Efficacy and Safety of Intranasal Ketamine for Acute Pain Management in the Emergency Setting: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Outcome Measures
2.4. Data Extraction and Quality Assessment
2.5. Data Synthesis and Analysis
3. Results
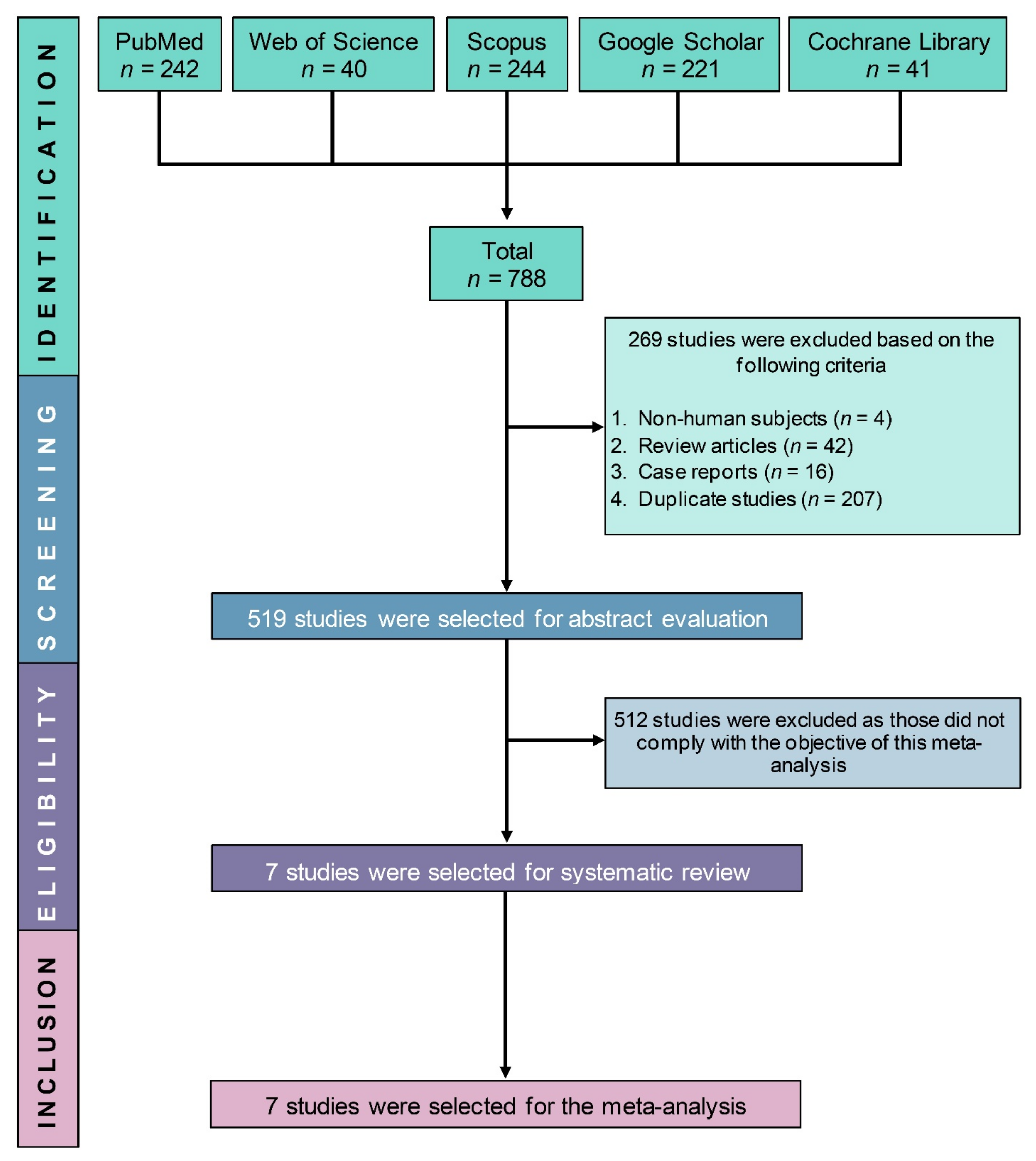
3.1. Study Selection
3.2. Study Characteristics
3.3. Study Quality
3.4. Primary Outcomes (Efficacy of IN Ketamine)
3.4.1. Pain Reduction from Baseline
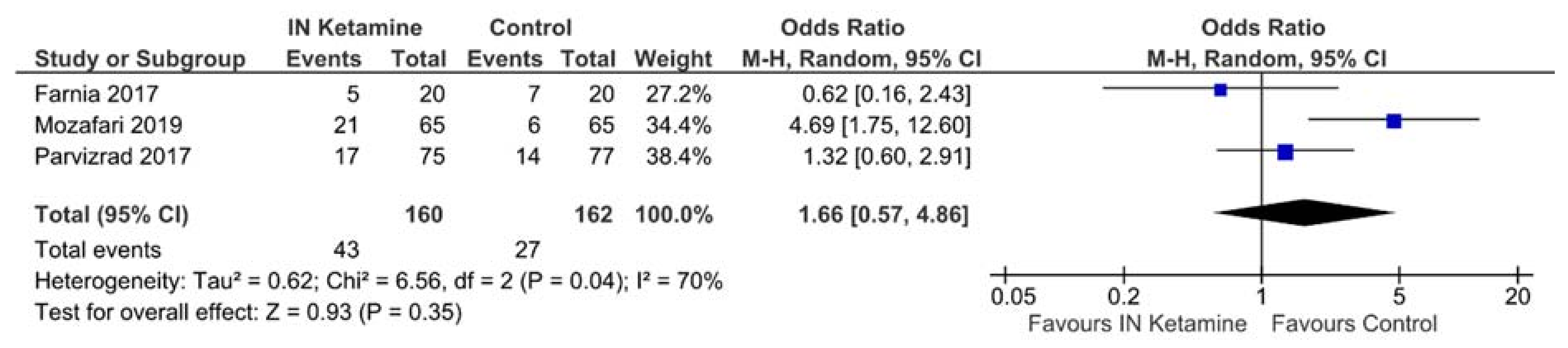
3.4.2. Requirements of Rescue Analgesics
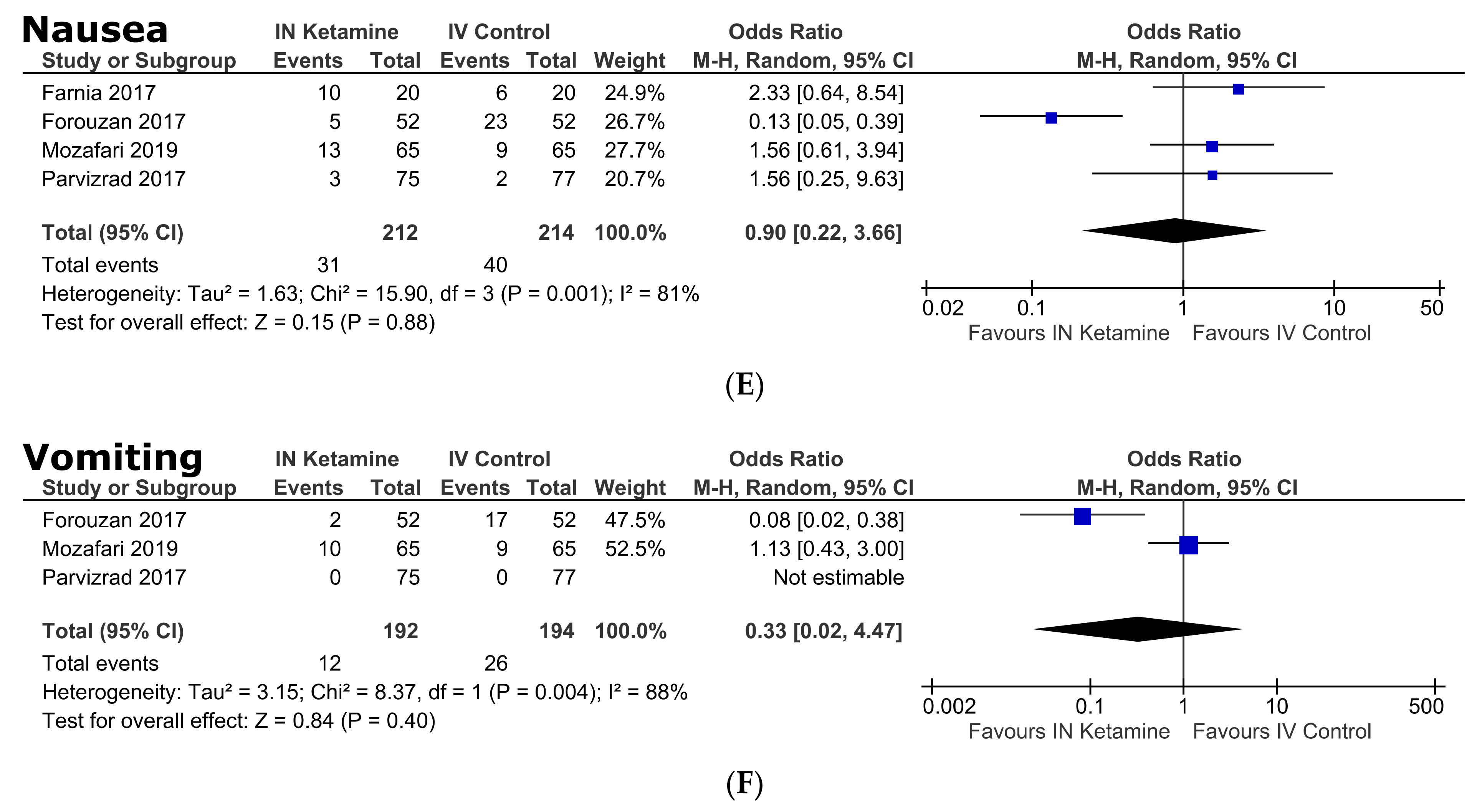
3.5. Secondary Outcomes (Safety of IN Ketamine)
Adverse Events
3.6. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, H.Y.; Daubresse, M.; Kruszewski, S.P.; Alexander, G.C. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am. J. Emerg. Med. 2014, 32, 421–431. [Google Scholar] [CrossRef]
- Samcam, I.; Papa, L. Acute Pain Management in the Emergency Department. In Pain Management; IntechOpen: London, UK, 2016; Available online: https://www.intechopen.com/chapters/50415 (accessed on 20 April 2021).
- Rupp, T.; Delaney, K.A. Inadequate analgesia in emergency medicine. Ann. Emerg. Med. 2004, 43, 494–503. [Google Scholar] [CrossRef]
- Sinatra, R. Causes and Consequences of Inadequate Management of Acute Pain. Pain Med. 2010, 11, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Kahsay, D.T.; Pitkäjärvi, M. Emergency nurses´ knowledge, attitude and perceived barriers regarding pain Management in Resource-Limited Settings: Cross-sectional study. BMC Nurs. 2019, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Todd, K.H.; Ducharme, J.; Choiniere, M.; Crandall, C.S.; Fosnocht, D.E.; Homel, P.; Tanabe, P. Pain in the emergency department: Results of the pain and emergency medicine initiative (PEMI) multicenter study. J. Pain 2007, 8, 460–466. [Google Scholar] [CrossRef]
- Vazirani, J.; Knott, J.C. Mandatory pain scoring at triage reduces time to analgesia. Ann. Emerg. Med. 2012, 59, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.B.; Charity, B.M. Use of night vision goggles and low-level light source in obtaining intravenous access in tactical conditions of darkness. Mil. Med. 2001, 166, 982–983. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hedegaard, H.; Miniño, A.M.; Warner, M. Drug Overdose Deaths in the United States, 1999–2017. 2018. NCHS Data Brief (Accession Number: CDC:84647). Available online: https://stacks.cdc.gov/view/cdc/84647 (accessed on 25 June 2021).
- Alexander, G.C.; Kruszewski, S.P.; Webster, D.W. Rethinking opioid prescribing to protect patient safety and public health. JAMA 2012, 308, 1865–1866. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W. Guide to prescribing in today’s management of severe pain. Prescriber 2012, 23, 25–40. [Google Scholar] [CrossRef]
- American College of Emergency Physicians. Optimizing the Treatment of Acute Pain in the Emergency Department. Ann. Emerg. Med. 2017, 70, 446–448. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Vlisides, P.E. Ketamine: 50 Years of Modulating the Mind. Front. Hum. Neurosci. 2016, 10, 612. [Google Scholar] [CrossRef]
- Pai, A.; Heining, M. Ketamine. BJA Educ. 2007, 7, 59–63. [Google Scholar] [CrossRef]
- Ahern, T.L.; Herring, A.A.; Anderson, E.S.; Madia, V.A.; Fahimi, J.; Frazee, B.W. The first 500: Initial experience with widespread use of low-dose ketamine for acute pain management in the ED. Am. J. Emerg. Med. 2015, 33, 197–201. [Google Scholar] [CrossRef]
- Hirlinger, W.K.; Dick, W. Intramuscular ketamine analgesia in emergency patients. II. Clinical study of traumatized patients. Anaesthesist 1984, 33, 272–275. [Google Scholar] [PubMed]
- Green, S.M.; Roback, M.G.; Kennedy, R.M.; Krauss, B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann. Emerg. Med. 2011, 57, 449–461. [Google Scholar] [CrossRef]
- Hurth, K.P.; Jaworski, A.; Thomas, K.B.; Kirsch, W.B.; Rudoni, M.A.; Wohlfarth, K.M. The Reemergence of Ketamine for Treatment in Critically Ill Adults. Crit. Care Med. 2020, 48, 899–911. [Google Scholar] [CrossRef]
- Carr, D.B.; Goudas, L.C.; Denman, W.T.; Brookoff, D.; Staats, P.S.; Brennen, L.; Green, G.; Albin, R.; Hamilton, D.; Rogers, M.C.; et al. Safety and efficacy of intranasal ketamine for the treatment of breakthrough pain in patients with chronic pain: A randomized, double-blind, placebo-controlled, crossover study. Pain 2004, 108, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Parvizrad, R.; Pakniyat, A.; Malekianzadeh, B.; Almasi-Hashiani, A. Comparing the analgesic effect of intranasal with intravenous ketamine in isolated orthopedic trauma: A randomized clinical trial. Turk. J. Emerg. Med. 2017, 17, 99–103. [Google Scholar] [CrossRef]
- McGuinness, S.K.; Wasiak, J.; Cleland, H.; Symons, J.; Hogan, L.; Hucker, T.; Mahar, P.D. A systematic review of ketamine as an analgesic agent in adult burn injuries. Pain Med. 2011, 12, 1551–1558. [Google Scholar] [CrossRef]
- Bouida, W.; Bel Haj Ali, K.; Ben Soltane, H.; Msolli, M.A.; Boubaker, H.; Sekma, A.; Beltaief, K.; Grissa, M.H.; Methamem, M.; Boukef, R.; et al. Effect on Opioids Requirement of Early Administration of Intranasal Ketamine for Acute Traumatic Pain. Clin. J. Pain 2020, 36, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetc, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In Joanna Briggs Institute Reviewer’s Manual; The Joanna Briggs Institute: Adelaide, Australia, 2020. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, S.S.; Kundu, S.; Hossan, T.; Kamal, M.A.; Cavestro, C. Prevalence of Headache in Patients with Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis of 14,275 Patients. Front. Neurol. 2020, 11, 562634. [Google Scholar] [CrossRef]
- Chia, Y.C.; Islam, M.A.; Hider, P.; Woon, P.Y.; Johan, M.F.; Hassan, R.; Ramli, M. The Prevalence of TET2 Gene Mutations in Patients with BCR-ABL-Negative Myeloproliferative Neoplasms (MPN): A Systematic Review and Meta-Analysis. Cancers 2021, 13, 3078. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Alam, F.; Islam, M.A.; Mohamed, M.; Ahmad, I.; Kamal, M.A.; Donnelly, R.; Idris, I.; Gan, S.H. Efficacy and Safety of Pioglitazone Monotherapy in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Sci. Rep. 2019, 9, 5389. [Google Scholar] [CrossRef]
- Chang, C.T.; Ang, J.Y.; Islam, M.A.; Chan, H.K.; Cheah, W.K.; Gan, S.H. Prevalence of Drug-Related Problems and Complementary and Alternative Medicine Use in Malaysia: A Systematic Review and Meta-Analysis of 37,249 Older Adults. Pharmaceuticals 2021, 14, 187. [Google Scholar] [CrossRef]
- Pouraghaei, M.; Moharamzadeh, P.; Paknezhad, S.P.; Rajabpour, Z.V.; Soleimanpour, H. Intranasal ketamine versus intravenous morphine for pain management in patients with renal colic: A double-blind, randomized, controlled trial. World J. Urol. 2021, 4, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, J.; Maleki Verki, M.; Motamed, H.; Sabouhi, A.; Tirandaz, F. Comparing intranasal ketamine with intravenous fentanyl in reducing pain in patients with renal colic: A double-blind randomized clinical trial. Am. J. Emerg. Med. 2020, 38, 549–553. [Google Scholar] [CrossRef]
- Farnia, M.R.; Jalali, A.; Vahidi, E.; Momeni, M.; Seyedhosseini, J.; Saeedi, M. Comparison of intranasal ketamine versus IV morphine in reducing pain in patients with renal colic. Am. J. Emerg. Med. 2017, 35, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Forouzan, A.; Masoumi, K.; Motamed, H.; Mozafari, J.; Gharibi, S. Comparison of intranasal ketamine versus intravenous morphine in pain relief of patient with bone fracture. Int. J. Adv. Biotechnol. Res. 2017, 8, 1636–1643. [Google Scholar]
- Shimonovich, S.; Gigi, R.; Shapira, A.; Sarig-Meth, T.; Nadav, D.; Rozenek, M.; West, D.; Halpern, P. Intranasal ketamine for acute traumatic pain in the Emergency Department: A prospective, randomized clinical trial of efficacy and safety. BMC Emerg. Med. 2016, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Hopper, A.B.; Vilke, G.M.; Castillo, E.M.; Campillo, A.; Davie, T.; Wilson, M.P. Ketamine Use for Acute Agitation in the Emergency Department. J. Emerg. Med. 2015, 48, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, A.; Mazer-Amirshahi, M.; Royall, C.; Alhawas, R.; Shesser, R. Low dose ketamine use in the emergency department, a new direction in pain management. Am. J. Emerg. Med. 2017, 35, 918–921. [Google Scholar] [CrossRef]
- Lee, E.N.; Lee, J.H. The Effects of Low-Dose Ketamine on Acute Pain in an Emergency Setting: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0165461. [Google Scholar] [CrossRef]
- Karlow, N.; Schlaepfer, C.H.; Stoll, C.R.T.; Doering, M.; Carpenter, C.R.; Colditz, G.A.; Motov, S.; Miller, J.; Schwarz, E.S. A Systematic Review and Meta-analysis of Ketamine as an Alternative to Opioids for Acute Pain in the Emergency Department. Acad. Emerg. Med. 2018, 25, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Yousefifard, M.; Askarian-Amiri, S.; Rafiei Alavi, S.N.; Sadeghi, M.; Saberian, P.; Baratloo, A.; Talebian, M.T. The Efficacy of Ketamine Administration in Prehospital Pain Management of Trauma Patients; a Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2019, 8, 1–11. [Google Scholar]
- Ghate, G.; Clark, E.; Vaillancourt, C. Systematic review of the use of low-dose ketamine for analgesia in the emergency department. CJEM 2018, 20, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Witting, M.D. IV access difficulty: Incidence and delays in an urban emergency department. J. Emerg. Med. 2012, 42, 483–487. [Google Scholar] [CrossRef]
- Kulbe, J. The use of ketamine nasal spray for short-term analgesia. Home Healthc. Nurse 1998, 16, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Rogers, E.; Green, G.A.; Hamilton, D.A.; Mermelstein, F.; Liao, E.; Wright, C.; Carr, D.B. Safety and efficacy of intranasal ketamine for acute postoperative pain. Acute Pain 2007, 9, 183–192. [Google Scholar] [CrossRef]
- Tsze, D.S.; Steele, D.W.; Machan, J.T.; Akhlaghi, F.; Linakis, J.G. Intranasal ketamine for procedural sedation in pediatric laceration repair: A preliminary report. Pediatr. Emerg. Care 2012, 28, 767–770. [Google Scholar] [CrossRef]
- Yeaman, F.; Meek, R.; Egerton-Warburton, D.; Rosengarten, P.; Graudins, A. Sub-dissociative-dose intranasal ketamine for moderate to severe pain in adult emergency department patients. Emerg. Med. Australas. 2014, 26, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Andolfatto, G.; Willman, E.; Joo, D.; Miller, P.; Wong, W.B.; Koehn, M.; Dobson, R.; Angus, E.; Moadebi, S. Intranasal ketamine for analgesia in the emergency department: A prospective observational series. Acad. Emerg. Med. 2013, 20, 1050–1054. [Google Scholar] [CrossRef]
- Andolfatto, G.; Innes, K.; Dick, W.; Jenneson, S.; Willman, E.; Stenstrom, R.; Zed, P.J.; Benoit, G. Prehospital Analgesia with Intranasal Ketamine (PAIN-K): A Randomized Double-Blind Trial in Adults. Ann. Emerg. Med. 2019, 74, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.E.S.L.; Lee, J.Y.; Bellolio, F.; Homme, J.L.; Anderson, J.L. Intranasal ketamine for acute pain management in children: A systematic review and meta-analysis. Am. J. Emerg. Med. 2020, 38, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Poonai, N.; Canton, K.; Ali, S.; Hendrikx, S.; Shah, A.; Miller, M.; Joubert, G.; Rieder, M.; Hartling, L. Intranasal ketamine for procedural sedation and analgesia in children: A systematic review. PLoS ONE 2017, 12, e0173253. [Google Scholar] [CrossRef]
- Singh, G.K.; Kim, I.E.; Girmay, M.; Perry, C.; Daus, G.P.; Vedamuthu, I.P.; De Los Reyes, A.A.; Ramey, C.T.; Martin, E.K.; Allender, M. Opioid Epidemic in the United States: Empirical Trends, and A Literature Review of Social Determinants and Epidemiological, Pain Management, and Treatment Patterns. Int. J. Matern. Child Health AIDS 2019, 8, 89–100. [Google Scholar] [CrossRef]
- Short, B.; Fong, J.; Galvez, V.; Shelker, W.; Loo, C.K. Side-effects associated with ketamine use in depression: A systematic review. Lancet Psychiatry 2018, 5, 65–78. [Google Scholar] [CrossRef]
- Soto, E.; Flores, A.; Eróstegui, C.; Vega, R. Evidence for NMDA receptor in the afferent synaptic transmission of the vestibular system. Brain Res. 1994, 633, 289–296. [Google Scholar] [CrossRef]
- Soto, E.; Vega, R. Neuropharmacology of vestibular system disorders. Curr. Neuropharm. 2010, 8, 26–40. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Farber, N.B.; Olney, J.W. NMDA receptor function, memory, and brain aging. Dialogues Clin. Neurosci. 2000, 2, 219–232. [Google Scholar] [PubMed]
- Durieux, M.E. Inhibition by ketamine of muscarinic acetylcholine receptor function. Anesth. Analg. 1995, 81, 57–62. [Google Scholar] [PubMed]
- Murrough, J.W.; Wan, L.-B.; Iacoviello, B.; Collins, K.A.; Solon, C.; Glicksberg, B.; Perez, A.M.; Mathew, S.J.; Charney, D.S.; Iosifescu, D.V.; et al. Neurocognitive effects of ketamine in treatment-resistant major depression: Association with antidepressant response. Psychopharmacology 2013, 3, 481–488. [Google Scholar] [CrossRef]
- Murrough, J.W.; Burdick, K.E.; Levitch, C.F.; Perez, A.M.; Brallier, J.W.; Chang, L.C.; Foulkes, A.; Charney, D.S.; Mathew, S.J.; Iosifescu, D.V. Neurocognitive effects of ketamine and association with antidepressant response in individuals with treatment-resistant depression: A randomized controlled trial. Neuropsychopharmacology 2015, 40, 1084–1090. [Google Scholar] [CrossRef]
- Balzer, N.; McLeod, S.L.; Walsh, C.; Grewal, K. Low—Dose ketamine for acute pain control in the emergency department: A systematic review and meta—analysis. Acad. Emerg. Med. 2021, 28, 444–454. [Google Scholar] [CrossRef]
- Li, X.; Hua, G.; Peng, F. Efficacy of intranasal ketamine for acute pain management in adults: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3286–3295. [Google Scholar] [PubMed]
- Mohammadshahi, A.; Abdolrazaghnejad, A.; Nikzamir, H.; Safaie, A. Intranasal ketamine administration for narcotic dose decrement in patients suffering from acute limb trauma in emergency department: A double-blind randomized placebo-controlled trial. Adv. J. Emerg. Med. 2018, 2, 1–7. [Google Scholar]




| Study ID | Country | Sample Size [Ketamine (% Female)/ Control (% Female)] | Age in Years (Mean ± SD) | Causes of Acute Pain | Pain Scale Used | Intervention | Comparator | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Route of Administration, Dose | ||||||||
| Bouida 2020 | Tunisia | 552 (59.6%)/ 550 (58.5%) | 37.2 ± 12.8 | Acute traumatic pain | VAS | IN Ketamine, 50 mg | IN Placebo |
|
| Pouraghaei 2020 | Iran | 95 (NR)/ 89 (NR) | 40.3 ± 4.6 | Renal colic | NRS | IN Ketamine, 1 mg/kg and IV Placebo | IV Morphine, 0.1 mg/kg and IN Placebo |
|
| Mozafari 2019 | Iran | 65 (NR)/ 65 (NR) | 36.9 ± 10.6 | Renal colic | VAS | IN Ketamine, 1 mg/kg and IV Placebo | IV Fentanyl, 1 μg/kg and IN Placebo |
|
| Farnia 2017 | Iran | 20 (40%)/ 20 (15%) | 37.0 ± 11.3 | Renal colic | VAS | IN Ketamine, 1 mg/kg and IV Placebo | IV Morphine, 0.1 mg/kg and IN Placebo |
|
| Forouzan 2017 | Iran | 52 (28.8%)/ 52 (17.3%) | 30.4 ± 11.5 | Acute traumatic pain | VAS | IN Ketamine, 1 mg/kg | IV Morphine, 0.1 mg/kg |
|
| Parvizrad 2017 | Iran | 75 (41.6%)/ 77 (59.8%) | 34.6 ± 12.2 | Acute traumatic pain | VAS | IN Ketamine, 0.4 mg/kg and IV Placebo | IV Ketamine, 0.2 mg/kg and IN Placebo |
|
| Shimonovich 2016 | Israel | 24 (29.2%)/ 24 (25%) | 39.4 ± NR | Acute traumatic pain | VAS | IN Ketamine, 1 mg/kg | IV Morphine, 0.1 mg/kg |
|
| Adverse Effects | Prevalence of Adverse Events [95% CIs] (%) | Number of Studies Analysed | Total Number of Patients | Heterogeneity | OR [95% CIs] | p-Value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|---|
| I2 | p-Value | I2 | p-Value | ||||||
| Dizziness | 21.7 [11.4–31.9] | 7 | 883 | 93% | <0.0001 | 1.9 [1.4–2.5] * | <0.0001 | 0% | 0.42 |
| Nausea | 17.0 [4.7–29.3] | 4 | 212 | 87% | <0.0001 | 1.0 [0.2–3.7] | 0.88 | 81% | 0.001 |
| Vomiting | 5.2 [0.0–12.3] | 3 | 192 | 84% | 0.003 | 0.3 [0.0–4.5] | 0.40 | 88% | 0.004 |
| Agitation | 2.0 [0.0–4.3] | 2 | 140 | 0% | 0.63 | 2.1 [0.3–16.8] | 0.47 | 0% | 0.43 |
| Difficulty concentrating | 58.3 [38.6–78.1] | 1 | 24 | NA | NA | 5.3 [1.5–19.0] | 0.01 | NA | NA |
| Confusion | 50 [30.0–70.0] | 1 | 24 | NA | NA | 7.0 [1.6–29.9] | 0.009 | NA | NA |
| Emergence phenomenon | 30.0 [9.9–50.1] | 1 | 20 | NA | NA | 18.4 [1.0–352.6] | 0.05 | NA | NA |
| Dry mouth | 25.0 [7.7–42.3] | 1 | 24 | NA | NA | 0.09 [0.02–0.3] | 0.0004 | NA | NA |
| Fatigue | 14.7 [6.7–22.7] | 1 | 75 | NA | NA | 0.9 [0.4–2.0] | 0.71 | NA | NA |
| Disorientation | 7.8 [5.6–10.0] | 1 | 552 | NA | NA | 9.2 [3.6–23.4] | <0.00001 | NA | NA |
| Drowsiness | 10.7 [3.7–17.7] | 1 | 75 | NA | NA | 3.0 [0.8–11.6] | 0.12 | NA | NA |
| Euphoria | 10.7 [3.7–17.7] | 1 | 75 | NA | NA | 0.5 [0.2–1.4] | 0.19 | NA | NA |
| Headache | 4.6 [0.0–9.7] | 1 | 65 | NA | NA | 3.1 [0.3–30.6] | 0.33 | NA | NA |
| Hypotension | 0.0 [0.0–6.5] | 1 | 20 | NA | NA | 0.04 [ 0.0–0.7] | 0.03 | NA | NA |
| Strategies of Sensitivity Analyses | Risk Ratio or Mean Difference [95% CIs] (%) | Number of Studies Analysed | Difference of Results | Total Number of Subjects | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 | p-Value | |||||
| Pain score (5 min) | ||||||
| Excluding low- or medium-quality studies | 0.81 [−1.84, 3.46] | 3 | 0.13 lower | 322 | 98% | <0.00001 |
| Using a fixed-effects model | 1.69 [1.41–1.97] | 5 | 0.75 higher | 474 | 96% | <0.00001 |
| Pain score (10 min) | ||||||
| Excluding low- or medium-quality studies | 2.45 [2.11–2.79] | 1 | 0.92 higher | 152 | NA | NA |
| Using a fixed-effects model | 2.19 [1.88–2.50] | 3 | 0.66 higher | 304 | 85% | 0.0001 |
| Pain score (15 min) | ||||||
| Excluding low- or medium-quality studies | −0.12 [−1.37–1.14] | 3 | 0.27 lower | 354 | 86% | 0.0008 |
| Using a fixed-effects model | 0.08 [−0.27–0.44] | 5 | 0.07 lower | 506 | 82% | 0.0001 |
| Pain score (20 min) | ||||||
| Excluding low- or medium-quality studies | 0.95 [0.61, 1.29] | 1 | 0.02 higher | 152 | NA | NA |
| Using a fixed-effects model | 0.93 [0.63–1.24] | 3 | No change | 304 | 0% | 0.96 |
| Pain score (25 min) | ||||||
| Using a fixed-effects model | 0.24 [−0.71–1.19] | 2 | No change | 152 | 0% | 0.79 |
| Pain score (30 min) | ||||||
| Excluding low- or medium-quality studies | 0.16 [−0.46-0.78] | 5 | 0.19 higher | 1608 | 73% | 0.005 |
| Using a fixed-effects model | −0.13 [−0.37–0.11] | 7 | 0.10 lower | 1760 | 76% | 0.0003 |
| Pain score (60 min) | ||||||
| Excluding low- or medium-quality studies | −0.85 [−2.24–0.54] | 2 | 0.45 lower | 1186 | 91% | 0.001 |
| Using a fixed-effects model | −0.73 [−1.14, −0.32] | 3 | 0.33 lower | 1234 | 87% | 0.0005 |
| Rescue analgesic requirement | ||||||
| Excluding low- or medium-quality studies | 1.66 [0.57–4.86] | 3 | No change | 322 | 70% | 0.04 |
| Using a fixed-effects model | 1.82 [1.06–3.12] | 3 | 0.16 higher | 322 | 70% | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seak, Y.S.; Nor, J.; Tuan Kamauzaman, T.H.; Arithra, A.; Islam, M.A. Efficacy and Safety of Intranasal Ketamine for Acute Pain Management in the Emergency Setting: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3978. https://doi.org/10.3390/jcm10173978
Seak YS, Nor J, Tuan Kamauzaman TH, Arithra A, Islam MA. Efficacy and Safety of Intranasal Ketamine for Acute Pain Management in the Emergency Setting: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(17):3978. https://doi.org/10.3390/jcm10173978
Chicago/Turabian StyleSeak, Yee Sin, Junainah Nor, Tuan Hairulnizam Tuan Kamauzaman, Ariff Arithra, and Md Asiful Islam. 2021. "Efficacy and Safety of Intranasal Ketamine for Acute Pain Management in the Emergency Setting: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 17: 3978. https://doi.org/10.3390/jcm10173978
APA StyleSeak, Y. S., Nor, J., Tuan Kamauzaman, T. H., Arithra, A., & Islam, M. A. (2021). Efficacy and Safety of Intranasal Ketamine for Acute Pain Management in the Emergency Setting: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(17), 3978. https://doi.org/10.3390/jcm10173978








