Impact of the 18F-FDG-PET/MRI on Metastatic Staging in Patients with Hepatocellular Carcinoma: Initial Results from 104 Patients
Abstract
:1. Introduction
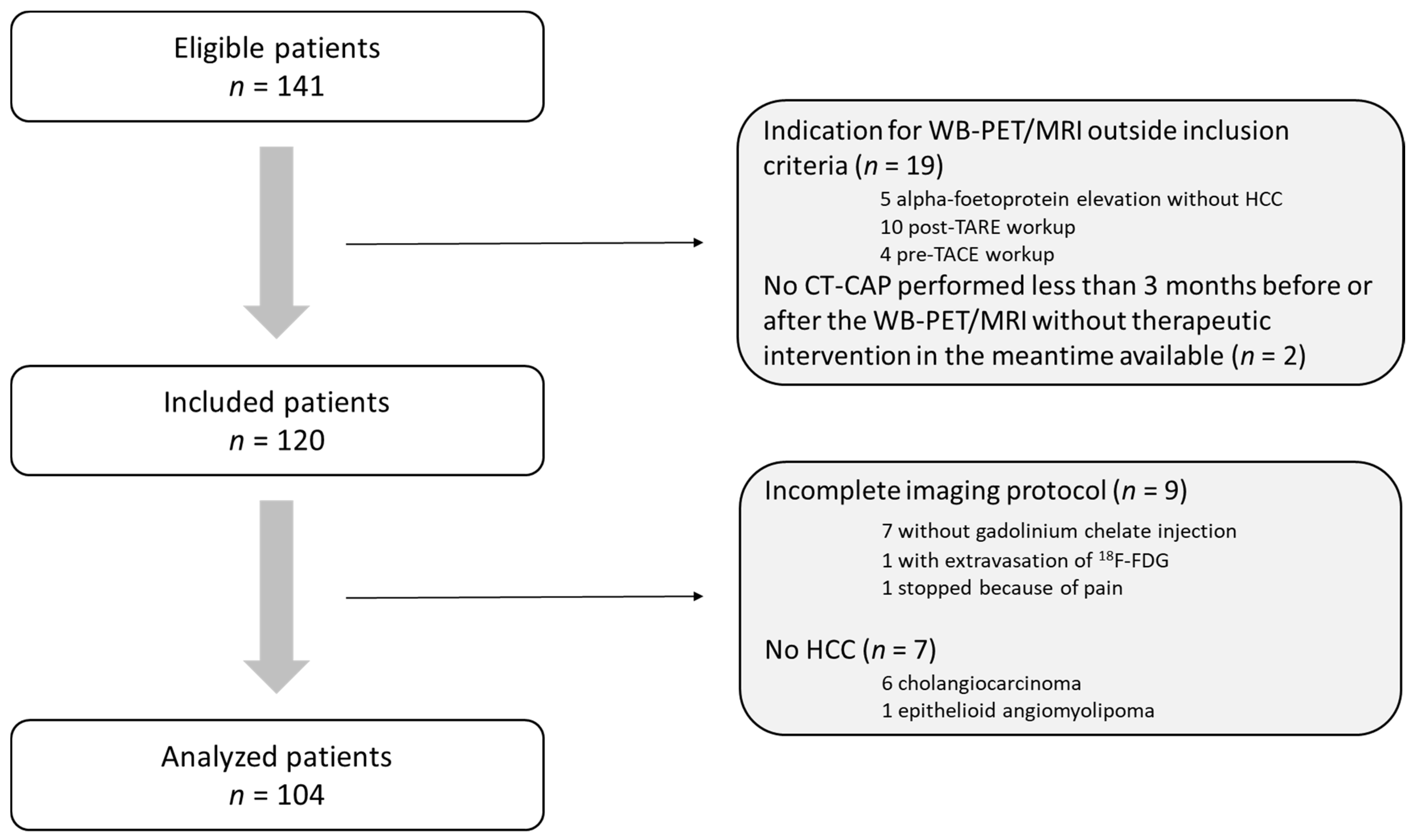
2. Materials and Methods
2.1. Patient Population
2.2. Clinical and Biological Data
2.3. Imaging Protocol
2.4. Imaging Evaluation
2.5. Reference Standard
2.6. Endpoint
2.7. Statistical Analyses
3. Results
3.1. Population Characteristics
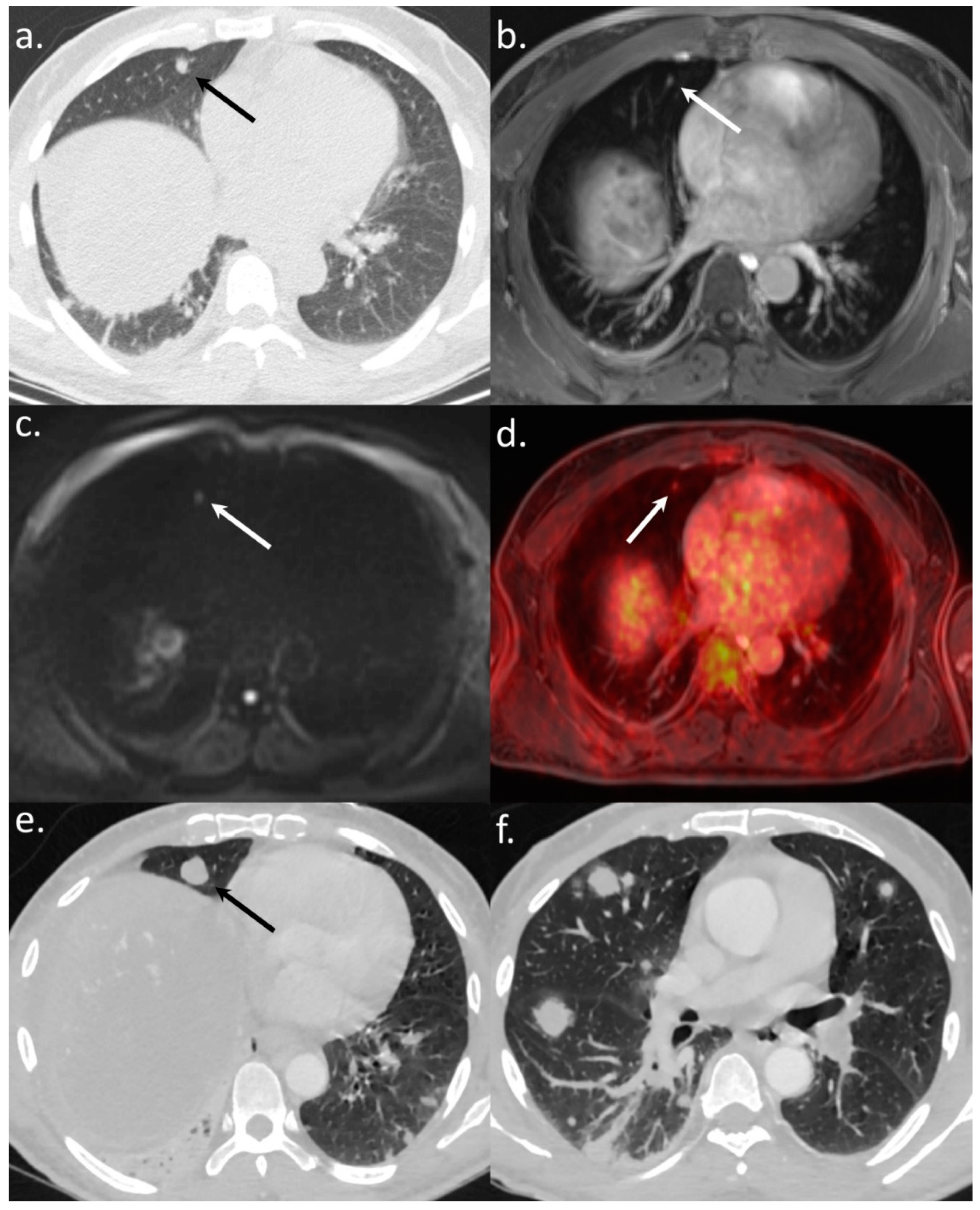
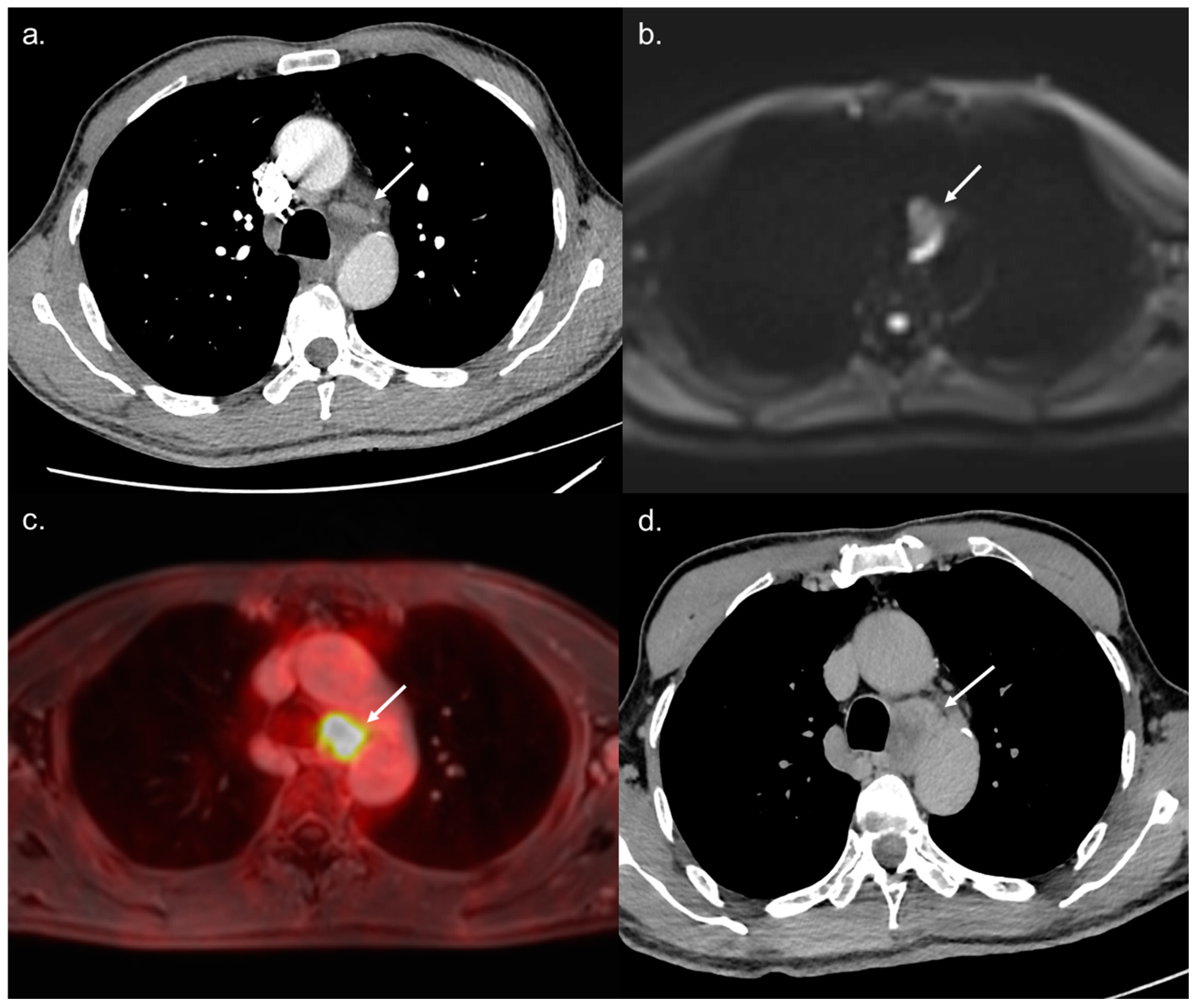
3.2. Diagnostic Accuracy of WB-PET/MRI for Metastatic Staging
3.3. Impact on Patient Management
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Roberts, L.R.; Sirlin, C.B.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Heimbach, J.K.; Murad, M.H.; Mohammed, K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology 2018, 67, 401–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primer 2016, 2, 16018. [Google Scholar] [CrossRef]
- Sarveazad, A.; Agah, S.; Babahajian, A.; Amini, N.; Bahardoust, M. Predictors of 5 year survival rate in hepatocellular carcinoma patients. J. Res. Med. Sci. 2019, 24, 86. [Google Scholar]
- Sangro, B.; Carpanese, L.; Cianni, R.; Golfieri, R.; Gasparini, D.; Ezziddin, S.; Paprottka, P.M.; Fiore, F.; Van Buskirk, M.; Bilbao, J.I.; et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology 2011, 54, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Lauenstein, T.C.; Semelka, R.C. Emerging techniques: Whole-body screening and staging with MRI. J. Magn. Reson. Imaging JMRI 2006, 24, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Morone, M.; Bali, M.A.; Tunariu, N.; Messiou, C.; Blackledge, M.; Grazioli, L.; Koh, D.-M. Whole-Body MRI: Current Applications in Oncology. AJR Am. J. Roentgenol. 2017, 209, W336–W349. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Q.; Nie, W.; Liu, S. Diagnostic value of whole-body diffusion-weighted magnetic resonance imaging for detection of primary and metastatic malignancies: A meta-analysis. Eur. J. Radiol. 2014, 83, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chalaye, J.; Luciani, A.; Enache, C.; Beaussart, P.; Lhermite, C.; Evangelista, E.; Sasanelli, M.; Safar, V.; Meignan, M.; Haioun, C.; et al. Clinical impact of contrast-enhanced computed tomography combined with low-dose (18)F-fluorodeoxyglucose positron emission tomography/computed tomography on routine lymphoma patient management. Leuk. Lymphoma 2014, 55, 2887–2892. [Google Scholar] [CrossRef]
- John, B.V.; Aubuchon, S.; Dahman, B.; Konjeti, V.R.; Heuman, D.; Hubert, J.; Thomas, S.; Deng, Y.; Solomon, C.; Sundaram, L.T.; et al. Addition of [18 F]Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography to Cross-Sectional Imaging Improves Staging and Alters Management in Hepatocellular Carcinoma. Liver Transpl. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transpl. Soc. 2020, 26, 774–784. [Google Scholar]
- Yoon, K.T.; Kim, J.K.; Kim, D.Y.; Ahn, S.H.; Lee, J.D.; Yun, M.; Rha, S.Y.; Chon, C.Y.; Han, K.-H. Role of 18F-fluorodeoxyglucose positron emission tomography in detecting extrahepatic metastasis in pretreatment staging of hepatocellular carcinoma. Oncology 2007, 72 (Suppl. 1), 104–110. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chen, J.-H.; Liang, J.-A.; Lin, C.-C.; Jeng, L.-B.; Kao, C.-H. 18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: A systematic review and meta-analysis. Eur. J. Radiol. 2012, 81, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Kim, S.H.; Kim, S.-K.; Kim, Y.-K.; Park, S.-J. Clinical Impact of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Living Donor Liver Transplantation for Advanced Hepatocellular Carcinoma. Transplantation 2015, 99, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Jreige, M.; Mitsakis, P.; Van Der Gucht, A.; Pomoni, A.; Silva-Monteiro, M.; Gnesin, S.; Boubaker, A.; Nicod-Lalonde, M.; Duran, R.; Prior, J.O.; et al. 18F-FDG PET/CT predicts survival after 90Y transarterial radioembolization in unresectable hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1215–1222. [Google Scholar] [CrossRef]
- Ho, C.L.; Chen, S.; Cheung, S.K.; Leung, T.W.T. Significant Value of 11C-Acetate and 18F-Fluorodeoxyglucose PET/Computed Tomography on 90Y Microsphere Radioembolization for Hepatocellular Carcinoma. PET Clin. 2019, 14, 459–467. [Google Scholar] [CrossRef]
- Catalano, O.A.; Rosen, B.R.; Sahani, D.V.; Hahn, P.F.; Guimaraes, A.R.; Vangel, M.G.; Nicolai, E.; Soricelli, A.; Salvatore, M. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: Initial experience in 134 patients—A hypothesis-generating exploratory study. Radiology 2013, 269, 857–869. [Google Scholar] [CrossRef]
- Sotoudeh, H.; Sharma, A.; Fowler, K.J.; McConathy, J.; Dehdashti, F. Clinical application of PET/MRI in oncology. J. Magn. Reson. Imaging JMRI 2016, 44, 265–276. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, J.M.; Chang, W.; Kang, H.-J.; Bandos, A.; Lim, H.-J.; Kang, S.Y.; Kang, K.W.; Ryoo, S.-B.; Jeong, S.-Y.; et al. Initial M Staging of Rectal Cancer: FDG PET/MRI with a Hepatocyte-specific Contrast Agent versus Contrast-enhanced CT. Radiology 2020, 294, 310–319. [Google Scholar] [CrossRef]
- Katyal, S.; Oliver, J.H.; Peterson, M.S.; Ferris, J.V.; Carr, B.S.; Baron, R.L. Extrahepatic metastases of hepatocellular carcinoma. Radiology 2000, 216, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Luciani, A.; Itti, E.; Rahmouni, A.; Meignan, M.; Clement, O. Lymph node imaging: Basic principles. Eur. J. Radiol. 2006, 58, 338–344. [Google Scholar] [CrossRef]
- Ghanem, N.; Uhl, M.; Brink, I.; Schäfer, O.; Kelly, T.; Moser, E.; Langer, M. Diagnostic value of MRI in comparison to scintigraphy, PET, MS-CT and PET/CT for the detection of metastases of bone. Eur. J. Radiol. 2005, 55, 41–55. [Google Scholar] [CrossRef]
- Kogan, F.; Broski, S.M.; Yoon, D.; Gold, G.E. Applications of PET-MRI in musculoskeletal disease. J. Magn. Reson. Imaging JMRI 2018, 48, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.A.; Nicolai, E.; Rosen, B.R.; Luongo, A.; Catalano, M.; Iannace, C.; Guimaraes, A.; Vangel, M.G.; Mahmood, U.; Soricelli, A.; et al. Comparison of CE-FDG-PET/CT with CE-FDG-PET/MR in the evaluation of osseous metastases in breast cancer patients. Br. J. Cancer 2015, 112, 1452–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, Y.; Koyama, H.; Yoshikawa, T.; Kishida, Y.; Seki, S.; Takenaka, D.; Yui, M.; Miyazaki, M.; Sugimura, K. Standard-, Reduced-, and No-Dose Thin-Section Radiologic Examinations: Comparison of Capability for Nodule Detection and Nodule Type Assessment in Patients Suspected of Having Pulmonary Nodules. Radiology 2017, 284, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Jang, J.Y.; Jeong, S.W.; Lee, S.H.; Kim, S.G.; Cha, S.-W.; Kim, Y.S.; Cho, Y.D.; Kim, H.S.; Kim, B.S.; et al. Diagnostic value for extrahepatic metastases of hepatocellular carcinoma in positron emission tomography/computed tomography scan. World J. Gastroenterol. 2012, 18, 2979–2987. [Google Scholar] [CrossRef] [PubMed]
- Biondetti, P.; Vangel, M.G.; Lahoud, R.M.; Furtado, F.S.; Rosen, B.R.; Groshar, D.; Canamaque, L.G.; Umutlu, L.; Zhang, E.W.; Mahmood, U.; et al. PET/MRI assessment of lung nodules in primary abdominal malignancies: Sensitivity and outcome analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1976–1986. [Google Scholar] [CrossRef]
- Groheux, D.; Quere, G.; Blanc, E.; Lemarignier, C.; Vercellino, L.; de Margerie-Mellon, C.; Merlet, P.; Querellou, S. FDG PET-CT for solitary pulmonary nodule and lung cancer: Literature review. Diagn. Interv. Imaging 2016, 97, 1003–1017. [Google Scholar] [CrossRef] [PubMed]



| Clinical and Biological Data | Subgroups | n (%)/Median [Range] |
|---|---|---|
| Gender | Men | 88/104 (84.6) |
| Age | 63 [35; 86] | |
| Chronic liver disease | 100/104 (96) | |
| HBV | 23/104 (22) | |
| HCV | 28/104 (27) | |
| HBV+HCV | 1/104 (1) | |
| Excessive alcohol consumption | 20/104 (19) | |
| NASH | 6/104 (6) | |
| Excessive alcohol consumption + NASH | 17/104 (16) | |
| Budd Chiari | 2/104 (2) | |
| Auto immune | 3/104 (3) | |
| Cirrhosis | 83/104 (80) | |
| αFP serum level (ng/mL) | 10 [1; 300,000] | |
| Indication | Pre surgery or pre percutaneous ablation workup | 33/104 (31.7) |
| Pre liver transplantation Workup | 34/104 (32.7) | |
| Pre TARE workup | 37/104 (35.6) | |
| BCLC (reference standard) | A | 38/104 (36.5) |
| B | 26/104 (25) | |
| C | 40/104 (38.5) |
| Reference Standard | CT-CAP/Liver MRI | WB-PET/MRI | MRI | PET | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Sensitivity (%) | n | Sensitivity (%) | p-Value | n | Sensitivity (%) | p-Value | n | Sensitivity (%) | p-Value | |||
| Lung Metastases | Per-Lesion Analysis | 16 | 16 | 100 | 7 | 44 | 0.0008 | 7 | 44 | 0.0008 | 6 | 38 | 0.0002 |
| Per-Patient Analysis | 5 | 5 | 100 | 5 | 100 | 1 | 5 | 100 | 1 | 5 | 100 | 1 | |
| Bone Metastases | Per-Lesion Analysis | 10 | 0 | 0 | 10 | 100 | <0.001 | 10 | 100 | <0.001 | 5 | 50 | 0.03 |
| Per-Patient Analysis | 5 | 0 | 0 | 5 | 100 | 0.008 | 5 | 100 | 0.008 | 4 | 80 | 0.047 | |
| Other Metastases | Per-Lesion Analysis | 4 | 1 | 25 | 4 | 100 | 0.14 | 4 | 100 | 0.14 | 4 | 100 | 0.14 |
| Per-Patient Analysis | 4 | 1 | 25 | 4 | 100 | 0.14 | 4 | 100 | 0.14 | 4 | 100 | 0.14 | |
| Total | Per-Lesion Analysis | 30 | 17 | 57 | 21 | 70 | 0.28 | 21 | 70 | 0.28 | 15 | 48 | 0.19 |
| Per-Site Analysis | 14 | 6 | 43 | 14 | 100 | 0.002 | 14 | 100 | 0.002 | 11 | 73 | 0.12 | |
| Per-Patient Analysis | 11 | 5 | 45 | 11 | 100 | 0.01 | 11 | 100 | 0.01 | 9 | 82 | 0.18 | |
| Patient Number | Inclusion Criteria | BCLC | CT-CAP/Liver MRI Findings | CT-CAP/Liver MRI Metastatic Staging | WB-PET/MRI Findings | WB-PET/MRI Metastatic Staging | Final Allocated Treatment |
|---|---|---|---|---|---|---|---|
| 26 | Pre TARE workup | C | Nonspecific mediastinal lymph nodes | Not Metastatic | Hypermetabolic mediastinal lymph nodes | Metastatic | Palliative care |
| 27 | Pre liver transplantation workup | C | Indeterminate 5 mm size lung nodule | Probably metastatic | Lung nodule visible on DWI with hypermetabolism + 5 bone lesions on MRI (including 2 hypermetabolic lesions) | Metastatic | Palliative care |
| 32 | Pre TARE workup | C | No metastasis | Not Metastatic | One 9 mm size hypermetabolic sternal bone lesion visible on MRI | Metastatic | Metastasis-centered stereotaxic radiotherapy |
| 37 | Pre liver transplantation workup | C | No metastasis | Not Metastatic | 2 bone lesions + 1 retroperitoneal hypermetabolic 8 mm size lymph node | Metastatic | Palliative care |
| 79 | Pre TARE workup | C | No metastasis | Not Metastatic | One 10 mm size hypermetabolic spinal bone lesion also visible on MRI | Metastatic | Metastasis-centered stereotaxic radiotherapy |
| 95 | Pre TARE workup | C | No metastasis | Not Metastatic | One 20 mm size hypermetabolic iliac bone lesion also visible on MRI | Metastatic | Metastasis-centered stereotaxic radiotherapy |
| 97 | Pre TARE workup | C | Nonspecific mediastinal lymph nodes | Not Metastatic | Hypermetabolic mediastinal lymph nodes | Metastatic | Palliative care |
| 5 | Pre surgery Workup | A | 11 mm rounded lung nodule | Metastatic | No metastatic site. Lung nodule not visible on DWI and no hypermetabolic | Not Metastatic | Liver surgery |
| 7 | Pre surgery Workup | A | 10 mm size lytic bone lesion | Metastatic | No metastatic site. Bone lesion typical of angioma on MRI and not hypermetabolic | Not Metastatic | Liver surgery |
| 76 | Pre surgery Workup | A | 10 mm size left adrenal lesion >10 UH without signal fall on out phase | Metastatic | Not hypermetabolic left adrenal lesion | Not Metastatic | Liver surgery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vermersch, M.; Mulé, S.; Chalaye, J.; Galletto Pregliasco, A.; Emsen, B.; Amaddeo, G.; Monnet, A.; Stemmer, A.; Baranes, L.; Laurent, A.; et al. Impact of the 18F-FDG-PET/MRI on Metastatic Staging in Patients with Hepatocellular Carcinoma: Initial Results from 104 Patients. J. Clin. Med. 2021, 10, 4017. https://doi.org/10.3390/jcm10174017
Vermersch M, Mulé S, Chalaye J, Galletto Pregliasco A, Emsen B, Amaddeo G, Monnet A, Stemmer A, Baranes L, Laurent A, et al. Impact of the 18F-FDG-PET/MRI on Metastatic Staging in Patients with Hepatocellular Carcinoma: Initial Results from 104 Patients. Journal of Clinical Medicine. 2021; 10(17):4017. https://doi.org/10.3390/jcm10174017
Chicago/Turabian StyleVermersch, Mathilde, Sébastien Mulé, Julia Chalaye, Athena Galletto Pregliasco, Berivan Emsen, Giuliana Amaddeo, Aurélien Monnet, Alto Stemmer, Laurence Baranes, Alexis Laurent, and et al. 2021. "Impact of the 18F-FDG-PET/MRI on Metastatic Staging in Patients with Hepatocellular Carcinoma: Initial Results from 104 Patients" Journal of Clinical Medicine 10, no. 17: 4017. https://doi.org/10.3390/jcm10174017
APA StyleVermersch, M., Mulé, S., Chalaye, J., Galletto Pregliasco, A., Emsen, B., Amaddeo, G., Monnet, A., Stemmer, A., Baranes, L., Laurent, A., Leroy, V., Itti, E., & Luciani, A. (2021). Impact of the 18F-FDG-PET/MRI on Metastatic Staging in Patients with Hepatocellular Carcinoma: Initial Results from 104 Patients. Journal of Clinical Medicine, 10(17), 4017. https://doi.org/10.3390/jcm10174017






