A Multicenter Randomized Controlled Prospective Study to Assess Efficacy of Laparoscopic Electrochemotherapy in the Treatment of Locally Advanced Pancreatic Cancer
Abstract
:1. Introduction
2. Trial Design
2.1. Objectives
2.1.1. Primary Endpoint
2.1.2. Secondary Endpoints
- (a)
- To evaluate the effect of ECT on disease progression-free time and survival;
- (b)
- To evaluate the impact of ECT on quality of life with particular attention to the effect on pain reduction;
- (c)
- To evaluate the ECT toxicity;
- (d)
- To evaluate by morphological and functional MRI parameters the conversion from locally advanced disease to resectable disease. The conversion rate will be calculated for each arm.
2.2. Subject’s Selection
2.3. Control Group
2.4. Experimental Group
2.5. Endpoints Evaluation Criteria
2.6. Secondary Endpoint of the Study
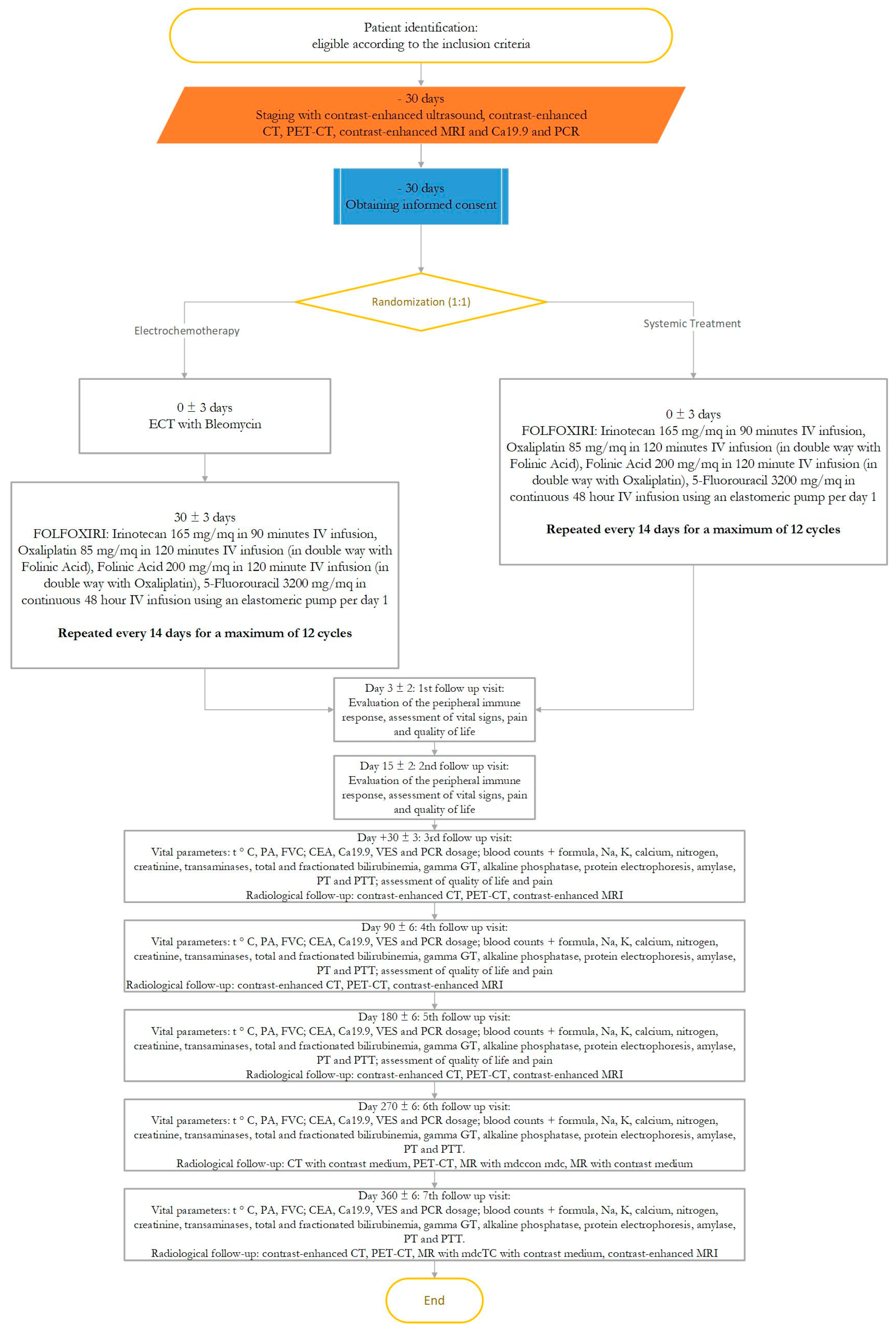
2.7. Description of Study Procedures
2.7.1. Visit 1
2.7.2. Visit 2
2.7.3. Visit 3 to Visit 9 (Follow-Up Visit)
2.8. Description of Sample Size Calculation
2.9. Statistical Analysis
3. Discussions
3.1. Preclinical Experiences
3.2. Clinical Experience
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics and Dissemination
References
- Belehradek, M.; Domenge, C.; Luboinski, B.; Orlowski, S.; Belehradek, J.; Mir, L.M. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer 1993, 72, 3694–3700. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Fusco, R.; D’Alessio, V.; Petrillo, A.; Lastoria, S.; Piccirillo, M.; Albino, V.; Belli, A.; Tafuto, S.; et al. Clinical Phase I/II Study: Local Disease Control and Survival in Locally Advanced Pancreatic Cancer Treated with Electrochemotherapy. J. Clin. Med. 2021, 10, 1305. [Google Scholar] [CrossRef]
- Mir, L.; Belehradek, M.; Domenge, C.; Orlowski, S.; Poddevin, B.; Belehradek, J.; Schwaab, G.; Luboinski, B.; Paoletti, C. Electrochemotherapy, a new antitumor treatment: First clinical trial. CR Acad. Sci. III 1991, 313, 613–618. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavčič, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4 (Suppl. S4), 3–13. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.-R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Campana, L.G.; Mocellin, S.; Basso, M.; Puccetti, O.; De Salvo, G.L.; Sileni, V.C.; Vecchiato, A.; Corti, L.; Rossi, C.R.; Nitti, D. Bleomycin-Based Electrochemotherapy: Clinical Outcome from a Single Institution’s Experience with 52 Patients. Ann. Surg. Oncol. 2009, 16, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.G.; Valpione, S.; Mocellin, S.; Sundararajan, R.; Granziera, E.; Sartore, L.; Sileni, V.C.; Rossi, C.R. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. BJS 2012, 99, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Mali, B.; Jarm, T.; Snoj, M.; Sersa, G.; Miklavcic, D. Antitumor effectiveness of electrochemotherapy: A systematic review and meta-analysis. Eur. J. Surg. Oncol. EJSO 2013, 39, 4–16. [Google Scholar] [CrossRef]
- Spratt, D.E.; Spratt, E.A.G.; Wu, S.; DeRosa, A.; Lee, N.Y.; Lacouture, M.E.; Barker, C.A. Efficacy of Skin-Directed Therapy for Cutaneous Metastases From Advanced Cancer: A Meta-Analysis. J. Clin. Oncol. 2014, 32, 3144–3155. [Google Scholar] [CrossRef] [Green Version]
- Aguado-Romeo, M.J.; Benot-López, S.; Romero-Tabares, A. Electrochemotherapy for the Treatment of Unresectable Locoregionally Advanced Cutaneous Melanoma: A Systematic Review. Actas Dermosifiliogr. 2017, 108, 91–97. [Google Scholar] [CrossRef]
- Plaschke, C.C.; Gothelf, A.; Gehl, J.; Wessel, I. Electrochemotherapy of mucosal head and neck tumors: A systematic review. Acta Oncol. 2016, 55, 1266–1272. [Google Scholar] [CrossRef]
- Rotunno, R.; Marenco, F.; Ribero, S.; Calvieri, S.; Amerio, P.; Curatolo, P.; Quaglino, P. Electrochemotherapy in non-melanoma head and neck skin cancers: A three-center experience and review of the literature. G. Ital. Dermatol. Venereol. 2016, 151, 610–618. [Google Scholar]
- Granata, V.; Grassi, R.; Fusco, R.; Belli, A.; Palaia, R.; Carrafiello, G.; Miele, V.; Petrillo, A.; Izzo, F. Local ablation of pancreatic tumors: State of the art and future perspectives. World J. Gastroenterol. 2021, 27, 3413–3428. [Google Scholar] [CrossRef] [PubMed]
- Tafuto, S.; von Arx, C.; De Divitiis, C.; Maura, C.T.; Palaia, R.; Albino, V.; Fusco, R.; Membrini, M.; Petrillo, A.; Granata, V.; et al. ENETS Center of Excellence Multidisciplinary Group for Neuroendocrine Tumors in Naples (Italy). Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int. J. Surg. 2015, 21 (Suppl. S1), S78–S82. [Google Scholar] [CrossRef] [PubMed]
- Cadossi, R.; Ronchetti, M.; Cadossi, M. Locally enhanced chemotherapy by electroporation: Clinical experiences and perspective of use of electrochemotherapy. Future Oncol. 2014, 10, 877–890. [Google Scholar] [CrossRef] [Green Version]
- Tarantino, L.; Busto, G.; Nasto, A.; Fristachi, R.; Cacace, L.; Talamo, M.; Accardo, C.; Bortone, S.; Gallo, P.; Tarantino, P.; et al. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: A feasibility study. World J. Gastroenterol. 2017, 23, 906–918. [Google Scholar] [CrossRef]
- Mali, B.; Gorjup, V.; Edhemovic, I.; Brecelj, E.; Cemazar, M.; Sersa, G.; Strazisar, B.; Miklavcic, D.; Jarm, T. Electrochemotherapy of colorectal liver metastases-An observational study of its effects on the electrocardiogram. Biomed. Eng. Online 2015, 14 (Suppl. S3), S5. [Google Scholar] [CrossRef] [Green Version]
- Edhemovic, I.; Brecelj, E.; Gasljevic, G.; Music, M.M.; Gorjup, V.; Mali, B.; Jarm, T.; Kos, B.; Pavliha, D.; Kuzmanov, B.G.; et al. Intraoperative electrochemotherapy of colorectal liver metastases. J. Surg. Oncol. 2014, 110, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Girelli, R.; Prejanò, S.; Cataldo, I.; Corbo, V.; Martini, L.; Scarpa, A.; Claudio, B. Feasibility and safety of electrochemotherapy (ECT) in the pancreas: A pre-clinical investigation. Radiol. Oncol. 2015, 49, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaroszeski, M.J.; Illingworth, P.; Pottinger, C.; Hyacinthe, M.; Heller, R. Electrically mediated drug delivery for treating subcutaneous and orthotopic pancreatic adenocarcinoma in a hamster model. Anticancer Res. 1999, 19, 989–994. [Google Scholar] [PubMed]
- Granata, V.; Fusco, R.; Piccirillo, M.; Palaia, R.; Petrillo, A.; Lastoria, S.; Izzo, F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int. J. Surg. 2015, 18, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Piccirillo, M.; Leongito, M.; Palaia, R.; Granata, F.; Lastoria, S.; Izzo, F.; Petrillo, A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J. Gastroenterol. 2017, 23, 4767–4778. [Google Scholar] [CrossRef] [PubMed]
- Grønvold, M.; Klee, M.C.; Sprangers, M.A.; Aaronson, N.K. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J. Clin. Epidemiol. 1997, 50, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Charnsangavej, C.; Faria, S.D.C.; Tamm, E.P.; Benjamin, R.S.; Johnson, M.M.; Macapinlac, H.A.; Podoloff, D.A. CT Evaluation of the Response of Gastrointestinal Stromal Tumors After Imatinib Mesylate Treatment:A Quantitative Analysis Correlated with FDG PET Findings. Am. J. Roentgenol. 2004, 183, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122S–150S. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Fusco, R.; Sansone, M.; Grassi, R.; Maio, F.; Palaia, R.; Tatangelo, F.; Botti, G.; Grimm, R.; Curley, S.; et al. Magnetic resonance imaging in the assessment of pancreatic cancer with quantitative parameter extraction by means of dynamic contrast-enhanced magnetic resonance imaging, diffusion kurtosis imaging and intravoxel incoherent motion diffusion-weighted imaging. Ther. Adv. Gastroenterol. 2020, 13, 1756284819885052. [Google Scholar] [CrossRef]
- Do, R.K.; Reyngold, M.; Paudyal, R.; Oh, J.H.; Konar, A.S.; LoCastro, E.; Goodman, K.A.; Shukla-Dave, A. Diffusion-Weighted and Dynamic Contrast-Enhanced MRI Derived Imaging Metrics for Stereotactic Body Radiotherapy of Pancreatic Ductal Adenocarcinoma: Preliminary Findings. Tomography 2020, 6, 261–271. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–656. [Google Scholar] [CrossRef]
- Chow, S.C.; Shao, J.; Wang, H. Sample Size Calculations in Clinical Research 2003, 3rd ed.; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Farrington, C.P.; Manning, G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat. Med. 1990, 9, 1447–1454. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, V.; Fusco, R.; Catalano, O.; Setola, S.V.; Castelguidone, E.D.L.D.; Piccirillo, M.; Palaia, R.; Grassi, R.; Granata, F.; Izzo, F.; et al. Multidetector computer tomography in the pancreatic adenocarcinoma assessment: An update. Infect. Agents Cancer 2016, 11, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- Arcidiacono, P.G.; Carrara, S.; Reni, M.; Petrone, M.C.; Cappio, S.; Balzano, G.; Boemo, C.; Cereda, S.; Nicoletti, R.; Enderle, M.D.; et al. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest. Endosc. 2012, 76, 1142–1151. [Google Scholar] [CrossRef]
- Pai, M.; Yang, J.; Zhang, X.; Jin, Z.; Wang, D.; Senturk, H.; Lakhtakia, S.; Reddy, D.N.; Kahaleh, M.; Habib, N.; et al. PWE-055 Endoscopic Ultrasound Guided Radiofrequency Ablation (EUS-RFA) for Pancreatic Ductal Adenocarcinoma. Gut 2013, 62, A153. [Google Scholar] [CrossRef]
- Pai, M.; Habib, N.; Senturk, H.; Lakhtakia, S.; Reddy, N.; Cicinnati, V.R.; Kaba, I.; Beckebaum, S.; Drymousis, P.; Kahaleh, M.; et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J. Gastrointest. Surg. 2015, 7, 52–59. [Google Scholar] [CrossRef]
- Carrafiello, G.; Ierardi, A.M.; Fontana, F.; Petrillo, M.; Floridi, C.; Lucchina, N.; Cuffari, S.; Dionigi, G.; Rotondo, A.; Fugazzola, C. Microwave Ablation of Pancreatic Head Cancer: Safety and Efficacy. J. Vasc. Interv. Radiol. 2013, 24, 1513–1520. [Google Scholar] [CrossRef]
- Crowley, J.M. Electrical Breakdown of Bimolecular Lipid Membranes as an Electromechanical Instability. Biophys. J. 1973, 13, 711–724. [Google Scholar] [CrossRef] [Green Version]
- Neumann, E.; Rosenheck, K. Permeability changes induced by electric impulses in vesicular membranes. J. Membr. Biol. 1972, 10, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, U.; Pilwat, G.; Riemann, F. Dielectric Breakdown of Cell Membranes. Biophys. J. 1974, 14, 881–899. [Google Scholar] [CrossRef] [Green Version]
- Sugar, I.; Neumann, E. Stochastic model for electric field-induced membrane pores electroporation. Biophys. Chem. 1984, 19, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Mir, L.M.; Orlowski, S. Mechanisms of electrochemotherapy. Adv. Drug Deliv. Rev. 1999, 35, 107–118. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Jaroszeski, M.J.; Dang, V.; Pottinger, C.; Hickey, J.; Gilbert, R.; Heller, R. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs 2000, 11, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Probst, U.; Fuhrmann, I.; Beyer, L.P.; Wiggermann, P. Electrochemotherapy as a New Modality in Interventional Oncology: A Review. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef] [Green Version]
- Laudicella, M.; Walsh, B.; Munasinghe, A.; Faiz, O. Impact of laparoscopic versus open surgery on hospital costs for colon cancer: A population-based retrospective cohort study. BMJ Open 2016, 6, e012977. [Google Scholar] [CrossRef] [PubMed]
- Buia, A.; Stockhausen, F.; Hanisch, E. Laparoscopic surgery: A qualified systematic review. World J. Methodol. 2015, 5, 238–254. [Google Scholar] [CrossRef]
- Dapri, G. 10-Year Experience with 1700 Single-Incision Laparoscopies. Surg. Technol. Int. 2019, 35, 71–83. [Google Scholar] [PubMed]
- Zhang, H.; Feng, Y.; Zhao, J.; Chen, R.; Chen, X.; Yin, X.; Cheng, W.; Li, D.; Li, J.; Huang, X.; et al. Total laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy (TJDBPS01): Study protocol for a multicentre, randomised controlled clinical trial. BMJ Open 2020, 10, e033490. [Google Scholar] [CrossRef] [PubMed]
- Bourke, M.; Salwa, S.; Forde, P.; Sadadcharam, M.; Larkin, J.; Collins, C.; Zeeshan, S.; Winter, D.; O’Sullivan, G.C.; Soden, D.; et al. P80. Endoscopically targeted electrochemotherapy for the treatment of colorectal cancer. Eur. J. Surg. Oncol. EJSO 2012, 38, 1127–1128. [Google Scholar] [CrossRef]
- Izzo, F.; Ionna, F.; Granata, V.; Albino, V.; Patrone, R.; Longo, F.; Guida, A.; DelRio, P.; Rega, D.; Scala, D.; et al. New Deployable Expandable Electrodes in the Electroporation Treatment in a Pig Model: A Feasibility and Usability Preliminary Study. Cancers 2020, 12, 515. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.; Primrose, J.N.; Langeberg, W.; Kelsh, M.; Mowat, F.; Alexander, D.; Choti, M.; Poston, G.; Kanas, G. Survival after liver resection in metastatic colorectal cancer: Review and meta-analysis of prognostic factors. Clin. Epidemiol. 2012, 4, 283–301. [Google Scholar] [CrossRef] [Green Version]
- Ierardi, A.M.; Lucchina, N.; Petrillo, M.; Floridi, C.; Piacentino, F.; Bacuzzi, A.; Fonio, P.; Fontana, F.; Fugazzola, C.; Brunese, L.; et al. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol. Med. 2014, 119, 483–498. [Google Scholar] [CrossRef]
- De Filippo, M.; Ziglioli, F.; Russo, U.; Pagano, P.; Brunese, L.; Bertelli, E.; Pagnini, F.; Maestroni, U. Radiofrequency ablation (RFA) of T1a renal cancer with externally cooled multitined expandable electrodes. Radiol. Med. 2020, 125, 790–797. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bruno, F.; Gianneramo, C.; Palumbo, P.; Zugaro, L.; Zoccali, C.; Barile, A.; Masciocchi, C. Evolution of the imaging features of osteoid osteoma treated with RFA or MRgFUS during a long-term follow-up: A pictorial review with clinical correlations. Radiol. Med. 2020, 125, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; DelRio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Castelguidone, E.D.L.C.; Fusco, R.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Gallipoli, A.D.; Petrillo, A. Irreversible electroporation of hepatocellular carcinoma: Preliminary report on the diagnostic accuracy of magnetic resonance, computer tomography, and contrast-enhanced ultrasound in evaluation of the ablated area. Radiol. Med. 2015, 121, 122–131. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, F.; Li, X.; Guan, Y.; Wang, M. Transcatheter arterial chemoembolization combined with simultaneous DynaCT-guided radiofrequency ablation in the treatment of solitary large hepatocellular carcinoma. Radiol. Med. 2019, 124, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calandri, M.; Ruggeri, V.; Carucci, P.; Mirabella, S.; Veltri, A.; Fonio, P.; Gazzera, C. Thermal ablation with fusion imaging guidance of hepatocellular carcinoma without conspicuity on conventional or contrast-enhanced US: Surrounding anatomical landmarks matter. Radiol. Med. 2019, 124, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, A.M.; Petrillo, M.; Coppola, A.; Angileri, S.A.; Galassi, A.; Padovano, B.; Volpi, A.; Cozzolino, M.; Carrafiello, G. Percutaneous microwave ablation of renal angiomyolipomas in tuberous sclerosis complex to improve the quality of life: Preliminary experience in an Italian center. Radiol. Med. 2018, 124, 176–183. [Google Scholar] [CrossRef]
- Bruno, F.; Catalucci, A.; Arrigoni, F.; Sucapane, P.; Cerone, D.; Cerrone, P.; Ricci, A.; Marini, C.; Masciocchi, C. An experience-based review of HIFU in functional interventional neuroradiology: Transcranial MRgFUS thalamotomy for treatment of tremor. Radiol. Med. 2020, 125, 877–886. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age ≥ 18 years | Age less than 18 years |
| Suitable mental health conditions | Absolute contraindication to surgery |
| Ability to sign a specific informed consent in order to be enrolled in the study | Visceral, bone, or diffuse metastases |
| Life expectancy in line with the follow-up indicated by the study | Presence of extrahepatic spread of the disease |
| Diagnosis of exocrine pancreatic cancer with histological confirmation | Clinically significant ascites |
| Preoperative Staging (CT and MRI) of locally advanced pancreatic cancer disease: stage III | Any serious and uncontrolled systemic illness |
| The subject is not eligible for the “gold-standard” treatment of surgical pancreatectomy and is eligible for a conventional systemic treatment (FOLFOXIRI) | Acute lung infection |
| Symptoms of poor lung function by clinical examination and Pulmonary function tests (PFT) | |
| Noncorrectable severe coagulation disorders | |
| Contraindications at the assumption of bleomycin | |
| Previous adverse reactions to bleomycin | |
| Cumulative dose of ≥250 mg/m2 of bleomycin | |
| Pregnancy or lactation |
| Visit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Month −1 (up to 1 Month Prior the Treatment) | Month 0 | Month 1 ± 1 Week | Month 2 ± 1 Week | Month 4 ± 1 Week | Month 6 ± 1 Week | Month 8 ± 1 Week | Month 10 ± 1 Week | Month 12 ± 1 Week | ||
| Visit Description | Restaging, Enrollment, Randomization | Day of Treatment Control Group: Cetuximab+Platinum+5- Fluorouracil Experimental Group: Electrochemotherapy | Post-Treatment Evaluation (Discharge Day ± 1 Week) | Follow-Up Visit | Follow-Up Visit Cut-Off Time to Evaluate Treatment Response | Follow-Up Visit | Follow-Up Visit | Follow-Up Visit | Follow-Up Visit | Follow-Up Visit | |
| Type of Assessment | |||||||||||
| Clinical evaluation | X | X | X | X | X | X | X | X | X | ||
| Duration of hospitalization | X | ||||||||||
| CT, MRI or PET-CT | X | Only estimation of Lesion size | X | X | X | ||||||
| Identification of the target lesion | X | X | X | ||||||||
| Photographic documentation | X | X | X | X | X | X | X | X | X | ||
| EORTC QLQ-C30, EORTC QLQ-H&N35, EQ-5D-5L questionnaires | X | X | X | X | X | X | X | X | X | ||
| Pain evaluation with VAS score | X | X | X | X | X | X | X | X | X | ||
| Blood samples as per normal clinical practice | X | X | X | X | X | X | X | X | X | ||
| CD8 and CD16 dosage | X | X | X | X | X | ||||||
| Recording of the drugs for pain control | X | X | X | X | X | X | X | ||||
| Recording of concomitant treatment | X | X | X | X | X | X | X | ||||
| ECOG status | X | X | X | X | X | X | X | X | |||
| Adverse Events/Complications | X | X | X | X | X | X | X | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzo, F.; Granata, V.; Fusco, R.; D’Alessio, V.; Petrillo, A.; Lastoria, S.; Piccirillo, M.; Albino, V.; Belli, A.; Nasti, G.; et al. A Multicenter Randomized Controlled Prospective Study to Assess Efficacy of Laparoscopic Electrochemotherapy in the Treatment of Locally Advanced Pancreatic Cancer. J. Clin. Med. 2021, 10, 4011. https://doi.org/10.3390/jcm10174011
Izzo F, Granata V, Fusco R, D’Alessio V, Petrillo A, Lastoria S, Piccirillo M, Albino V, Belli A, Nasti G, et al. A Multicenter Randomized Controlled Prospective Study to Assess Efficacy of Laparoscopic Electrochemotherapy in the Treatment of Locally Advanced Pancreatic Cancer. Journal of Clinical Medicine. 2021; 10(17):4011. https://doi.org/10.3390/jcm10174011
Chicago/Turabian StyleIzzo, Francesco, Vincenza Granata, Roberta Fusco, Valeria D’Alessio, Antonella Petrillo, Secondo Lastoria, Mauro Piccirillo, Vittorio Albino, Andrea Belli, Guglielmo Nasti, and et al. 2021. "A Multicenter Randomized Controlled Prospective Study to Assess Efficacy of Laparoscopic Electrochemotherapy in the Treatment of Locally Advanced Pancreatic Cancer" Journal of Clinical Medicine 10, no. 17: 4011. https://doi.org/10.3390/jcm10174011
APA StyleIzzo, F., Granata, V., Fusco, R., D’Alessio, V., Petrillo, A., Lastoria, S., Piccirillo, M., Albino, V., Belli, A., Nasti, G., Avallone, A., Patrone, R., Grassi, F., Leongito, M., & Palaia, R. (2021). A Multicenter Randomized Controlled Prospective Study to Assess Efficacy of Laparoscopic Electrochemotherapy in the Treatment of Locally Advanced Pancreatic Cancer. Journal of Clinical Medicine, 10(17), 4011. https://doi.org/10.3390/jcm10174011







