The Occurrence of Pain-Induced Depression Is Different between Rat Models of Inflammatory and Neuropathic Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. SNI Model of Neuropathic Pain
2.3. CFA Model of Inflammatory Pain
2.4. Mechanical Allodynia Test
2.5. Cold Allodynia Test
2.6. Hind Paw Dorsoventral Thickness
2.7. Measurement of TIMP-1 and CXCL1 Levels in Footpad Tissues
2.8. FST Procedures
2.9. SPT Procedures
2.10. PET Imaging of the SERT
2.11. Statistical Analysis
3. Results
3.1. Mechanical and Cold Allodynia Tests after SNI and CFA Injection
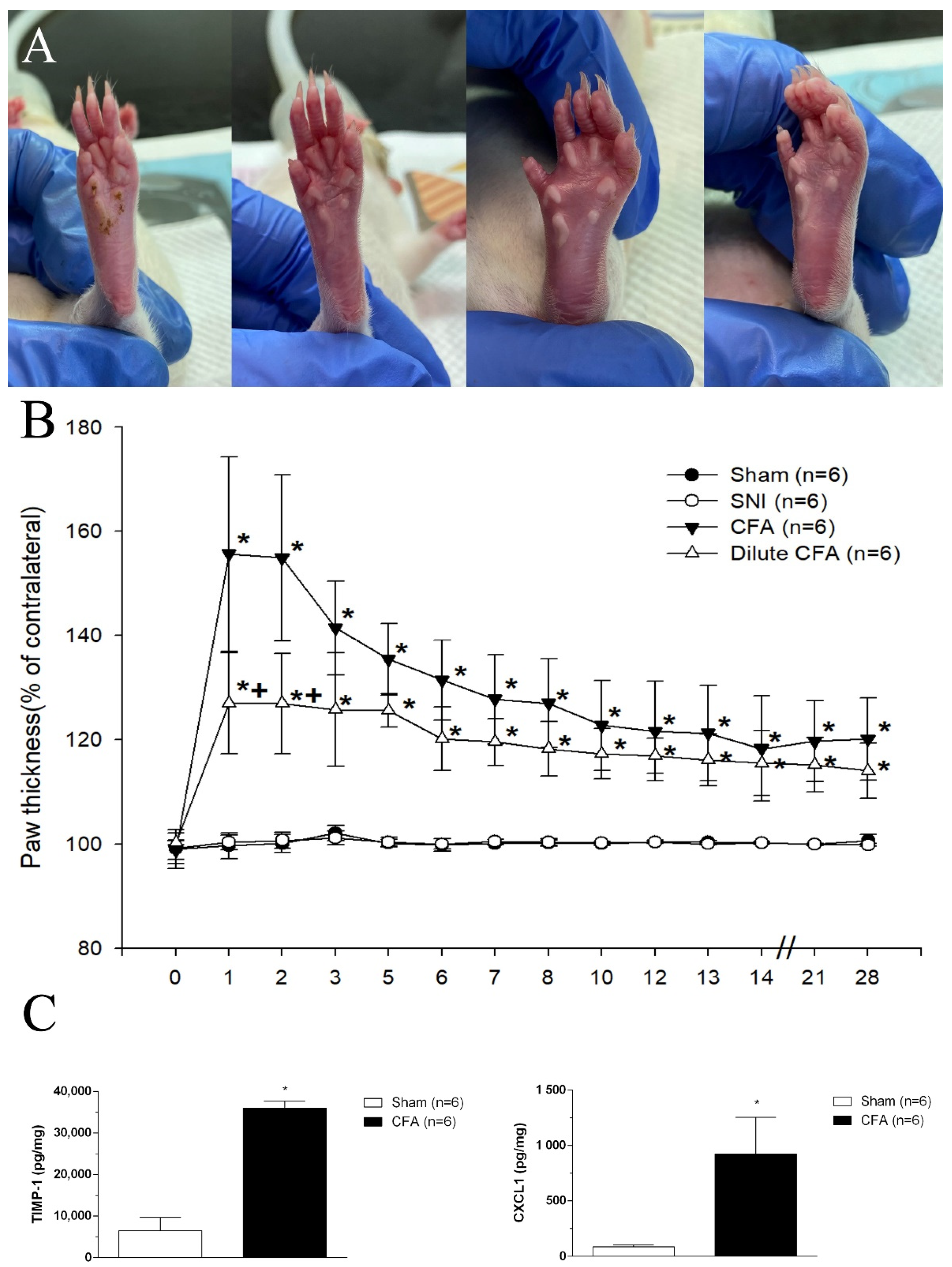
3.2. Hind Paw Thickness (Dorsoventral)
3.3. CFA Induced the Increases of TIMP-1 and CXCL1 Levels in Footpad Tissues
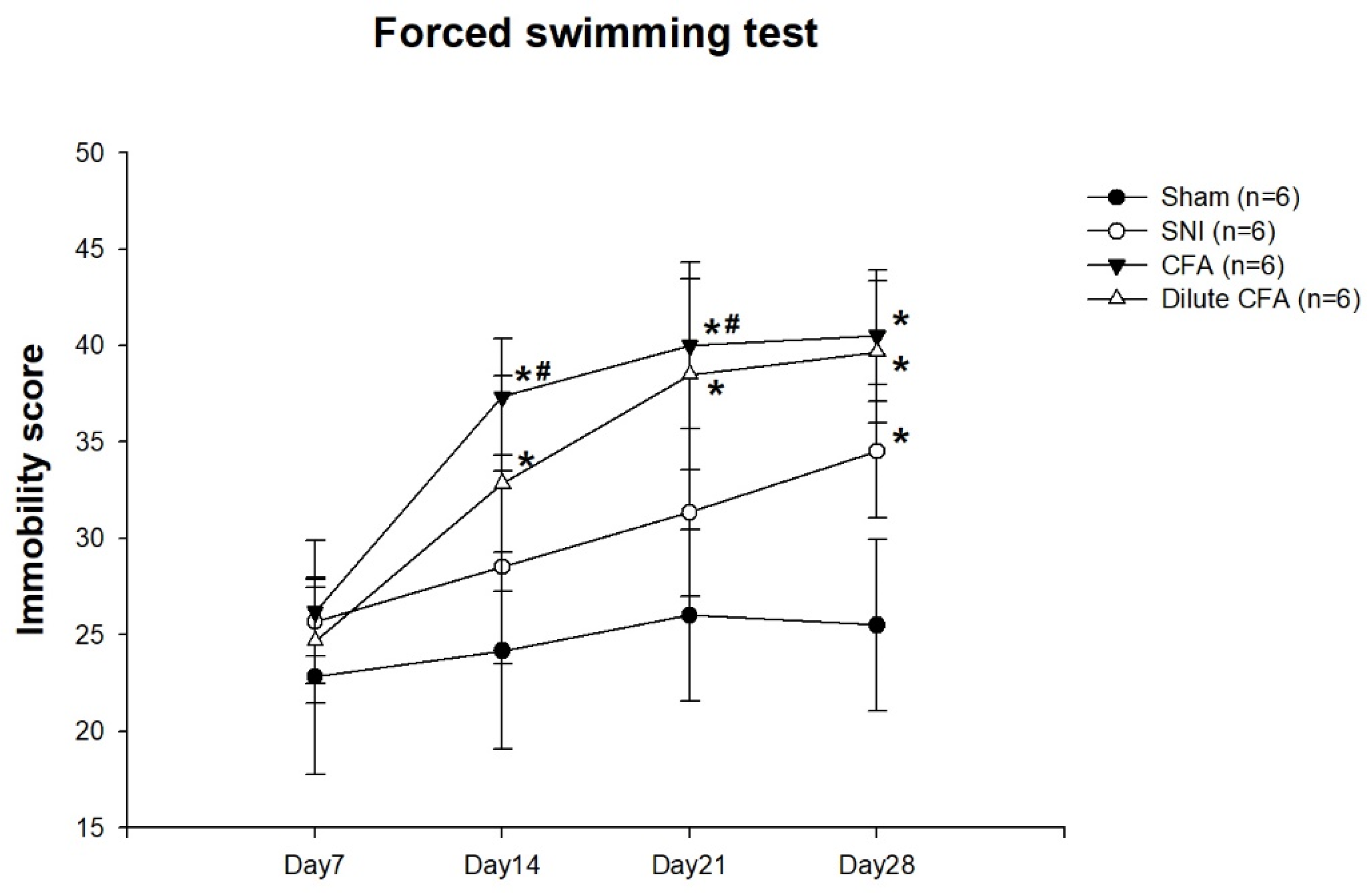
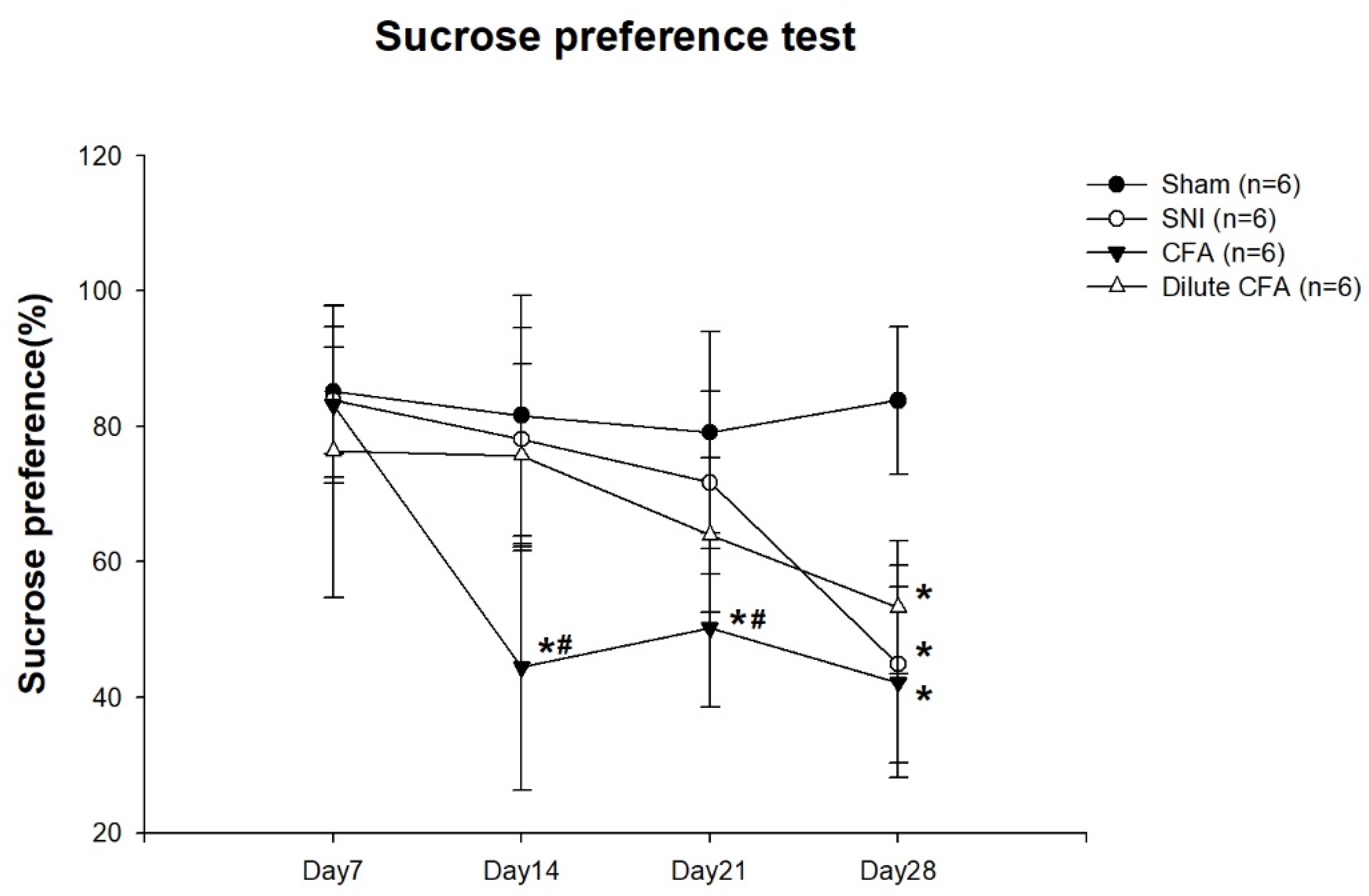
3.4. Depression-like Behavior
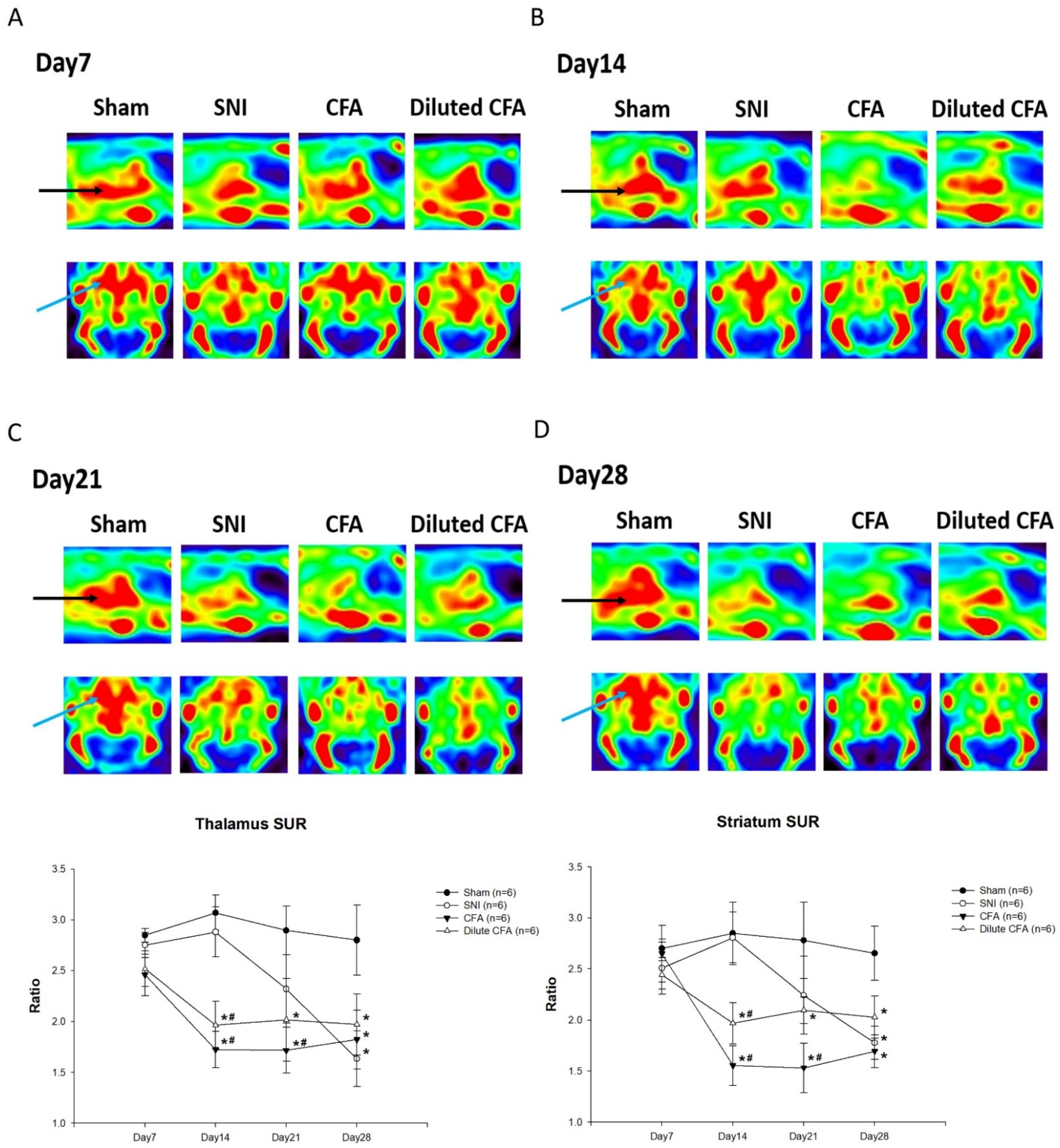
3.5. PET Images of SERT Expression in the Brains of Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ojeda, B.; Salazar, A.; Calahorro, M.; Dueñas, M.; Mico, J.; de Sola, H.; Failde, I. Understanding the different relationships between mood and sleep disorders in several groups of non-oncological patients with chronic pain. Curr. Med. Res. Opin. 2018, 34, 669–676. [Google Scholar] [CrossRef]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.-R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muley, M.M.; Krustev, E.; McDougall, J.J. Preclinical assessment of inflammatory pain. CNS Neurosci. Ther. 2016, 22, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Corallo, F.; De Salvo, S.; Floridia, D.; Bonanno, L.; Muscarà, N.; Cerra, F.; Cannistraci, C.; Di Cara, M.; Buono, V.L.; Bramanti, P. Assessment of spinal cord stimulation and radiofrequency: Chronic pain and psychological impact. Medicine 2020, 99, e18633. [Google Scholar] [CrossRef]
- Cruccu, G.; Truini, A. A review of neuropathic pain: From guidelines to clinical practice. Pain Ther. 2017, 6, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.J.; Huang, Y.-Y.; Underwood, M.D.; Kassir, S.A.; Oppenheim, S.; Kelly, T.M.; Dwork, A.J.; Arango, V. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch. Gen. Psychiatry 2000, 57, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Hains, B.C.; Everhart, A.W.; Fullwood, S.D.; Hulsebosch, C.E. Changes in serotonin, serotonin transporter expression and serotonin denervation supersensitivity: Involvement in chronic central pain after spinal hemisection in the rat. Exp. Neurol. 2002, 175, 347–362. [Google Scholar] [CrossRef]
- Weng, S.-J.; Shiue, C.-Y.; Huang, W.-S.; Cheng, C.-Y.; Huang, S.-Y.; Li, I.-H.; Tao, C.-C.; Chou, T.-K.; Liao, M.-H.; Chang, Y.-P. PET imaging of serotonin transporters with 4-[18F]-ADAM in a Parkinsonian rat model. Cell Transplant. 2013, 22, 1295–1305. [Google Scholar] [CrossRef] [Green Version]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- McCarson, K.E. Models of Inflammation: Carrageenan-or Complete Freund’s Adjuvant (CFA)–Induced Edema and Hypersensitivity in the Rat. Curr. Protoc. Pharmacol. 2015, 70, 5.4.1–5.4.9. [Google Scholar] [CrossRef]
- Kalmar, B.; Greensmith, L.; Malcangio, M.; McMahon, S.; Csermely, P.; Burnstock, G. The effect of treatment with BRX-220, a co-inducer of heat shock proteins, on sensory fibers of the rat following peripheral nerve injury. Exp. Neurol. 2003, 184, 636–647. [Google Scholar] [CrossRef]
- Dowdall, T.; Robinson, I.; Meert, T.F. Comparison of five different rat models of peripheral nerve injury. Pharmacol. Biochem. Behav. 2005, 80, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-J.; Shih, C.-C.; Chang, K.-Y.; Liao, M.-H.; Liaw, W.-J.; Wu, C.-C.; Tsao, C.-M. Angiotensin-(1–7) treatment blocks lipopolysaccharide-induced organ damage, platelet dysfunction, and IL-6 and nitric oxide production in rats. Sci. Rep. 2021, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, e52587. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.K.; Arnt, J.; Sánchez, C. Intracranial self-stimulation and sucrose intake differ as hedonic measures following chronic mild stress: Interstrain and interindividual differences. Behav. Brain Res. 2000, 107, 21–33. [Google Scholar] [CrossRef]
- Peng, C.-J.; Huang, Y.-Y.; Huang, W.-S.; Shiue, C.-Y. An automated synthesis of N,N-dimethyl-2-(2-amino-4-[18F] fluorophenylthio) benzylamine (4-[18F]-ADAM) for imaging serotonin transporters. Appl. Radiat. Isot. 2008, 66, 625–631. [Google Scholar] [CrossRef]
- Ma, K.-H.; Huang, W.-S.; Kuo, Y.-Y.; Peng, C.-J.; Liou, N.-H.; Liu, R.-S.; Hwang, J.-J.; Liu, J.-C.; Chen, H.-J.; Shiue, C.-Y. Validation of 4-[18F]-ADAM as a SERT imaging agent using micro-PET and autoradiography. Neuroimage 2009, 45, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.J.; Lu, D.; Sparer, T.E.; Masi, T.; Goff, M.R.; Karlstad, M.D.; Collier, J.J. NF-κB and STAT1 control CXCL1 and CXCL2 gene transcription. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E131–E149. [Google Scholar] [CrossRef] [Green Version]
- Almeida, L.S.; Castro-Lopes, J.M.; Neto, F.L.; Potes, C.S. Amylin, a peptide expressed by nociceptors, modulates chronic neuropathic pain. Eur. J. Pain 2019, 23, 784–799. [Google Scholar] [CrossRef] [Green Version]
- Leiguarda, C.; Potilinski, C.; Rubione, J.; Tate, P.; Villar, M.J.; Montaner, A.; Bisagno, V.; Constandil, L.; Brumovsky, P.R. IMT504 Provides Analgesia by Modulating Cell Infiltrate and Inflammatory Milieu in a Chronic Pain Model. J. Neuroimmune Pharmacol. 2020, 16, 651–666. [Google Scholar] [CrossRef]
- Szabo-Pardi, T.A.; Agalave, N.M.; Burton, M.D. The role of microglia versus peripheral macrophages in maladaptive plasticity after nerve injury. Neural Regen. Res. 2021, 16, 1202. [Google Scholar]
- Cook, A.D.; Christensen, A.D.; Tewari, D.; McMahon, S.B.; Hamilton, J.A. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018, 39, 240–255. [Google Scholar] [CrossRef]
- Xu, Q.; Yaksh, T.L. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr. Opin. Anaesthesiol. 2011, 24, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The link between depression and chronic pain: Neural mechanisms in the brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef] [PubMed]
- Pandya, M.; Altinay, M.; Malone, D.A.; Anand, A. Where in the brain is depression? Curr. Psychiatry Rep. 2012, 14, 634–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-Y.; Wan, Y.; Tang, S.-J.; Guan, Y.; Wei, F.; Ma, D. Maladaptive plasticity and neuropathic pain. Neural Plast. 2016, 2016, 4842159. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Wang, J.; Han, J.-F.; Guo, J.; Xie, Z.-M.; Pan, W.; Yang, J.-J.; Sun, K.-J. Acute single dose of ketamine relieves mechanical allodynia and consequent depression-like behaviors in a rat model. Neurosci. Lett. 2016, 631, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Xu, X.; Lin, X.; Liu, R. Downregulated spinal IRF8 and BDNF in NAC are involved in neuropathic pain-induced depression relief via pulsed radiofrequency on dorsal root ganglion in rat SNI model. Brain Res. Bull. 2019, 146, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H. Five things to know about inflammation and depression. Psychiatr. Times 2018, 35, 2018. [Google Scholar]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capuron, L.; Miller, A.H. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther. 2011, 130, 226–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, A.M.; Lee, M.; Su, C.; Zou, A.; Wang, J. AMPAkines have novel analgesic properties in rat models of persistent neuropathic and inflammatory pain. Anesthesiology 2014, 121, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Carvajal-Oliveros, A.; Campusano, J.M. Studying the Contribution of Serotonin to Neurodevelopmental Disorders. Can This Fly? Front. Behav. Neurosci. 2021, 14, 262. [Google Scholar] [CrossRef]
- Selvaraj, S.; Murthy, N.V.; Bhagwagar, Z.; Bose, S.K.; Hinz, R.; Grasby, P.M.; Cowen, P.J. Diminished brain 5-HT transporter binding in major depression: A positron emission tomography study with [11 C] DASB. Psychopharmacology 2011, 213, 555–562. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-C.; Ma, K.-H.; Guo, S.-L.; Lin, B.-F.; Tsai, C.-S.; Yeh, C.-C. The Occurrence of Pain-Induced Depression Is Different between Rat Models of Inflammatory and Neuropathic Pain. J. Clin. Med. 2021, 10, 4016. https://doi.org/10.3390/jcm10174016
Hsu Y-C, Ma K-H, Guo S-L, Lin B-F, Tsai C-S, Yeh C-C. The Occurrence of Pain-Induced Depression Is Different between Rat Models of Inflammatory and Neuropathic Pain. Journal of Clinical Medicine. 2021; 10(17):4016. https://doi.org/10.3390/jcm10174016
Chicago/Turabian StyleHsu, Yung-Chi, Kuo-Hsing Ma, Shu-Lin Guo, Bo-Feng Lin, Chien-Sung Tsai, and Chun-Chang Yeh. 2021. "The Occurrence of Pain-Induced Depression Is Different between Rat Models of Inflammatory and Neuropathic Pain" Journal of Clinical Medicine 10, no. 17: 4016. https://doi.org/10.3390/jcm10174016
APA StyleHsu, Y.-C., Ma, K.-H., Guo, S.-L., Lin, B.-F., Tsai, C.-S., & Yeh, C.-C. (2021). The Occurrence of Pain-Induced Depression Is Different between Rat Models of Inflammatory and Neuropathic Pain. Journal of Clinical Medicine, 10(17), 4016. https://doi.org/10.3390/jcm10174016





