Abstract
Fatigue is a complex and multifactorial phenomenon which is often neglected by clinicians. The aim of this review was to analyze the impact, determinants and management of fatigue in patients with Systemic Lupus Erythematosus (SLE). Fatigue is one of the most prevalent symptoms in SLE, reported by 67% to 90% of patients. It is also described as the most bothersome symptom, considering that it may impair key aspects of health-related quality of life, while also leading to employment disability. It is a multifactorial phenomenon involving psychological factors, pain, lifestyle factors such as reduced physical activity, whereas the contribution of disease activity remains controversial. The management of fatigue in patients with SLE should rely upon a person-centered approach, with targeted interventions. Some pharmacological treatments used to control disease activity have demonstrated beneficial effects upon fatigue and non-pharmacological therapies such as psychological interventions, pain reduction and lifestyle changes, and each of these should be incorporated into fatigue management in SLE.
1. Introduction
Fatigue is a universal symptom experienced by nearly everyone in the general population. However, we lack a consensual definition of fatigue. Fatigue can be described as a subjective unpleasant sensation of exhaustion with physical and mental components, which interferes with individuals’ ability to function at their normal capacity. Fatigue impairs quality of life, and may lead to irritability, inability to concentrate, and poor motivation [1,2]. In chronic conditions such as Systemic Lupus Erythematosus (SLE), but also in other autoimmune diseases such as Sjögren’s syndrome or systemic sclerosis, the experience of fatigue seems to differ from ‘everyday tiredness’, as being more frequent, unpredictable and typically unresolved by rest [3]. This symptom remains a complex, multidimensional and poorly understood concept, often neglected by clinicians who prefer to focus on objective manifestations. The aim of this review was to report upon the impact, determinants and management of fatigue in patients with SLE.
2. The Most Frequent and Disabling Symptom
Fatigue is recognized as one of the most prevalent symptoms in SLE, reported by 67% to 90% of patients, depending on the series [4]. In a 2020 survey analyzing the burden of SLE from the patients’ perspective in European countries [4], fatigue was described as the most common symptom (affecting 85.3% of the 4375 respondents) and the most bothersome symptom, which is consistent with previous studies. Fatigue is reported as severe in intensity in more than a third of SLE patients [4].
Fatigue may impair several key aspects of the patient’s quality of life, with repercussions on both physical and mental health. Indeed, SLE patients report that fatigue has a negative impact on emotions, cognition, work, activities of daily living, leisure activities, social activities and family activities. They describe physical impairment, with walking and exercising difficulties. Emotional consequences of fatigue, such as frustrations and stress due to being unable to accomplish tasks, sadness or loss of motivation, are also common [5,6,7,8,9]. Fatigue in SLE has a significantly negative impact on work ability and work productivity, as it can lead to limitations in workplace activities by affecting endurance, mobility, concentration, or interactions with employees and coworkers. Fatigue in SLE is also associated with a higher risk of absenteeism and unemployment [10]. Altogether, fatigue is an important determinant in the perception of SLE impact upon patients’ daily living, even for those in remission.
3. A Multifactorial Manifestation
Altogether, fatigue is a highly multifactorial manifestation (Figure 1), caused by a complex interplay between disease itself, psychosocial, behavioral and personal variables. A recent study from our group described 3 main clusters of fatigue in SLE patients: (1) the most frequent profile (67.5% of the patients) was represented by patients with moderate fatigue, low disease activity and low anxiety and depression; (2) a quarter of the patients had very high fatigue, high depression and anxiety but low disease activity; and (3) less than 10% of the patients had high levels of fatigue, with high disease activity, low anxiety and no depression [11]. This suggests that the mental health status is an important predictor of fatigue in SLE, that disease activity plays a weaker role in SLE fatigue, and that, most of the time, other factors contribute to fatigue in SLE.
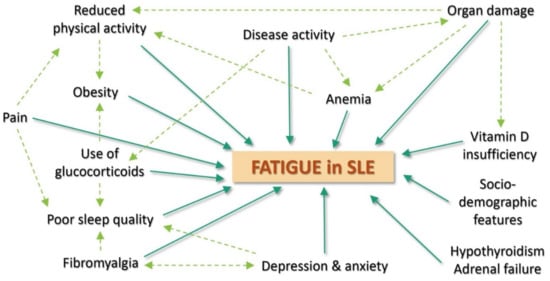
Figure 1.
Main determinants of fatigue in Systemic Lupus Erythematosus.
3.1. Lupus-Related Determinants
The association between fatigue and disease activity in SLE has been widely studied and debated for a long time, with controversial results [11,12,13,14,15,16,17,18,19]. Type I interferons, which are key cytokines in SLE, are associated with fatigue and may provide a clue towards a pathogenic explanation for fatigue in SLE. Disease activity seems to play a role in the genesis of fatigue but it cannot fully explain fatigue by itself. Indeed, a study based on an inception cohort of adult patients with SLE found that fatigue and disease activity followed distinct trajectories over 10 years [20]. Additionally, in our recent FATILUP studies [21], the association between fatigue and disease activity was significant, but weak (OR: 1.05 (95% CI: 1.00–1.12) per 1 point increase in SELENA-SLEDAI score). Therefore, it is likely that disease activity has a complex and potentially indirect contribution to fatigue, such as by influencing other major determinants of fatigue, for example pain and psychological factors. Some specific organ involvements such as neurological impairment and painful manifestations such as arthritis or oral ulcers have been found to be associated with fatigue in some studies [14,21,22]. Pain has been reported to have a specific role in SLE fatigue, and chronic pain treatment is essential to the management of fatigue in SLE [12,14,17,23]. Organ damage, especially renal or cardiac failure, can also be important causes of fatigue in SLE patients [24,25,26,27]. Furthermore, the use of glucocorticoid has been shown to be independently associated with fatigue in SLE [21].
3.2. Psychological Determinants
Mental health status, emotional and functional wellbeing have shown to be major determinants of fatigue in SLE patients. Depression and anxiety appear to be among the strongest predictors of fatigue in SLE patients [11,12,13,21,23,28,29]. There is a clear association between fatigue and depression in general, and scales assessing depression often include fatigue-related items. Depression affects both physical and mental dimensions of fatigue in SLE. Additionally, depression is frequent in SLE patients (between 17 and 75% of patients), and some authors have mentioned that SLE contributes to depression through its neurological involvement, an autoimmune effect, and the emotional consequences of pain and disability [30,31,32]. Stress, which is a subjective negative perception of life events, which may be influenced by sociological and psychological factors and SLE burden, seems to mediate the relationship between depression and fatigue over time in SLE patients. Decline in stress has been associated with a meaningful improvement in fatigue in SLE [31]. Sleep disorders have also been shown to be common and significant predictors of fatigue, occurring in more than half of SLE patients [28,29,33,34]. SLE may contribute to sleep disorders because of pain and inflammation, and steroid use has been associated with sleep disorders [35]. In addition, helplessness (a state in which a person remains passive in negative situations), coping disability (difficulties in facing problems in an adequate manner) and abnormal illness-related behavior have been associated, although not independently, with fatigue in SLE in some series [14,15,19]. The role of psychological determinants is therefore major in SLE fatigue. Consequently, it is crucial to suggest a thorough psychological assessment of SLE patients reporting severe fatigue, especially for those with no or low disease activity, since mood disorders are frequent in patients with SLE [4,21,30,31,32] and multifactorial.
3.3. Comorbidities
Fibromyalgia is a major predictor of fatigue in SLE [14,18,36]. In a study conducted by Touma et al., trajectories with higher fatigue scores were associated with a higher prevalence of fibromyalgia [20]. Fibromyalgia is common in SLE patients (from 6.2% to 30% of patients) but may be underdiagnosed by physicians [21,37]. Consequently, the role of fibromyalgia should be considered in SLE patients who complain about fatigue and widespread pain. Other frequent comorbidities such as anemia, hypothyroidism, or adrenal failure are risk factors in fatigue. Vitamin D insufficiency was associated with fatigue in SLE in some but not all studies [38,39]. SLE patients have a high risk of vitamin D deficiency because of photoprotection as well as in case of renal failure.
3.4. Behavioral and Socio-Demographic Features
Reduced levels of physical activity and aerobic capacity significantly increase fatigue in SLE. SLE patients have many actual and perceived barriers to exercise. It has been shown that, compared to sedentary controls, SLE patients have reduced levels of aerobic fitness, reduced exercise capacity and reduced muscle strength, which further leads to a reduced ability to perform physical activity. Furthermore, SLE patients are limited by arthralgia, anemia, and other SLE organ involvements. For all of those reasons, SLE patients often have limited physical activity and assume a sedentary lifestyle [40,41,42]. Obesity and smoking are other potential behavioral determinants of fatigue in this population [14,43]. The role of sociodemographic features is contradictory, but some studies found higher levels of fatigue in SLE patients with low annual income, low education level, or difficulty in accessing health care [14,15]. In some studies, a low level of perceived social support was also associated with fatigue [12].
4. Interventions to Improve Fatigue
Recently, an increasing number of interventional studies focused on fatigue in SLE, and some pharmacologic and non-pharmacologic therapies have demonstrated beneficial effects on fatigue. Improving disease activity is associated with significant reduction in fatigue in randomized controlled trials of belimumab, blisibimod, and hydroxychloroquine [44,45,46]. This effect is likely to be observed with any treatment improving disease activity in SLE, although this has not been formally proven. N-acetyl-cysteine has also been shown to improve fatigue in SLE. A double-blind, placebo controlled, randomized trial found that a 2.4 g/day dose of N-acetyl-cysteine is effective for reducing fatigue and improving disease activity, and is safe and well-tolerated [47]. Vitamin D supplementation also seems to have positive effects on fatigue in SLE patients. An observational study found significantly lower fatigue scores after vitamin D supplementation in 80 SLE patients, and a randomized double-blind placebo-controlled trial showed a decrease in fatigue in juvenile-onset SLE patient receiving vitamin D supplementation [48,49]. Physical activities such as supervised training, home training, and appropriately prescribed graded aerobic exercise, have been associated with favorable improvements in patient-reported fatigue in different studies. Importantly, exercise was reported to be safe and well tolerated, with rare adverse effects, and no reported deleterious effects on disease activity or inflammation [50,51,52]. Physical activity should therefore be generally recommended for the management of fatigue in SLE patients, especially since it also leads to less pain interference, better physical function, cardiovascular risk reduction, and even positive impact on anxiety and depression. A trial conducted by Davies et al. indicates that a low glycemic index diet and a low-calorie diet were both associated with reduction in fatigue in SLE, indicating the role of weight loss in the improvement in fatigue [53].
Different psychosocial interventions have been associated with significant improvement in fatigue in SLE: cognitive behavioral therapy, psychoeducation, psychotherapy, relaxation and self-management. Those interventions focus on coping ability improvement, cognitive restructuring and perceived social supports [52,54,55,56]. Even if the effect in reducing fatigue has been shown to be weak in most of these studies, such interventions can decrease psychological distress and pain and therefore might be integrated into the general management of SLE patients. Some interventions targeting pain have also shown their ability to improve fatigue in patients with SLE. A randomized trial found a significant decrease in fatigue in SLE patients receiving transcutaneous auricular vagus nerve stimulation [57]. Additionally, a randomized controlled trial indicates benefits of acupuncture in reducing fatigue in patients with SLE [58].
5. The Need for a Personalized Management
At this time, there is no validated recommendation for the management of fatigue in SLE. Since fatigue may be influenced by a variety of factors and because of the diverse profiles of fatigue in SLE, the management of fatigue should rely upon an individualized person-centered approach (Figure 2). Women with SLE have reported the need for fatigue acknowledgement by clinicians, as well as conversations about fatigue, with information about coping strategies [59]. Fatigue management in SLE would start with an assessment of the intensity and the characteristics of fatigue using validated scales, enabling an individual follow-up of fatigue over time. In recent years, there has been an increasing interest in using Patient Reported Outcomes (PROs), because they place the patients at the center of their health management and help to establish a trusting physician-patient relationship. The most commonly PROs used to evaluate fatigue in SLE are the Fatigue Severity Scale (FSS), the FACIT-fatigue score, which we use in clinical practice, the Fatigue-VAS, which are unidimensional scales measuring fatigue intensity, and the Multi-dimensional Fatigue Inventory (MFI), which analyze general fatigue, physical and mental components of fatigue as well as the reduction in activities and motivation [60]. A personalized investigation of fatigue predictors is needed, with evaluation of disease activity, search for intricate causes (major organ damage, chronic pain, anemia…) and psychosocial factors, assessment of life habits (physical activity, quality of sleep, smoking, obesity…). Common medical causes of fatigue, such as pregnancy, infections, metabolic diseases or drug-induced fatigue must not be forgotten. Finally, optimal management of fatigue for patients with SLE should be based on providing targeted interventions, according to the patient profile [61].
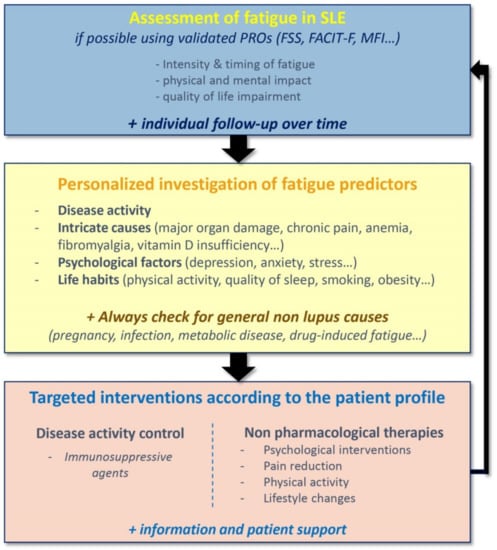
Figure 2.
Personalized strategy for the assessment of fatigue in Systemic Lupus Erythematosus. FSS: Fatigue Severity Scale; FACIT-F: Functional Assessment of Chronic Illness Therapy—Fatigue; MFI: Multidimensional Fatigue Inventory.
6. Conclusions
According to patients, fatigue is the most common and disabling symptom in SLE, and this may impair patients’ physical and mental health and reduce patients’ quality of life by impacting upon their emotions, work, and daily life activities. Fatigue must therefore be adequately assessed and managed in SLE. It is a complex and multifactorial phenomenon, possessing many patterns. Psychological factors seem to be the most important fatigue predictors in SLE patients. Pain and fibromyalgia are also major fatigue determinants, along with lifestyle, especially reduced physical activity. Disease activity seems to have a complex contribution to fatigue, and its role remains controversial. Consequently, the management of fatigue in patients with SLE should rely upon a person-centered approach, with a personalized assessment, and targeted interventions. Some pharmacological treatments used to control disease activity, such as Belimumab, have demonstrated beneficial effects on fatigue. Non-pharmacological therapies, such as psychological interventions, pain reduction and lifestyle changes should be integrated into fatigue management in SLE. In recent years, the scientific community seems to have increased their understanding of the importance of fatigue management in SLE, and we can hope for a better understanding and treatment of fatigue in patients with SLE in the future.
Funding
This study was supported by the EU-funded (ERDF) project INTERREG IV and V Rhin and the Centre National de Référence des Maladies Autoimmunes Systémiques Rares Est Sud-Ouest (RESO).
Acknowledgments
The authors wish to thank the European Reference Networks (ERNs) ReCONNET and RITA.
Conflicts of Interest
Laurent ARNAUD has acted as a consultant for Alexion, Amgen, Astra-Zeneca, Biogen, BMS, Boehringer-Ingelheim, GSK, Grifols, Janssen-Cilag, LFB, Lilly, Menarini France, Medac, Novartis, Pfizer, Roche-Chugaï, UCB. Lou KAWKA, Aurélien SCHLENCKER, Philippe MERTZ have no relationship to disclose. Thierry MARTIN has acted as a consultant for GSK. The authors declare no conflict of interest.
References
- Ream, E.; Richardson, A. Fatigue: A concept analysis. Int. J. Nurs. Stud. 1996, 33, 519–529. [Google Scholar] [CrossRef]
- Hardy, S.E.; Studenski, S.A. Qualities of Fatigue and Associated Chronic Conditions among Older Adults. J. Pain Symptom Manag. 2010, 39, 1033–1042. [Google Scholar] [CrossRef]
- Finsterer, J.; Mahjoub, S.Z. Fatigue in Healthy and Diseased Individuals. Am. J. Hosp. Palliat. Med. 2014, 31, 562–575. [Google Scholar] [CrossRef]
- Cornet, A.; Andersen, J.; Myllys, K.; Edwards, A.; Arnaud, L. Living with systemic lupus erythematosus in 2020: A European patient survey. Lupus Sci. Med. 2021, 8, e000469. [Google Scholar] [CrossRef]
- Sterling, K.; Gallop, K.; Swinburn, P.; Flood, E.; French, A.; Al Sawah, S.; Iikuni, N.; Naegeli, A.; Nixon, A. Patient-reported fatigue and its impact on patients with systemic lupus erythematosus. Lupus 2014, 23, 124–132. [Google Scholar] [CrossRef]
- Raymond, K.; Park, J.; Joshi, A.V.; White, M.K. Patient Experience with Fatigue and Qualitative Interview-Based Evidence of Content Validation of The FACIT-Fatigue in Systemic Lupus Erythematosus. Rheumatol. Ther. 2021, 8, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Mathias, S.D.; Berry, P.; De Vries, J.; Pascoe, K.; Colwell, H.H.; Chang, D.J.; Askanase, A.D. Patient experience in systemic lupus erythematosus: Development of novel patient-reported symptom and patient-reported impact measures. J. Patient-Rep. Outcomes 2017, 2, 11. [Google Scholar] [CrossRef]
- Elefante, E.; Tani, C.; Stagnaro, C.; Ferro, F.; Parma, A.; Carli, L.; Signorini, V.; Zucchi, D.; Peta, U.; Santoni, A.; et al. Impact of fatigue on health-related quality of life and illness perception in a monocentric cohort of patients with systemic lupus erythematosus. RMD Open 2020, 6, e001133. [Google Scholar] [CrossRef]
- Du, X.; Zhao, Q.; Zhuang, Y.; Chen, H.; Shen, B. Fatigue of systemic lupus erythematosus in China: Contributors and effects on the quality of life. Patient Prefer. Adherence 2018, 12, 1729–1735. [Google Scholar] [CrossRef]
- Basta, F.; Margiotta, D.P.E.; Vadacca, M.; Vernuccio, A.; Mazzuca, C.; Picchianti Diamanti, A.; Afeltra, A. Is fatigue a cause of work disability in systemic lupus erythematosus? Results from a systematic literature review. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4589–4597. [Google Scholar]
- Arnaud, L.; Mertz, P.; Amoura, Z.; Voll, R.E.; Schwarting, A.; Maurier, F.; Blaison, G.; Bonnotte, B.; Poindron, V.; Fiehn, C.; et al. Patterns of fatigue and association with disease activity and clinical manifestations in systemic lupus erythematosus. Rheumatology 2020, 60, 2672–2677. [Google Scholar] [CrossRef]
- Jump, R.L.; Robinson, M.E.; Armstrong, A.E.; Barnes, E.V.; Kilbourn, K.M.; Richards, H.B. Fatigue in systemic lupus erythematosus: Contributions of disease activity, pain, depression, and perceived social support. J. Rheumatol. 2005, 32, 1699–1705. [Google Scholar]
- Yilmaz-Oner, S.; Ilhan, B.; Can, M.; Alibaz-Oner, F.; Polat-Korkmaz, O.; Ozen, G.; Mumcu, G.; Kremers, H.M.; Tuglular, S.; Direskeneli, H. Fatigue in systemic lupus erythematosus: Association with disease activity, quality of life and psychosocial factors. Z. Rheumatol. 2017, 76, 913–919. [Google Scholar] [CrossRef]
- Burgos, P.I.; Alarcón, G.S.; McGwin, G.; Crews, K.Q.; Reveille, J.D.; Vilá, L.M. Disease Activity and Damage are not Associated with Increased Levels of Fatigue in Systemic Lupus Erythematosus Patients from LUMINA LXVII, a Multiethnic Cohort. Arthritis Care Res. 2009, 61, 1179–1186. [Google Scholar] [CrossRef]
- Zonana-Nacach, A.; Roseman, J.M.; McGwin, G.; Friedman, A.W.; Baethge, B.A.; Reveille, J.D. Systemic lupus erythematosus in three ethnic groups. VI: Factors associated with fatigue within 5 years of criteria diagnosis. Lupus 2000, 9, 101–109. [Google Scholar] [CrossRef]
- Li, H.J.; Du, Q.; Wang, S.Y.; Guan, S.Q.; Zhan, H.H.; Tian, W.; Shao, Y.X.; Zhang, Z.Y.; Mei, Y.F. The application and influence factors of FACIT Fatigue Scale in SLE patients. Ann. Rheum. Dis. 2017, 97, 2775–2778. [Google Scholar]
- Bruce, I.; Mak, V.; Hallett, D.; Gladman, D.; Urowitz, M. Factors associated with fatigue in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 1999, 58, 379–381. [Google Scholar] [CrossRef]
- Wang, B.; Gladman, D.D.; Urowitz, M.B. Fatigue in lupus is not correlated with disease activity. J. Rheumatol. 1998, 25, 892–895. [Google Scholar]
- Tayer, W.G.; Nicassio, P.M.; Weisman, M.H.; Schuman, C.; Daly, J. Disease status predicts fatigue in systemic lupus erythematosus. J. Rheumatol. 2001, 28, 1999–2007. [Google Scholar]
- Moazzami, M.; Strand, V.; Su, J.; Touma, Z. Dual trajectories of fatigue and disease activity in an inception cohort of adults with systemic lupus erythematosus over 10 years. Lupus 2021, 30, 578–586. [Google Scholar] [CrossRef]
- Arnaud, L.; Gavand, P.E.; Voll, R.; Schwarting, A.; Maurier, F.; Blaison, G.; Magy-Bertrand, N.; Pennaforte, J.-L.; Peter, H.-H.; Kieffer, P.; et al. Predictors of fatigue and severe fatigue in a large international cohort of patients with systemic lupus erythematosus and a systematic review of the literature. Rheumatology 2019, 58, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Harboe, E.; Greve, O.J.; Beyer, M.; Goransson, L.G.; Tjensvoll, A.B.; Maroni, S.; Omdal, R. Fatigue is associated with cerebral white matter hyperintensities in patients with systemic lupus erythematosus. J. Neurol. Neurosurg. Psychiatry 2008, 79, 199–201. [Google Scholar] [CrossRef]
- Moldovan, I.; Cooray, D.; Carr, F.; Katsaros, E.; Torralba, K.; Shinada, S.; Ishimori, M.; Jolly, M.; Wilson, A.; Wallace, D.; et al. Pain and depression predict self-reported fatigue/energy in lupus. Lupus 2013, 22, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Gregg, L.P.; Jain, N.; Carmody, T.; Minhajuddin, A.T.; Rush, A.J.; Trivedi, M.H.; Hedayati, S.S. Fatigue in Nondialysis Chronic Kidney Disease: Correlates and Association with Kidney Outcomes. Am. J. Nephrol. 2019, 50, 37–47. [Google Scholar] [CrossRef]
- Artom, M.; Moss-Morris, R.; Caskey, F.; Chilcot, J. Fatigue in advanced kidney disease. Kidney Int. 2014, 86, 497–505. [Google Scholar] [CrossRef]
- Barnes, S.; Gott, M.; Payne, S.; Parker, C.; Seamark, D.; Gariballa, S.; Small, N. Prevalence of symptoms in a community-based sample of heart failure patients. J. Pain Symptom Manag. 2006, 32, 208–216. [Google Scholar] [CrossRef]
- Conley, S.; Feder, S.; Redeker, N.S. The Relationship between Pain, Fatigue, Depression and Functional Performance in Stable Heart Failure. Heart Lung. J. Crit. Care 2015, 44, 107–112. [Google Scholar] [CrossRef]
- Da Costa, D.; Dritsa, M.; Bernatsky, S.; Pineau, C.; Ménard, H.A.; Dasgupta, K.; Keschani, A.; Rippen, N.; Clarke, A.E. Dimensions of fatigue in systemic lupus erythematosus: Relationship to disease status and behavioral and psychosocial factors. J. Rheumatol. 2006, 33, 1282–1288. [Google Scholar]
- Iaboni, A.; Ibanez, D.; Gladman, D.D.; Urowitz, M.B.; Moldofsky, H. Fatigue in systemic lupus erythematosus: Contributions of disordered sleep, sleepiness, and depression. J. Rheumatol. 2006, 33, 2453–2457. [Google Scholar]
- Figueiredo-Braga, M.; Cornaby, C.; Cortez, A.; Bernardes, M.; Terroso, G.; Figueiredo, M.; Santos Mesquita, C.D.; Costa, L.; Poole, B.D. Depression and anxiety in systemic lupus erythematosus: The crosstalk between immunological, clinical, and psychosocial factors. Medicine 2018, 97, e11376. [Google Scholar] [CrossRef]
- Azizoddin, D.R.; Jolly, M.; Arora, S.; Yelin, E.; Katz, P. Longitudinal Study of Fatigue, Stress, and Depression: Role of Reduction in Stress toward Improvement in Fatigue. Arthritis Care Res. 2020, 72, 1440–1448. [Google Scholar] [CrossRef]
- Palagini, L.; Mosca, M.; Tani, C.; Gemignani, A.; Mauri, M.; Bombardieri, S. Depression and systemic lupus erythematosus: A systematic review. Lupus 2013, 22, 409–416. [Google Scholar] [CrossRef]
- Mckinley, P.S.; Ouellette, S.C.; Winkel, G.H. The contributions of disease activity, sleep patterns, and depression to fatigue in systemic lupus erythematosus. Arthritis Rheum. 1995, 38, 826–834. [Google Scholar] [CrossRef]
- Da Costa, D.; Bernatsky, S.; Dritsa, M.; Clarke, A.E.; Dasgupta, K.; Keshani, A.; Pineau, C. Determinants of sleep quality in women with systemic lupus erythematosus. Arthritis Rheum. 2005, 53, 272–278. [Google Scholar] [CrossRef]
- Palagini, L.; Tani, C.; Mauri, M.; Carli, L.; Vagnani, S.; Bombardieri, S.; Gemignani, A.; Mosca, M. Sleep disorders and systemic lupus erythematosus. Lupus 2014, 23, 115–123. [Google Scholar] [CrossRef]
- Taylor, J.; Skan, J.; Erb, N.; Carruthers, D.; Bowman, S.; Gordon, C.; Isenberg, D. Lupus patients with fatigue—Is there a link with fibromyalgia syndrome? Rheumatology 2000, 39, 620–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, F.F.; Fang, R.; Nguyen, M.H.; Bryant, K.; Gibson, K.A.; O’Neill, S.G. Identifying co-morbid fibromyalgia in patients with systemic lupus erythematosus using the Multi-Dimensional Health Assessment Questionnaire. Lupus 2020, 29, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Stockton, K.A.; Kandiah, D.A.; Paratz, J.D.; Bennell, K.L. Fatigue, muscle strength and vitamin D status in women with systemic lupus erythematosus compared with healthy controls. Lupus 2012, 21, 271–278. [Google Scholar] [CrossRef]
- Carrión-Barberà, I.; Salman-Monte, T.C.; Castell, S.; Castro, F.; Ojeda, F.; Carbonell, J. Prevalence and factors associated with fatigue in female patients with systemic lupus erythematosus. Med. Clin. 2018, 151, 353–358. [Google Scholar] [CrossRef]
- Mancuso, C.A.; Perna, M.; Sargent, A.B.; Salmon, J.E. Perceptions and measurements of physical activity in patients with systemic lupus erythematosus. Lupus 2011, 20, 231–242. [Google Scholar] [CrossRef]
- Tench, C.; Bentley, D.; Vleck, V.; McCurdie, I.; White, P.; D’Cruz, D. Aerobic fitness, fatigue, and physical disability in systemic lupus erythematosus. J. Rheumatol. 2002, 29, 474–481. [Google Scholar]
- Balsamo, S.; da Mota, L.M.H.; de Carvalho, J.F.; da Nascimento, D.C.; Tibana, R.A.; de Santana, F.S.; Moreno, R.L.; Gualano, B.; dos Santos-Neto, L. Low dynamic muscle strength and its associations with fatigue, functional performance, and quality of life in premenopausal patients with systemic lupus erythematosus and low disease activity: A case-control study. BMC Musculoskelet. Disord. 2013, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.L.; Schmajuk, G.; Jafri, K.; Yazdany, J.; Katz, P. Obesity is Independently Associated With Worse Patient-Reported Outcomes in Women with Systemic Lupus Erythematosus. Arthritis Care Res. 2019, 71, 126–133. [Google Scholar] [CrossRef]
- Strand, V.; Berry, P.; Lin, X.; Asukai, Y.; Punwaney, R.; Ramachandran, S. Long-Term Impact of Belimumab on Health-Related Quality of Life and Fatigue in Patients With Systemic Lupus Erythematosus: Six Years of Treatment. Arthritis Care Res. 2019, 71, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.A.; Martin, R.S.; Scheinberg, M.A.; Furie, R.A. Assessments of fatigue and disease activity in patients with systemic lupus erythematosus enrolled in the Phase 2 clinical trial with blisibimod. Lupus 2017, 26, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, N.; Eto, H.; Tanikawa, A.; Ikeda, T.; Yamamoto, K.; Takahashi, T.; Mizukami, H.; Sato, T.; Yokota, N.; Furukawa, F. Effects of Hydroxychloroquine in Patients With Cutaneous Lupus Erythematosus: A Multicenter, Double-Blind, Randomized, Parallel-Group Trial. Arthritis Rheumatol. 2017, 69, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.-W.; Hanczko, R.; Bonilla, E.; Caza, T.N.; Clair, B.; Bartos, A.; Miklossy, G.; Jimah, J.; Doherty, E.; Tily, H.; et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012, 64, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.L.; Paupitz, J.; Aikawa, N.E.; Takayama, L.; Bonfa, E.; Pereira, R.M.R. Vitamin D Supplementation in Adolescents and Young Adults With Juvenile Systemic Lupus Erythematosus for Improvement in Disease Activity and Fatigue Scores: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Care Res. 2016, 68, 91–98. [Google Scholar] [CrossRef]
- Ruiz-Irastorza, G.; Gordo, S.; Olivares, N.; Egurbide, M.-V.; Aguirre, C. Changes in vitamin D levels in patients with systemic lupus erythematosus: Effects on fatigue, disease activity, and damage. Arthritis Care Res. 2010, 62, 1160–1165. [Google Scholar] [CrossRef]
- O’Dwyer, T.; Durcan, L.; Wilson, F. Exercise and physical activity in systemic lupus erythematosus: A systematic review with meta-analyses. Semin. Arthritis Rheum. 2017, 47, 204–215. [Google Scholar] [CrossRef]
- Mahieu, M.A.; Ahn, G.E.; Chmiel, J.S.; Dunlop, D.D.; Helenowski, I.B.; Semanik, P.; Song, J.; Yount, S.; Chang, R.W.; Ramsey-Goldman, R. Fatigue, patient reported outcomes, and objective measurement of physical activity in systemic lupus erythematosus. Lupus 2016, 25, 1190–1199. [Google Scholar] [CrossRef]
- Tench, C.M.; McCarthy, J.; McCurdie, I.; White, P.D.; D’Cruz, D.P. Fatigue in systemic lupus erythematosus: A randomized controlled trial of exercise. Rheumatology 2003, 42, 1050–1054. [Google Scholar] [CrossRef]
- Davies, R.J.; Lomer, M.C.E.; Yeo, S.I.; Avloniti, K.; Sangle, S.R.; D’Cruz, D.P. Weight loss and improvements in fatigue in systemic lupus erythematosus: A controlled trial of a low glycaemic index diet versus a calorie restricted diet in patients treated with corticosteroids. Lupus 2012, 21, 649–655. [Google Scholar] [CrossRef]
- Karlson, E.W.; Liang, M.H.; Eaton, H.; Huang, J.; Fitzgerald, L.; Rogers, M.P.; Daltroy, L.H. A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum. 2004, 50, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Health Outcome Improvements in Patients with Systemic Lupus Erythematosus Using Two Telephone Counseling Interventions—Austin—1996—Arthritis & Rheumatism—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/1529-0131(199610)9:5%3C391::AID-ANR1790090508%3E3.0.CO;2-V (accessed on 19 July 2021).
- Sohng, K.Y. Effects of a self-management course for patients with systemic lupus erythematosus. J. Adv. Nurs. 2003, 42, 479–486. [Google Scholar] [CrossRef]
- Aranow, C.; Atish-Fregoso, Y.; Lesser, M.; Mackay, M.; Anderson, E.; Chavan, S.; Zanos, T.P.; Datta-Chaudhuri, T.; Bouton, C.; Tracey, K.J.; et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: A randomised, double-blind, sham-controlled pilot trial. Ann. Rheum. Dis. 2021, 80, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Kao, A.; Maksimowicz-McKinnon, K.; Glick, R.; Houze, M.; Sereika, S.; Balk, J.; Manzi, S. Acupuncture for systemic lupus erythematosus: A pilot RCT feasibility and safety study. Lupus 2008, 17, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Kier, A.Ø.; Midtgaard, J.; Hougaard, K.S.; Berggreen, A.; Bukh, G.; Hansen, R.B.; Dreyer, L. How do women with lupus manage fatigue? A focus group study. Clin. Rheumatol. 2016, 35, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Barbacki, A.; Petri, M.; Aviña-Zubieta, A.; Alarcón, G.S.; Bernatsky, S. Fatigue Measurements in Systemic Lupus Erythematosus. J. Rheumatol. 2019, 46, 1470–1477. [Google Scholar] [CrossRef]
- Mertz, P.; Schlencker, A.; Schneider, M.; Gavand, P.-E.; Martin, T.; Arnaud, L. Towards a practical management of fatigue in systemic lupus erythematosus. Lupus Sci. Med. 2020, 7, e000441. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).