reijman, M.; Verhaar, J.A.N. Ten year follow-up study comparing conservative versus operative treatment of anterior cruciate ligament ruptures. A matched-pair analysis of high level athletes. Br. J. Sports Med. 2009, 43, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Brophy, R.H.; Zeltser, D.; Wright, R.W.; Flanigan, D. Anterior cruciate ligament reconstruction and concomitant articular cartilage injury: Incidence and treatment. Arthrosc.-J. Arthrosc. Relat. Surg. 2010, 26, 112–120. [Google Scholar] [CrossRef] [PubMed]
- John, R.; Dhillon, M.S.; Sharma, S.; Prabhakar, S.; Bhandari, M. Is there a genetic predisposition to anterior cruciate ligament tear? A systematic review. Am. J. Sports Med. 2016, 44, 3262–3269. [Google Scholar] [CrossRef]
- Kaynak, M.; Nijman, F.; van Meurs, J.; Reijman, M.; Meuffels, D.E. Genetic variants and anterior cruciate ligament rupture: A systematic review. Sport. Med. 2017, 47, 1637–1650. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Q.; Lu, Q.; Hong, C.; Luo, T.; Duan, Q.; Shu, S.; Lv, J.; Zhao, W. Correlations between the genetic variations in the COL1A1, COL5A1, COL12A1, and b-fibrinogen genes and anterior cruciate ligament injury in Chinese patients. J. Athl. Train. 2020, 55, 515–521. [Google Scholar] [CrossRef]
- John, R.; Prabhakar, S.; Dhillon, M.S.; Anand, A.; Minhas, G. Association of ACL tears and single nucleotide polymorphisms in the collagen 12 A1 gene in the Indian population—A preliminary case-control study. Muscles Ligaments Tendons J. 2016, 6, 253–257. [Google Scholar] [CrossRef]
- Ficek, K.; Stepien-Slodkowska, M.; Kaczmarczyk, M.; Maciejewska-Karlowska, A.; Sawczuk, M.; Cholewinski, J.; Leonska-Duniec, A.; Zarebska, A.; Cieszczyk, P.; Zmijewski, P. Does the A9285G polymorphism in collagen type XII α1 gene associate with the risk of anterior cruciate ligament ruptures? Balk. J. Med. Genet. 2014, 17, 41–46. [Google Scholar] [CrossRef]
- Chiquet, M.; Birk, D.E.; Bönnemann, C.G.; Koch, M. Collagen XII: Protecting bone and muscle integrity by organizing collagen fibrils. Int. J. Biochem. Cell Biol. 2014, 53, 51–54. [Google Scholar] [CrossRef]
- Izu, Y.; Sun, M.; Zwolanek, D.; Veit, G.; Williams, V.; Cha, B.; Jepsen, K.J.; Koch, M.; Birk, D.E. Type XII collagen regulates osteoblast polarity and communication during bone formation. J. Cell Biol. 2011, 193, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 26, 8–26. [Google Scholar] [CrossRef]
- Jakobsen, J.R.; Mackey, A.L.; Knudsen, A.B.; Koch, M.; Kjær, M.; Krogsgaard, M.R. Composition and adaptation of human myotendinous junction and neighboring muscle fibers to heavy resistance training. Scand. J. Med. Sci. Sport. 2017, 27, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zwolanek, D.; Izu, Y.; Gandhy, S.; Schreiber, G.; Brockmann, K.; Devoto, M.; Tian, Z.; Hu, Y.; Veit, G.; et al. Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice. Hum. Mol. Genet. 2014, 23, 2339–2352. [Google Scholar] [CrossRef] [PubMed]
- Hicks, D.; Farsani, G.T.; Laval, S.; Collins, J.; Sarkozy, A.; Martoni, E.; Shah, A.; Zou, Y.; Koch, M.; Bönnemann, C.G.; et al. Mutations in the collagen XII gene define a new form of extracellular matrix-related myopathy. Hum. Mol. Genet. 2014, 23, 2353–2363. [Google Scholar] [CrossRef]
- Izu, Y.; Adams, S.M.; Connizzo, B.K.; Beason, D.P.; Soslowsky, L.J.; Koch, M.; Birk, D.E. Collagen XII mediated cellular and extracellular mechanisms regulate establishment of tendon structure and function. Matrix Biol. 2021, 95, 52–67. [Google Scholar] [CrossRef]
- Akula, S.K.; McCullough, K.B.; Weichselbaum, C.; Dougherty, J.D.; Maloney, S.E. The trajectory of gait development in mice. Brain Behav. 2020, 10, e01636. [Google Scholar] [CrossRef]
- O’Connell, K.; Knight, H.; Ficek, K.; Leonska-Duniec, A.; Maciejewska-Karlowska, A.; Sawczuk, M.; Stepien-Slodkowska, M.; O’Cuinneagain, D.; van der Merwe, W.; Posthumus, M.; et al. Interactions between collagen gene variants and risk of anterior cruciate ligament rupture. Eur. J. Sport Sci. 2015, 15, 341–350. [Google Scholar] [CrossRef]
- Agarwal, P.; Zwolanek, D.; Keene, D.R.; Schulz, J.N.; Blumbach, K.; Heinegard, D.; Zaucke, F.; Paulsson, M.; Krieg, T.; Koch, M.; et al. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J. Biol. Chem. 2012, 287, 22549–22559. [Google Scholar] [CrossRef] [PubMed]
- Mannion, S.; Mtintsilana, A.; Posthumus, M.; Van Der Merwe, W.; Hobbs, H.; Collins, M.; September, A.V. Genes encoding proteoglycans are associated with the risk of anterior cruciate ligament ruptures. Br. J. Sports Med. 2014, 48, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, S.; Dhooge, T.; Syx, D.; Petit, F.; Goemans, N.; Destrée, A.; Vanakker, O.; De Rycke, R.; Symoens, S.; Malfait, F. Novel defects in collagen XII and VI expand the mixed myopathy/Ehlers-Danlos syndrome spectrum and lead to variant-specific alterations in the extracellular matrix. Genet. Med. 2019, 22, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ezura, Y.; Chervoneva, I.; Robinson, P.S.; Beason, D.P.; Carine, E.T.; Soslowsky, L.J.; Iozzo, R.V.; Birk, D.E. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell. Biochem. 2006, 98, 1436–1449. [Google Scholar] [CrossRef] [PubMed]
- Ezura, Y.; Chakravarti, S.; Oldberg, Å.; Chervoneva, I.; Birk, D.E. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 2000, 151, 779–787. [Google Scholar] [CrossRef]
- Juneja, S.C.; Veillette, C. Defects in tendon, ligament, and enthesis in response to genetic alterations in key proteoglycans and glycoproteins: A review. Arthritis 2013, 2013, 154812. [Google Scholar] [CrossRef]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef]
- Birk, D.E.; Bruckner, P. Collagens, suprastructures and collagen fibril assembly. In The Extracellular Matrix: An Overview; Springer: Cham, Switzerland, 2011; pp. 77–115. [Google Scholar]
- Smith, S.M.; Zhang, G.; Birk, D.E. Collagen V localizes to pericellular sites during tendon collagen fibrillogenesis. Matrix Biol. 2013, 33, 47–53. [Google Scholar] [CrossRef]
- Symoens, S.; Renard, M.; Bonod-Bidaud, C.; Syx, D.; Vaganay, E.; Malfait, F.; Ricard-Blum, S.; Kessler, E.; Van Laer, L.; Coucke, P.; et al. Identification of binding partners interacting with the α1-N-propeptide of type V collagen. Biochem. J. 2011, 433, 371–381. [Google Scholar] [CrossRef]
- Izu, Y.; Ezura, Y.; Koch, M.; Birk, D.E.; Noda, M. Collagens VI and XII form complexes mediating osteoblast interactions during osteogenesis. Cell Tissue Res. 2016, 364, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Trächslin, J.; Koch, M.; Chiquet, M. Rapid and reversible regulation of collagen XII expression by changes in tensile stress. Exp. Cell Res. 1999, 247, 320–328. [Google Scholar] [CrossRef]
- Fluck, M.; Giraud, M.N.; Tunc, V.; Chiquet, M. Tensile stress-dependent collagen XII and fibronectin production by fibroblasts requires separate pathways. Biochim. Biophys. Acta 2003, 593, 239–248. [Google Scholar] [CrossRef]
- Karimbux, N.Y.; Nishimura, I. Temporal and spatial expressions of type XII collagen in the remodeling periodontal ligament during experimental tooth movement. J. Dent. Res. 1995, 74, 313–318. [Google Scholar] [CrossRef]
- Marieswaran, M.; Jain, I.; Garg, B.; Sharma, V.; Kalyanasundaram, D. A review on biomechanics of anterior cruciate ligament and materials for reconstruction. Appl. Bionics Biomech. 2018, 2018, 4657824. [Google Scholar] [CrossRef] [PubMed]
| Young Stage (5-Week-Old) | Mature Stage (17–19-Week-Old) | |
|---|---|---|
| Col12a1+/+ | 0% (0/4) | 0% (0/8) |
| Col12a1-/- | 20% (1/5) | 53% (8/15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukusato, S.; Nagao, M.; Fujihara, K.; Yoneda, T.; Arai, K.; Koch, M.; Kaneko, K.; Ishijima, M.; Izu, Y. Collagen XII Deficiency Increases the Risk of Anterior Cruciate Ligament Injury in Mice. J. Clin. Med. 2021, 10, 4051. https://doi.org/10.3390/jcm10184051
Fukusato S, Nagao M, Fujihara K, Yoneda T, Arai K, Koch M, Kaneko K, Ishijima M, Izu Y. Collagen XII Deficiency Increases the Risk of Anterior Cruciate Ligament Injury in Mice. Journal of Clinical Medicine. 2021; 10(18):4051. https://doi.org/10.3390/jcm10184051
Chicago/Turabian StyleFukusato, Shin, Masashi Nagao, Kei Fujihara, Taiju Yoneda, Kiyotaka Arai, Manuel Koch, Kazuo Kaneko, Muneaki Ishijima, and Yayoi Izu. 2021. "Collagen XII Deficiency Increases the Risk of Anterior Cruciate Ligament Injury in Mice" Journal of Clinical Medicine 10, no. 18: 4051. https://doi.org/10.3390/jcm10184051
APA StyleFukusato, S., Nagao, M., Fujihara, K., Yoneda, T., Arai, K., Koch, M., Kaneko, K., Ishijima, M., & Izu, Y. (2021). Collagen XII Deficiency Increases the Risk of Anterior Cruciate Ligament Injury in Mice. Journal of Clinical Medicine, 10(18), 4051. https://doi.org/10.3390/jcm10184051






