Genetic Factors of Non-Obstructive Azoospermia: Consequences on Patients’ and Offspring Health
Abstract
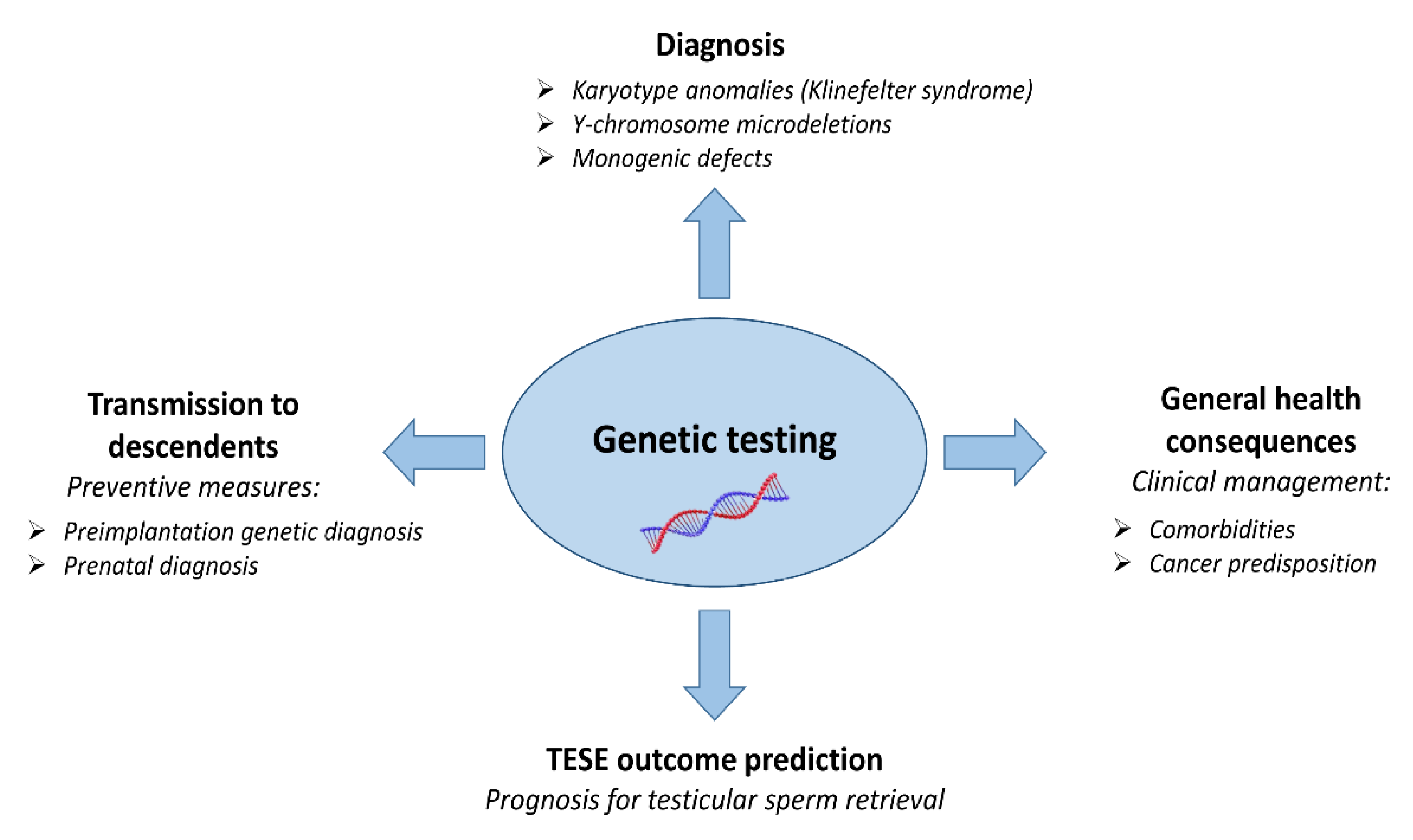
1. Introduction
2. Consequences of Chromosomal Anomalies
2.1. Klinefelter Syndrome (47,XXY)
2.2. 46,XX Testicular/ovo-Testicular Disorder of Sex Development (DSD)
3. Consequences of Y-Chromosome Microdeletions
4. Consequences of Monogenic Defects
4.1. AR Gene
4.2. TEX11 Gene
4.3. Shared Genes between Spermatogenesis and Tumorigenesis
4.3.1. Rare Pathogenic Mutations
4.3.2. Genetic Polymorphisms
5. Health Issues in ICSI Offspring from NOA Fathers
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tournaye, H.; Krausz, C.; Oates, R.D. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol. 2017, 5, 544–553. [Google Scholar] [CrossRef]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Cioppi, F.; Rosta, V.; Krausz, C. Genetics of Azoospermia. Int. J. Mol. Sci. 2021, 22, 3264. [Google Scholar] [CrossRef]
- Kasak, L.; Laan, M. Monogenic causes of non-obstructive azoospermia: Challenges, established knowledge, limitations and perspectives. Hum. Genet. 2021, 140, 135–154. [Google Scholar] [CrossRef]
- Capalbo, A.; Poli, M.; Riera-Escamilla, A.; Shukla, V.; Høffding, M.K.; Krausz, C.; Hoffmann, E.R.; Simon, C. Preconception genome medicine: Current state and future perspectives to improve infertility diagnosis and reproductive and health outcomes based on individual genomic data. Hum. Reprod. Update 2021, 27, 254–279. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.K.; Jacobsen, R.; Christensen, K.; Nielsen, N.C.; Bostofte, E. Good Semen Quality and Life Expectancy: A Cohort Study of 43,277 Men. Am. J. Epidemiol. 2009, 170, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Matloob, R.; Gallina, A.; Abdollah, F.; Saccà, A.; Briganti, A.; Suardi, N.; Colombo, R.; Rocchini, L.; Guazzoni, G.; et al. Are Infertile Men Less Healthy than Fertile Men? Results of a Prospective Case-Control Survey. Eur. Urol. 2009, 56, 1025–1032. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Betts, P.; Herder, D.; Lamb, D.J.; Lipshultz, L.I. Increased risk of cancer among azoospermic men. Fertil. Steril. 2013, 100, 681–685.e1. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Li, S.; Behr, B.; Cullen, M.R.; Galusha, D.; Lamb, D.J.; Lipshultz, L.I. Semen quality, infertility and mortality in the USA. Hum. Reprod. 2014, 29, 1567–1574. [Google Scholar] [CrossRef]
- Ventimiglia, E.; Capogrosso, P.; Boeri, L.; Serino, A.; Colicchia, M.; Ippolito, S.; Scano, R.; Papaleo, E.; Damiano, R.; Montorsi, F.; et al. Infertility as a proxy of general male health: Results of a cross-sectional survey. Fertil. Steril. 2015, 104, 48–55. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Shufeng, L.; Cullen, M.R.; Baker, L.C. Increased risk of incident chronic medical conditions in infertile men: Analysis of United States claims data. Fertil. Steril. 2016, 105, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.T.; Eisenberg, M.L. Male infertility as a window to health. Fertil. Steril. 2018, 110, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Glazer, C.H.; Eisenberg, M.L.; Tøttenborg, S.S.; Giwercman, A.; Flachs, E.M.; Bräuner, E.V.; Vassard, D.; Pinborg, A.; Schmidt, L.; Bonde, J.P. Male factor infertility and risk of death: A nationwide record-linkage study. Hum. Reprod. 2019, 34, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F.; Kasman, A.M.; Li, S.; Belladelli, F.; Ferro, M.; de Cobelli, O.; De Bernardinis, E.; Busetto, G.M.; Eisenberg, M.L. Increased Mortality among Men Diagnosed with Impaired Fertility: Analysis of US Claims Data. Urology 2021, 147, 143–149. [Google Scholar] [CrossRef]
- Bobjer, J.; Naumovska, M.; Giwercman, Y.L.; Giwercman, A. High prevalence of androgen deficiency and abnormal lipid profile in infertile men with non-obstructive azoospermia. Int. J. Androl. 2012, 35, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Garolla, A.; Ghezzi, M.; Selice, R.; Pelago, P.; Caretta, N.; Di Mambro, A.; Valente, U.; De Rocco Ponce, M.; Dipresa, S.; et al. Sperm Count and Hypogonadism as Markers of General Male Health. Eur. Urol. Focus 2021, 7, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Rastrelli, G.; Vignozzi, L.; Mannucci, E.; Maggi, M. Testosterone, cardiovascular disease and the metabolic syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 337–353. [Google Scholar] [CrossRef]
- Baillargeon, J.; Al Snih, S.; Raji, M.A.; Urban, R.J.; Sharma, G.; Sheffield-Moore, M.; Lopez, D.S.; Baillargeon, G.; Kuo, Y.F. Hypogonadism and the risk of rheumatic autoimmune disease. Clin. Rheumatol. 2016, 35, 2983–2987. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Li, S.; Brooks, J.D.; Cullen, M.R.; Baker, L.C. Increased Risk of Cancer in Infertile Men: Analysis of U.S. Claims Data. J. Urol. 2015, 193, 1596–1601. [Google Scholar] [CrossRef]
- Del Giudice, F.; Kasman, A.M.; Ferro, M.; Sciarra, A.; De Bernardinis, E.; Belladelli, F.; Salonia, A.; Eisenberg, M.L. Clinical correlation among male infertility and overall male health: A systematic review of the literature. Investig. Clin. Urol. 2020, 61, 355–371. [Google Scholar] [CrossRef]
- Nagirnaja, L.; Aston, K.I.; Conrad, D.F. Genetic intersection of male infertility and cancer. Fertil. Steril. 2018, 109, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Vloeberghs, V.; Verheyen, G.; Santos-Ribeiro, S.; Staessen, C.; Verpoest, W.; Gies, I.; Tournaye, H. Is genetic fatherhood within reach for all azoospermic Klinefelter men? PLoS ONE 2018, 13, e0200300. [Google Scholar] [CrossRef]
- Zitzmann, M.; Aksglaede, L.; Corona, G.; Isidori, A.M.; Juul, A.; T’Sjoen, G.; Kliesch, S.; D’Hauwers, K.; Toppari, J.; Słowikowska-Hilczer, J.; et al. European academy of andrology guidelines on Klinefelter Syndrome Endorsing Organization: European Society of Endocrinology. Andrology 2021, 9, 145–167. [Google Scholar] [CrossRef]
- Visootsak, J.; Graham, J.M. Klinefelter syndrome and other sex chromosomal aneuploidies. Orphanet J. Rare Dis. 2006, 1, 42. [Google Scholar] [CrossRef] [PubMed]
- Gravholt, C.H.; Chang, S.; Wallentin, M.; Fedder, J.; Moore, P.; Skakkebæk, A. Klinefelter syndrome: Integrating genetics, neuropsychology, and endocrinology. Endocr. Rev. 2018, 39, 389–423. [Google Scholar] [CrossRef]
- Corona, G.; Pizzocaro, A.; Lanfranco, F.; Garolla, A.; Pelliccione, F.; Vignozzi, L.; Ferlin, A.; Foresta, C.; Jannini, E.A.; Maggi, M.; et al. Sperm recovery and ICSI outcomes in Klinefelter syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2017, 23, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Rohayem, J.; Nieschlag, E.; Zitzmann, M.; Kliesch, S. Testicular function during puberty and young adulthood in patients with Klinefelter’s syndrome with and without spermatozoa in seminal fluid. Andrology 2016, 4, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Franik, S.; Hoeijmakers, Y.; D’Hauwers, K.; Braat, D.D.; Nelen, W.L.; Smeets, D.; Claahsen-van der Grinten, H.L.; Ramos, L.; Fleischer, K. Klinefelter syndrome and fertility: Sperm preservation should not be offered to children with Klinefelter syndrome. Hum. Reprod. 2016, 31, 1952–1959. [Google Scholar] [CrossRef]
- Seminog, O.O.; Seminog, A.B.; Yeates, D.; Goldacre, M.J. Associations between Klinefelter’s syndrome and autoimmune diseases: English national record linkage studies. Autoimmunity 2015, 48, 125–128. [Google Scholar] [CrossRef]
- Panimolle, F.; Tiberti, C.; Granato, S.; Semeraro, A.; Gianfrilli, D.; Anzuini, A.; Lenzi, A.; Radicioni, A. Screening of endocrine organ-specific humoral autoimmunity in 47,XXY Klinefelter’s syndrome reveals a significant increase in diabetes-specific immunoreactivity in comparison with healthy control men. Endocrine 2016, 52, 157–164. [Google Scholar] [CrossRef]
- Nieschlag, E.; Ferlin, A.; Gravholt, C.H.; Gromoll, J.; Köhler, B.; Lejeune, H.; Rogol, A.D.; Wistuba, J. The Klinefelter syndrome: Current management and research challenges. Andrology 2016, 4, 545–549. [Google Scholar] [CrossRef]
- Greco, E.; Scarselli, F.; Minasi, M.G.; Casciani, V.; Zavaglia, D.; Dente, D.; Tesarik, J.; Franco, G. Birth of 16 healthy children after ICSI in cases of nonmosaic Klinefelter syndrome. Hum. Reprod. 2013, 28, 1155–1160. [Google Scholar] [CrossRef]
- Denschlag, D.; Tempfer, C.; Kunze, M.; Wolff, G.; Keck, C. Assisted reproductive techniques in patients with Klinefelter syndrome: A critical review. Fertil. Steril. 2004, 82, 775–779. [Google Scholar] [CrossRef]
- Fullerton, G.; Hamilton, M.; Maheshwari, A. Should non-mosaic Klinefelter syndrome men be labelled as infertile in 2009? Hum. Reprod. 2010, 25, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Brilli, S.; Forti, G. Managing infertility in patients with Klinefelter syndrome. Expert Rev. Endocrinol. Metab. 2014, 9, 239–250. [Google Scholar] [CrossRef]
- McElreavey, K.; Vilain, E.; Abbas, N.; Herskowitz, I.; Fellous, M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc. Natl. Acad. Sci. USA 1993, 90, 3368–3372. [Google Scholar] [CrossRef]
- Vorona, E.; Zitzmann, M.; Gromoll, J.; Schüring, A.N.; Nieschlag, E. Clinical, endocrinological, and epigenetic features of the 46,XX male syndrome, compared with 47,XXY Klinefelter patients. J. Clin. Endocrinol. Metab. 2007, 92, 3458–3465. [Google Scholar] [CrossRef]
- Kousta, E.; Papathanasiou, A.; Skordis, N. Sex determination and disorders of sex development according to the revised nomenclature and classification in 46,XX individuals. Hormones 2010, 9, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Hoefsloot, L.; Simoni, M.; Tüttelmann, F.; European Academy of Andrology. European Molecular Genetics Quality Network EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions: State-of-the-art 2013. Andrology 2014, 2, 5–19. [Google Scholar] [CrossRef]
- Tiepolo, L.; Zuffardi, O. Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum. Genet. 1976, 34, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Vogt, P.H.; Edelmann, A.; Kirsch, S.; Henegariu, O.; Hirschmann, P.; Kiesewetter, F.; Köhn, F.M.; Schill, W.B.; Farah, S.; Ramos, C.; et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum. Mol. Genet. 1996, 5, 933–943. [Google Scholar] [CrossRef]
- Skaletsky, H.; Kuroda-Kawaguchi, T.; Minx, P.J.; Cordum, H.S.; Hillier, L.; Brown, L.G.; Repping, S.; Pyntikova, T.; Ali, J.; Bieri, T.; et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003, 423, 825–837. [Google Scholar] [CrossRef]
- Lo Giacco, D.; Chianese, C.; Sánchez-Curbelo, J.; Bassas, L.; Ruiz, P.; Rajmil, O.; Sarquella, J.; Vives, A.; Ruiz-Castañé, E.; Oliva, R.; et al. Clinical relevance of Y-linked CNV screening in male infertility: New insights based on the 8-year experience of a diagnostic genetic laboratory. Eur. J. Hum. Genet. 2014, 22, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.G.; Marszalek, J.D.; Irenze, K.; Skaletsky, H.; Brown, L.G.; Oates, R.D.; Silber, S.J.; Ardlie, K.; Page, D.C. AZFc Deletions and Spermatogenic Failure: A Population-Based Survey of 20,000 Y Chromosomes. Am. J. Hum. Genet. 2012, 91, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Casamonti, E. Spermatogenic failure and the Y chromosome. Hum. Genet. 2017, 136, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Stouffs, K.; Vloeberghs, V.; Gheldof, A.; Tournaye, H.; Seneca, S. Are AZFb deletions always incompatible with sperm production? Andrology 2017, 5, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Jorgez, C.J.; Weedin, J.W.; Sahin, A.; Tannour-Louet, M.; Han, S.; Bournat, J.C.; Mielnik, A.; Cheung, S.W.; Nangia, A.K.; Schlegel, P.N.; et al. Aberrations in pseudoautosomal regions (PARs) found in infertile men with Y-chromosome microdeletions. J. Clin. Endocrinol. Metab. 2011, 96, E674–E679. [Google Scholar] [CrossRef] [PubMed]
- Chianese, C.; Lo Giacco, D.; Tüttelmann, F.; Ferlin, A.; Ntostis, P.; Vinci, S.; Balercia, G.; Ars, E.; Ruiz-Castañé, E.; Giglio, S.; et al. Y-chromosome microdeletions are not associated with SHOX haploinsufficiency. Hum. Reprod. 2013, 28, 3155–3160. [Google Scholar] [CrossRef]
- Castro, A.; Rodríguez, F.; Flórez, M.; López, P.; Curotto, B.; Martínez, D.; Maturana, A.; Lardone, M.C.; Palma, C.; Mericq, V.; et al. Pseudoautosomal abnormalities in terminal AZFb+c deletions are associated with isochromosomes Yp and may lead to abnormal growth and neuropsychiatric function. Hum. Reprod. 2017, 32, 465–475. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Sun, Y.; Han, J.; Ma, H.; Sun, Y. Effect of Y Chromosome Microdeletions on the Pregnancy Outcome of Assisted Reproduction Technology: A Meta-analysis. Reprod. Sci. 2021, 28, 2413–2421. [Google Scholar] [CrossRef]
- Siffroi, J.P.; Le Bourhis, C.; Krausz, C.; Barbaux, S.; Quintana-Murci, L.; Kanafani, S.; Rouba, H.; Bujan, L.; Bourrouillou, G.; Seifer, I.; et al. Sex chromosome mosaicism in males carrying Y chromosome long arm deletions. Hum. Reprod. 2000, 15, 2559–2562. [Google Scholar] [CrossRef]
- Jaruzelska, J.; Korcz, A.; Wojda, A.; Jedrzejczak, P.; Bierla, J.; Surmacz, T.; Pawelczyk, L.; Page, D.C.; Kotecki, M. Mosaicism for 45,X cell line may accentuate the severity of spermatogenic defects in men with AZFc deletion. J. Med. Genet. 2001, 38, 798–802. [Google Scholar] [CrossRef]
- Papadimas, J.; Goulis, D.G.; Giannouli, C.; Papanicolaou, A.; Tarlatzis, B.; Bontis, J.N. Ambiguous genitalia, 45,X/46,XY mosaic karyotype, and Y chromosome microdeletions in a 17-year-old man. Fertil. Steril. 2001, 76, 1261–1263. [Google Scholar] [CrossRef]
- Papanikolaou, A.D.; Goulis, D.G.; Giannouli, C.; Gounioti, C.; Bontis, J.N.; Papadimas, J. Intratubular germ cell neoplasia in a man with ambiguous genitalia, 45,X/46,XY mosaic karyotype, and Y chromosome microdeletions. Endocr. Pathol. 2003, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Patsalis, P.C.; Sismani, C.; Quintana-Murci, L.; Taleb-Bekkouche, F.; Krausz, C.; McElreavey, K. Effects of transmission of Y chromosome AZFc deletions. Lancet 2002, 360, 1222–1224. [Google Scholar] [CrossRef]
- Patsalis, P.C.; Skordis, N.; Sismani, C.; Kousoulidou, L.; Koumbaris, G.; Eftychi, C.; Stavrides, G.; Ioulianos, A.; Kitsiou-Tzeli, S.; Galla-Voumvouraki, A.; et al. Identification of high frequency of Y chromosome deletions in patients with sex chromosome mosaicism and correlation with the clinical phenotype and Y-chromosome instability. Am. J. Med. Genet. 2005, 135, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Mateu, E.; Rodrigo, L.; Martínez, M.C.; Peinado, V.; Milán, M.; Gil-Salom, M.; Martínez-Jabaloyas, J.M.; Remohí, J.; Pellicer, A.; Rubio, C. Aneuploidies in embryos and spermatozoa from patients with Y chromosome microdeletions. Fertil. Steril. 2010, 94, 2874–2877. [Google Scholar] [CrossRef] [PubMed]
- Stouffs, K.; Lissens, W.; Tournaye, H.; Van Steirteghem, A.; Liebaers, I. The choice and outcome of the fertility treatment of 38 couples in whom the male partner has a Yq microdeletion. Hum. Reprod. 2005, 20, 1887–1896. [Google Scholar] [CrossRef]
- Golin, A.P.; Yuen, W.; Flannigan, R. The effects of Y chromosome microdeletions on in vitro fertilization outcomes, health abnormalities in offspring and recurrent pregnancy loss. Transl. Androl. Urol. 2021, 10, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Francomano, D.; Greco, E.A.; Lenzi, A.; Aversa, A. CAG repeat testing of androgen receptor polymorphism: Is this necessary for the best clinical management of hypogonadism? J. Sex. Med. 2013, 10, 2373–2381. [Google Scholar] [CrossRef]
- Krausz, C.; Cioppi, F.; Riera-Escamilla, A. Testing for genetic contributions to infertility: Potential clinical impact. Expert Rev. Mol. Diagn. 2018, 18, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, P.; Bogaert, V.; De Bacquer, D.; Goemaere, S.; Zmierczak, H.; Kaufman, J.M. Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: Contribution of the androgen receptor polyglutamine tract polymorphism. J. Clin. Endocrinol. Metab. 2007, 92, 3604–3610. [Google Scholar] [CrossRef] [PubMed]
- Davis-Dao, C.A.; Tuazon, E.D.; Sokol, R.Z.; Cortessis, V.K. Male Infertility and Variation in CAG Repeat Length in the Androgen Receptor Gene: A Meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 4319–4326. [Google Scholar] [CrossRef]
- Zitzmann, M.; Brune, M.; Kornmann, B.; Gromoll, J.; von Eckardstein, S.; von Eckardstein, A.; Nieschlag, E. The CAG repeat polymorphism in the AR gene affects high density lipoprotein cholesterol and arterial vasoreactivity. J. Clin. Endocrinol. Metab. 2001, 86, 4867–4873. [Google Scholar] [CrossRef]
- Schneider, G.; Nienhaus, K.; Gromoll, J.; Heuft, G.; Nieschlag, E.; Zitzmann, M. Sex hormone levels, genetic androgen receptor polymorphism, and anxiety in ≥50-year-old males. J. Sex. Med. 2011, 8, 3452–3464. [Google Scholar] [CrossRef]
- Mitsumori, K.; Terai, A.; Oka, H.; Segawa, T.; Ogura, K.; Yoshida, O.; Ogawa, O. Androgen Receptor CAG Repeat Length Polymorphism in Benign Prostatic Hyperplasia (BPH): Correlation with Adenoma Growth. Prostate 1999, 41, 253–257. [Google Scholar] [CrossRef]
- Zitzmann, M.; Depenbusch, M.; Gromoll, J.; Nieschlag, E. Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: A longitudinal pharmacogenetic study. J. Clin. Endocrinol. Metab. 2003, 88, 2049–2054. [Google Scholar] [CrossRef][Green Version]
- La Spada, A.R.; Wilson, E.M.; Lubahn, D.B.; Harding, A.E.; Fischbeck, K.H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 1991, 352, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Gelmann, E.P. Molecular biology of the androgen receptor. J. Clin. Oncol. 2002, 20, 3001–3015. [Google Scholar] [CrossRef]
- Sobue, G.; Doyu, M.; Morishima, T.; Mukai, E.; Yasuda, T.; Kachi, T.; Mitsuma, T. Aberrant androgen action and increased size of tandem CAG repeat in androgen receptor gene in X-linked recessive bulbospinal neuronopathy. J. Neurol. Sci. 1994, 121, 167–171. [Google Scholar] [CrossRef]
- Paulson, H. Repeat expansion diseases. Handb. Clin. Neurol. 2018, 147, 105. [Google Scholar] [CrossRef] [PubMed]
- Yatsenko, A.N.; Georgiadis, A.P.; Röpke, A.; Berman, A.J.; Jaffe, T.; Olszewska, M.; Westernströer, B.; Sanfilippo, J.; Kurpisz, M.; Rajkovic, A.; et al. X-linked TEX11 mutations, meiotic arrest, and azoospermia in infertile men. N. Engl. J. Med. 2015, 372, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Silber, S.; Leu, N.A.; Oates, R.D.; Marszalek, J.D.; Skaletsky, H.; Brown, L.G.; Rozen, S.; Page, D.C.; Wang, P.J. TEX11 is mutated in infertile men with azoospermia and regulates genome-wide recombination rates in mouse. EMBO Mol. Med. 2015, 7, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Zheng, L.; Ji, Z.; Mei, L.; Ding, L.; Lin, S.; Wang, X.; Yang, X.; Li, P. A novel TEX11 mutation induces azoospermia: A case report of infertile brothers and literature review. BMC Med. Genet. 2018, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Riera-Escamilla, A.; Moreno-Mendoza, D.; Holleman, K.; Cioppi, F.; Algaba, F.; Pybus, M.; Friedrich, C.; Wyrwoll, M.J.; Casamonti, E.; et al. Genetic dissection of spermatogenic arrest through exome analysis: Clinical implications for the management of azoospermic men. Genet. Med. 2020, 22, 1956–1966. [Google Scholar] [CrossRef]
- Chalmel, F.; Lardenois, A.; Primig, M. Toward understanding the core meiotic transcriptome in mammals and its implications for somatic cancer. Ann. N. Y. Acad. Sci. 2007, 1120, 1–15. [Google Scholar] [CrossRef]
- Hanson, H.A.; Anderson, R.E.; Aston, K.I.; Carrell, D.T.; Smith, K.R.; Hotaling, J.M. Subfertility increases risk of testicular cancer: Evidence from population-based semen samples. Fertil. Steril. 2016, 105, 322–328. [Google Scholar] [CrossRef]
- Kasak, L.; Punab, M.; Nagirnaja, L.; Grigorova, M.; Minajeva, A.; Lopes, A.M.; Punab, A.M.; Aston, K.I.; Carvalho, F.; Laasik, E.; et al. Bi-allelic Recessive Loss-of-Function Variants in FANCM Cause Non-obstructive Azoospermia. Am. J. Hum. Genet. 2018, 103, 200–212. [Google Scholar] [CrossRef]
- Krausz, C.; Riera-Escamilla, A.; Chianese, C.; Moreno-Mendoza, D.; Ars, E.; Rajmil, O.; Pujol, R.; Bogliolo, M.; Blanco, I.; Rodríguez, I.; et al. From exome analysis in idiopathic azoospermia to the identification of a high-risk subgroup for occult Fanconi anemia. Genet. Med. 2019, 21, 189–194. [Google Scholar] [CrossRef]
- Yin, H.; Ma, H.; Hussain, S.; Zhang, H.; Xie, X.; Jiang, L.; Jiang, X.; Iqbal, F.; Bukhari, I.; Jiang, H.; et al. A homozygous FANCM frameshift pathogenic variant causes male infertility. Genet. Med. 2019, 21, 62–70. [Google Scholar] [CrossRef]
- Liu, C.C.; Tseng, Y.T.; Li, W.; Wu, C.Y.; Mayzus, I.; Rzhetsky, A.; Sun, F.; Waterman, M.; Chen, J.J.; Chaudhary, P.M.; et al. DiseaseConnect: A comprehensive web server for mechanism-based disease-disease connections. Nucleic Acids Res. 2014, 42, W137–W146. [Google Scholar] [CrossRef]
- Tarín, J.J.; García-Pérez, M.A.; Hamatani, T.; Cano, A. Infertility etiologies are genetically and clinically linked with other diseases in single meta-diseases. Reprod. Biol. Endocrinol. 2015, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Ceccaldi, R.; Sarangi, P.; D’Andrea, A.D. The Fanconi anaemia pathway: New players and new functions. Nat. Rev. Mol. Cell Biol. 2016, 17, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Nepal, M.; Che, R.; Zhang, J.; Ma, C.; Fei, P. Fanconi Anemia Signaling and Cancer. Trends Cancer 2017, 3, 840–856. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, J.; Dai, L.; Zhu, Y.; Hu, H.; Tan, L.; Chen, W.; Liang, D.; He, J.; Tu, M.; et al. XRCC2 mutation causes meiotic arrest, azoospermia and infertility. J. Med. Genet. 2018, 55, 628–636. [Google Scholar] [CrossRef]
- Kuznetsov, S.; Pellegrini, M.; Shuda, K.; Fernandez-Capetillo, O.; Liu, Y.; Martin, B.K.; Burkett, S.; Southon, E.; Pati, D.; Tessarollo, L.; et al. RAD51C deficiency in mice results in early prophase I arrest in males and sister chromatid separation at metaphase II in females. J. Cell Biol. 2007, 176, 581–592. [Google Scholar] [CrossRef]
- Cavaillé, M.; Uhrhammer, N.; Privat, M.; Ponelle-Chachuat, F.; Gay-Bellile, M.; Lepage, M.; Molnar, I.; Viala, S.; Bidet, Y.; Bignon, Y.J. Analysis of 11 candidate genes in 849 adult patients with suspected hereditary cancer predisposition. Genes. Chromosomes Cancer 2021, 60, 73–78. [Google Scholar] [CrossRef]
- Bakker, S.T.; van de Vrugt, H.J.; Rooimans, M.A.; Oostra, A.B.; Steltenpool, J.; Delzenne-Goette, E.; van der Wal, A.; van der Valk, M.; Joenje, H.; te Riele, H.; et al. Fancm-deficient mice reveal unique features of Fanconi anemia complementation group M. Hum. Mol. Genet. 2009, 18, 3484–3495. [Google Scholar] [CrossRef]
- Luo, Y.; Hartford, S.A.; Zeng, R.; Southard, T.L.; Shima, N.; Schimenti, J.C. Hypersensitivity of Primordial Germ Cells to Compromised Replication-Associated DNA Repair Involves ATM-p53-p21 Signaling. PLoS Genet. 2014, 10, e1004730. [Google Scholar] [CrossRef]
- Tenenbaum-Rakover, Y.; Weinberg-Shukron, A.; Renbaum, P.; Lobel, O.; Eideh, H.; Gulsuner, S.; Dahary, D.; Abu-Rayyan, A.; Kanaan, M.; Levy-Lahad, E.; et al. Minichromosome maintenance complex component 8 (MCM8) gene mutations result in primary gonadal failure. J. Med. Genet. 2015, 52, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; He, X.J.; Du, W.D.; Chen, G.; Zhou, Y.; Xu, S.; Zuo, X.B.; Fang, L.B.; Cao, Y.X.; Zhang, X.J. Genetic variants in TEX15 gene conferred susceptibility to spermatogenic failure in the Chinese Han population. Reprod. Sci. 2012, 19, 1190–1196. [Google Scholar] [CrossRef]
- Okutman, O.; Muller, J.; Baert, Y.; Serdarogullari, M.; Gultomruk, M.; Piton, A.; Rombaut, C.; Benkhalifa, M.; Teletin, M.; Skory, V.; et al. Exome sequencing reveals a nonsense mutation in TEX15 causing spermatogenic failure in a Turkish family. Hum. Mol. Genet. 2015, 24, 5581–5588. [Google Scholar] [CrossRef]
- Colombo, R.; Pontoglio, A.; Bini, M. Two novel TEX15 mutations in a family with nonobstructive azoospermia. Gynecol. Obstet. Investig. 2017, 82, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Golubicki, M.; Bonjoch, L.; Acuña-Ochoa, J.G.; Díaz-Gay, M.; Muñoz, J.; Cuatrecasas, M.; Ocaña, T.; Iseas, S.; Mendez, G.; Cisterna, D.; et al. Germline biallelic Mcm8 variants are associated with early-onset Lynch-like syndrome. JCI Insight 2020, 5, e140698. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, Z.; Gao, P.; Gao, Z.; Chen, H.; Qi, J.; Liu, F.; Ye, D.; Jiang, H.; Na, R.; et al. TEX15: A DNA repair gene associated with prostate cancer risk in Han Chinese. Prostate 2017, 77, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.; Jordan, P. Emerging roles for WNK kinases in cancer. Cell. Mol. Life Sci. 2010, 67, 1265–1276. [Google Scholar] [CrossRef]
- Fakhro, K.A.; Elbardisi, H.; Arafa, M.; Robay, A.; Rodriguez-Flores, J.L.; Al-Shakaki, A.; Syed, N.; Mezey, J.G.; Abi Khalil, C.; Malek, J.A.; et al. Point-of-care whole-exome sequencing of idiopathic male infertility. Genet. Med. 2018, 20, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xing, B.; Liu, W.; Li, J.; Wang, X.; Li, J.; Yang, J.; Ji, C.; Li, Z.; Dong, B.; et al. Molecularly annotation of mouse avatar models derived from patients with colorectal cancer liver metastasis. Theranostics 2019, 9, 3485–3500. [Google Scholar] [CrossRef] [PubMed]
- Gallinger, S.; Aronson, M.; Shayan, K.; Ratcliffe, E.M.; Gerstle, J.T.; Parkin, P.C.; Rothenmund, H.; Croitoru, M.; Baumann, E.; Durie, P.R.; et al. Gastrointestinal cancers and neurofibromatosis type 1 features in children with a germline homozygous MLH1 mutation. Gastroenterology 2004, 126, 576–585. [Google Scholar] [CrossRef]
- Alotaibi, H.; Ricciardone, M.D.; Ozturk, M. Homozygosity at variant MLH1 can lead to secondary mutation in NF1, neurofibromatosis type I and early onset leukemia. Mutat. Res. 2008, 637, 209–214. [Google Scholar] [CrossRef]
- Ji, G.; Long, Y.; Zhou, Y.; Huang, C.; Gu, A.; Wang, X. Common variants in mismatch repair genes associated with increased risk of sperm DNA damage and male infertility. BMC Med. 2012, 10, 49. [Google Scholar] [CrossRef]
- Duraturo, F.; Liccardo, R.; Izzo, P. Coexistence of MLH3 germline variants in colon cancer patients belonging to families with Lynch syndrome-associated brain tumors. J. Neurooncol. 2016, 129, 577–578. [Google Scholar] [CrossRef]
- Vymetalkova, V.; Pardini, B.; Rosa, F.; Di Gaetano, C.; Novotny, J.; Levy, M.; Buchler, T.; Slyskova, J.; Vodickova, L.; Naccarati, A.; et al. Variations in mismatch repair genes and colorectal cancer risk and clinical outcome. Mutagenesis 2014, 29, 259–265. [Google Scholar] [CrossRef][Green Version]
- Xu, K.; Lu, T.; Zhou, H.; Bai, L.; Xiang, Y. The role of MSH5 C85T and MLH3 C2531T polymorphisms in the risk of male infertility with azoospermia or severe oligozoospermia. Clin. Chim. Acta. 2010, 411, 49–52. [Google Scholar] [CrossRef]
- Saunders, E.J.; Dadaev, T.; Leongamornlert, D.A.; Al Olama, A.A.; Benlloch, S.; Giles, G.G.; Wiklund, F.; Gronberg, H.; Haiman, C.A.; Schleutker, J.; et al. Gene and pathway level analyses of germline DNA-repair gene variants and prostate cancer susceptibility using the iCOGS-genotyping array. Br. J. Cancer 2016, 114, 945–952. [Google Scholar] [CrossRef]
- Scarbrough, P.M.; Weber, R.P.; Iversen, E.S.; Brhane, Y.; Amos, C.I.; Kraft, P.; Hung, R.J.; Sellers, T.A.; Witte, J.S.; Pharoah, P.; et al. A Cross-Cancer Genetic Association Analysis of the DNA Repair and DNA Damage Signaling Pathways for Lung, Ovary, Prostate, Breast, and Colorectal Cancer. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 193–200. [Google Scholar] [CrossRef]
- Ni, B.; Lin, Y.; Sun, L.; Zhu, M.; Li, Z.; Wang, H.; Yu, J.; Guo, X.; Zuo, X.; Dong, J.; et al. Low-frequency germline variants across 6p22.2-6p21.33 are associated with non-obstructive azoospermia in Han Chinese men. Hum. Mol. Genet. 2015, 24, 5628–5636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramchander, N.C.; Ryan, N.A.; Crosbie, E.J.; Evans, D.J. Homozygous germ-line mutation of the PMS2 mismatch repair gene: A unique case report of constitutional mismatch repair deficiency (CMMRD). BMC Med. Genet. 2017, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Clendenning, M.; Senter, L.; Hampel, H.; Lagerstedt Robinson, K.; Sun, S.; Buchanan, D.; Walsh, M.D.; Nilbert, M.; Green, J.; Potter, J.; et al. A frame-shift mutation of PMS2 is a widespread cause of Lynch syndrome. J. Med. Genet. 2008, 45, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Swift, M. Mortality rates among carriers of ataxia-telangiectasia mutant alleles. Ann. Intern. Med. 2000, 133, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Pilié, P.G.; Johnson, A.M.; Hanson, K.L.; Dayno, M.E.; Kapron, A.L.; Stoffel, E.M.; Cooney, K.A. Germline genetic variants in men with prostate cancer and one or more additional cancers. Cancer 2017, 123, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, J.; Zhang, T.; Li, H.; Ni, Y. rs189037, a functional variant in ATM gene promoter, is associated with idiopathic nonobstructive azoospermia. Fertil. Steril. 2013, 100, 1536–1541. [Google Scholar] [CrossRef]
- Li, S.; Peng, Q.; Chen, Y.; You, J.; Chen, Z.; Deng, Y.; Lao, X.; Wu, H.; Qin, X.; Zeng, Z. DNA repair gene XRCC1 polymorphisms, smoking, and bladder cancer risk: A meta-analysis. PLoS ONE 2013, 8, e73448. [Google Scholar] [CrossRef]
- Gu, A.H.; Liang, J.; Lu, N.X.; Wu, B.; Xia, Y.K.; Lu, C.C.; Song, L.; Wang, S.L.; Wang, X.R. Association of XRCC1 gene polymorphisms with idiopathic azoospermia in a Chinese population. Asian J. Androl. 2007, 9, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.R.; Wang, X.F.; Zhou, D.X.; Zhang, J.; Huo, Y.W.; Tian, H. Association between XRCC1 single-nucleotide polymorphisms and infertility with idiopathic azoospermia in northern Chinese Han males. Reprod. Biomed. Online 2012, 25, 402–407. [Google Scholar] [CrossRef]
- Yi, L.; Xiao-Feng, H.; Yun-Tao, L.; Hao, L.; Ye, S.; Song-Tao, Q. Association between the XRCC1 Arg399Gln polymorphism and risk of cancer: Evidence from 297 case-control studies. PLoS ONE 2013, 8, e78071. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Bansal, S.K.; Sudhakar, D.V.; Neelabh; Chakraborty, A.; Trivedi, S.; Gupta, G.; Thangaraj, K.; Rajender, S.; Singh, K. SNPs in ERCC1, ERCC2, and XRCC1 genes of the DNA repair pathway and risk of male infertility in the Asian populations: Association study, meta-analysis, and trial sequential analysis. J. Assist. Reprod. Genet. 2019, 36, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Akbas, H.; Balkan, M.; Binici, M.; Gedik, A. The Possible Role of XRCC1 Gene Polymorphisms with Idiopathic Non-obstructive Azoospermia in Southeast Turkey. Urol. J. 2019, 16, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Gu, A.; Xia, Y.; Lu, C.; Liang, J.; Wang, S.; Ma, J.; Peng, Y.; Wang, X. ERCC1 and ERCC2 polymorphisms and risk of idiopathic azoospermia in a Chinese population. Reprod. Biomed. Online 2008, 17, 36–41. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, W.; Gao, L.; Xing, B. Association between ERCC1 C8092A and ERCC2 K751Q polymorphisms and risk of adult glioma: A meta-analysis. Tumour Biol. 2014, 35, 3211–3221. [Google Scholar] [CrossRef]
- Ding, Y.W.; Gao, X.; Ye, D.X.; Liu, W.; Wu, L.; Sun, H.Y. Association of ERCC1 polymorphisms (rs3212986 and rs11615) with the risk of head and neck carcinomas based on case-control studies. Clin. Transl. Oncol. 2015, 17, 710–719. [Google Scholar] [CrossRef]
- Guo, X.G.; Wang, Q.; Xia, Y.; Zheng, L. The C8092A polymorphism in the ERCC1 gene and breast carcinoma risk: A meta-analysis of case-control studies. Int. J. Clin. Exp. Med. 2015, 8, 3691–3699. [Google Scholar] [PubMed]
- Bonduelle, M.; Legein, J.; Derde, M.P.; Buysse, A.; Schietecatte, J.; Wisanto, A.; Devroey, P.; Van Steirteghem, A.; Liebaers, I. Comparative follow-up study of 130 children born after intracytoplasmic sperm injection and 130 children born after in-vitro fertilization. Hum. Reprod. 1995, 10, 3327–3331. [Google Scholar] [CrossRef] [PubMed]
- In’t Veld, P.; Brandenburg, H.; Verhoeff, A.; Dhont, M.; Los, F. Sex chromosomal abnormalities and intracytoplasmic sperm injection. Lancet 1995, 346, 773. [Google Scholar] [CrossRef]
- Tournaye, H.; Liu, J.; Nagy, Z.; Joris, H.; Wisanto, A.; Bonduelle, M.; Van der Elst, J.; Staessen, C.; Smitz, J.; Silber, S. Intracytoplasmic sperm injection (ICSI): The Brussels experience. Reprod. Fertil. Dev. 1995, 7, 269–279. [Google Scholar] [CrossRef]
- Foresta, C.; Rossato, M.; Garolla, A.; Ferlin, A. Male infertility and ICSI: Are there limits? Hum. Reprod. 1996, 11, 2347–2348. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.G.; Hoegerman, S.F.; Cuticchia, A.J.; Moon, S.Y.; Doncel, G.F.; Acosta, A.A.; Kearns, W.G. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum. Reprod. 1999, 14, 1266–1273. [Google Scholar] [CrossRef]
- Moosani, N.; Pattinson, H.A.; Carter, M.D.; Cox, D.M.; Rademaker, A.W.; Martin, R.H. Chromosomal analysis of sperm from men with idiopathic infertility using sperm karyotyping and fluorescence in situ hybridization. Fertil. Steril. 1995, 64, 811–817. [Google Scholar] [CrossRef]
- Esteves, S.C.; Roque, M.; Bedoschi, G.; Haahr, T.; Humaidan, P. Intracytoplasmic sperm injection for male infertility and consequences for offspring. Nat. Rev. Urol. 2018, 15, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Jiang, J.; Ding, C.; Dai, J.; Liu, Y.; Xia, Y.; Liu, J.; Hu, Z. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: A meta-analysis. Fertil. Steril. 2012, 97, 1331–1337.e4. [Google Scholar] [CrossRef]
- Lacamara, C.; Ortega, C.; Villa, S.; Pommer, R.; Schwarze, J.E. Are children born from singleton pregnancies conceived by ICSI at increased risk for congenital malformations when compared to children conceived naturally? A systematic review and meta-analysis. JBRA Assist. Reprod. 2017, 21, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Ferlin, A. Offspring conceived by intracytoplasmic sperm injection. Lancet 2001, 358, 1270. [Google Scholar] [CrossRef]
- Massaro, P.A.; MacLellan, D.L.; Anderson, P.A.; Romao, R.L. Does intracytoplasmic sperm injection pose an increased risk of genitourinary congenital malformations in offspring compared to in vitro fertilization? A systematic review and meta-analysis. J. Urol. 2015, 193, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Halliday, J. Outcomes for offspring of men having ICSI for male factor infertility. Asian J. Androl. 2012, 14, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, A.R.; Sevoyan, A.; Oswald, T.K.; Fernandez, R.C.; Davies, M.J.; Moore, V.M. Impact of male factor infertility on offspring health and development. Fertil. Steril. 2019, 111, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Nygren, K.G.; Iliadou, A.; Hultman, C.M.; Reichenberg, A. Autism and mental retardation among offspring born after in vitro fertilization. JAMA 2013, 310, 75–84. [Google Scholar] [CrossRef]
- Hvidtjørn, D.; Schieve, L.; Schendel, D.; Jacobsson, B.; Svaerke, C.; Thorsen, P. Cerebral palsy, autism spectrum disorders, and developmental delay in children born after assisted conception: A systematic review and meta-analysis. Arch. Pediatr. Adolesc. Med. 2009, 163, 72–83. [Google Scholar] [CrossRef]
- Bay, B.; Mortensen, E.L.; Hvidtjørn, D.; Kesmodel, U.S. Fertility treatment and risk of childhood and adolescent mental disorders: Register based cohort study. BMJ 2013, 347, f3978. [Google Scholar] [CrossRef] [PubMed]
- Kissin, D.M.; Zhang, Y.; Boulet, S.L.; Fountain, C.; Bearman, P.; Schieve, L.; Yeargin-Allsopp, M.; Jamieson, D.J. Association of assisted reproductive technology (ART) treatment and parental infertility diagnosis with autism in ART-conceived children. Hum. Reprod. 2015, 30, 454–465. [Google Scholar] [CrossRef]
- Sutcliffe, A.G.; Saunders, K.; McLachlan, R.; Taylor, B.; Edwards, P.; Grudzinskas, G.; Leiberman, B.; Thornton, S. A retrospective case-control study of developmental and other outcomes in a cohort of Australian children conceived by intracytoplasmic sperm injection compared with a similar group in the United Kingdom. Fertil. Steril. 2003, 79, 512–516. [Google Scholar] [CrossRef]
- Bonduelle, M.; Ponjaert, I.; Van Steirteghem, A.; Derde, M.P.; Devroey, P.; Liebaers, I. Developmental outcome at 2 years of age for children born after ICSI compared with children born after IVF. Hum. Reprod. 2003, 18, 342–350. [Google Scholar] [CrossRef]
- Wennerholm, U.B.; Bonduelle, M.; Sutcliffe, A.; Bergh, C.; Niklasson, A.; Tarlatzis, B.; Kai, C.M.; Peters, C.; Victorin Cederqvist, A.; Loft, A. Paternal sperm concentration and growth and cognitive development in children born with a gestational age more than 32 weeks after assisted reproductive therapy. Hum. Reprod. 2006, 21, 1514–1520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belva, F.; Henriet, S.; Liebaers, I.; Van Steirteghem, A.; Celestin-Westreich, S.; Bonduelle, M. Medical outcome of 8-year-old singleton ICSI children (born ≥32 weeks’ gestation) and a spontaneously conceived comparison group. Hum. Reprod. 2007, 22, 506–515. [Google Scholar] [CrossRef][Green Version]
- Belva, F.; Painter, R.; Bonduelle, M.; Roelants, M.; Devroey, P.; De Schepper, J. Are ICSI adolescents at risk for increased adiposity? Hum. Reprod. 2012, 27, 257–264. [Google Scholar] [CrossRef]
- Belva, F.; Roelants, M.; De Schepper, J.; Roseboom, T.J.; Bonduelle, M.; Devroey, P.; Painter, R.C. Blood pressure in ICSI-conceived adolescents. Hum. Reprod. 2012, 27, 3100–3108. [Google Scholar] [CrossRef]
- Belva, F.; Bonduelle, M.; Provyn, S.; Painter, R.C.; Tournaye, H.; Roelants, M.; De Schepper, J. Metabolic Syndrome and Its Components in Young Adults Conceived by ICSI. Int. J. Endocrinol. 2018, 2018, 8170518. [Google Scholar] [CrossRef] [PubMed]
- Kettner, L.O.; Matthiesen, N.B.; Ramlau-Hansen, C.H.; Kesmodel, U.S.; Bay, B.; Henriksen, T.B. Fertility treatment and childhood type 1 diabetes mellitus: A nationwide cohort study of 565,116 live births. Fertil. Steril. 2016, 106, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, B.; Eisemann, N.; Elsner, S.; Ludwig, A.K.; Katalinic, A.; Kixmüller, D.; Ludwig, M. Pubertal development and reproductive hormone levels of singleton ICSI offspring in adolescence: Results of a prospective controlled study. Hum. Reprod. 2020, 35, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, Y.; Neri, Q.V.; Takeuchi, T.; Schlegel, P.N.; Megid, W.A.; Kent-First, M.; Rosenwaks, Z.; Palermo, G.D. Y chromosome assessment and its implications for the development of ICSI children. Reprod. Biomed. Online 2004, 8, 307–318. [Google Scholar] [CrossRef]
- Palermo, G.D.; Neri, Q.V.; Takeuchi, T.; Squires, J.; Moy, F.; Rosenwaks, Z. Genetic and epigenetic characteristics of ICSI children. Reprod. Biomed. Online 2008, 17, 820–833. [Google Scholar] [CrossRef]
- Belva, F.; Bonduelle, M.; Roelants, M.; Michielsen, D.; Van Steirteghem, A.; Verheyen, G.; Tournaye, H. Semen quality of young adult ICSI offspring: The first results. Hum. Reprod. 2016, 31, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Giachini, C.; Lo Giacco, D.; Daguin, F.; Chianese, C.; Ars, E.; Ruiz-Castane, E.; Forti, G.; Rossi, E. High Resolution X Chromosome-Specific Array-CGH Detects New CNVs in Infertile Males. PLoS ONE 2012, 7, e44887. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.M.; Aston, K.I.; Thompson, E.; Carvalho, F.; Gonçalves, J.; Huang, N.; Matthiesen, R.; Noordam, M.J.; Quintela, I.; Ramu, A.; et al. Human Spermatogenic Failure Purges Deleterious Mutation Load from the Autosomes and Both Sex Chromosomes, including the Gene DMRT1. PLoS Genet. 2013, 9, e1003349. [Google Scholar] [CrossRef] [PubMed]
- Tüttelmann, F.; Simoni, M.; Kliesch, S.; Ledig, S.; Dworniczak, B.; Wieacker, P.; Röpke, A. Copy Number Variants in Patients with Severe Oligozoospermia and Sertoli-Cell-Only Syndrome. PLoS ONE 2011, 6, e19426. [Google Scholar] [CrossRef]
- Krausz, C. Editorial for the special issue on the molecular genetics of male infertility. Hum. Genet. 2021, 140, 1–5. [Google Scholar] [CrossRef]
| Increased Mortality Rate (HR) | Increased Morbidity Rate (Yes/No) | Reference |
|---|---|---|
| n.a. | Yes * | [8] |
| 2.29, 95% CI: 1.12–4.65 | n.a. | [9] |
| n.a. | Yes ** | [11] |
| 3.66, 95% CI: 2.18–6.16 | n.a. | [13] |
| 2.01, 95% CI: 1.60–2.53 | n.a. | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krausz, C.; Cioppi, F. Genetic Factors of Non-Obstructive Azoospermia: Consequences on Patients’ and Offspring Health. J. Clin. Med. 2021, 10, 4009. https://doi.org/10.3390/jcm10174009
Krausz C, Cioppi F. Genetic Factors of Non-Obstructive Azoospermia: Consequences on Patients’ and Offspring Health. Journal of Clinical Medicine. 2021; 10(17):4009. https://doi.org/10.3390/jcm10174009
Chicago/Turabian StyleKrausz, Csilla, and Francesca Cioppi. 2021. "Genetic Factors of Non-Obstructive Azoospermia: Consequences on Patients’ and Offspring Health" Journal of Clinical Medicine 10, no. 17: 4009. https://doi.org/10.3390/jcm10174009
APA StyleKrausz, C., & Cioppi, F. (2021). Genetic Factors of Non-Obstructive Azoospermia: Consequences on Patients’ and Offspring Health. Journal of Clinical Medicine, 10(17), 4009. https://doi.org/10.3390/jcm10174009






