Liver Transplantation in Patients with Hepatocellular Carcinoma beyond the Milan Criteria: A Comprehensive Review
Abstract
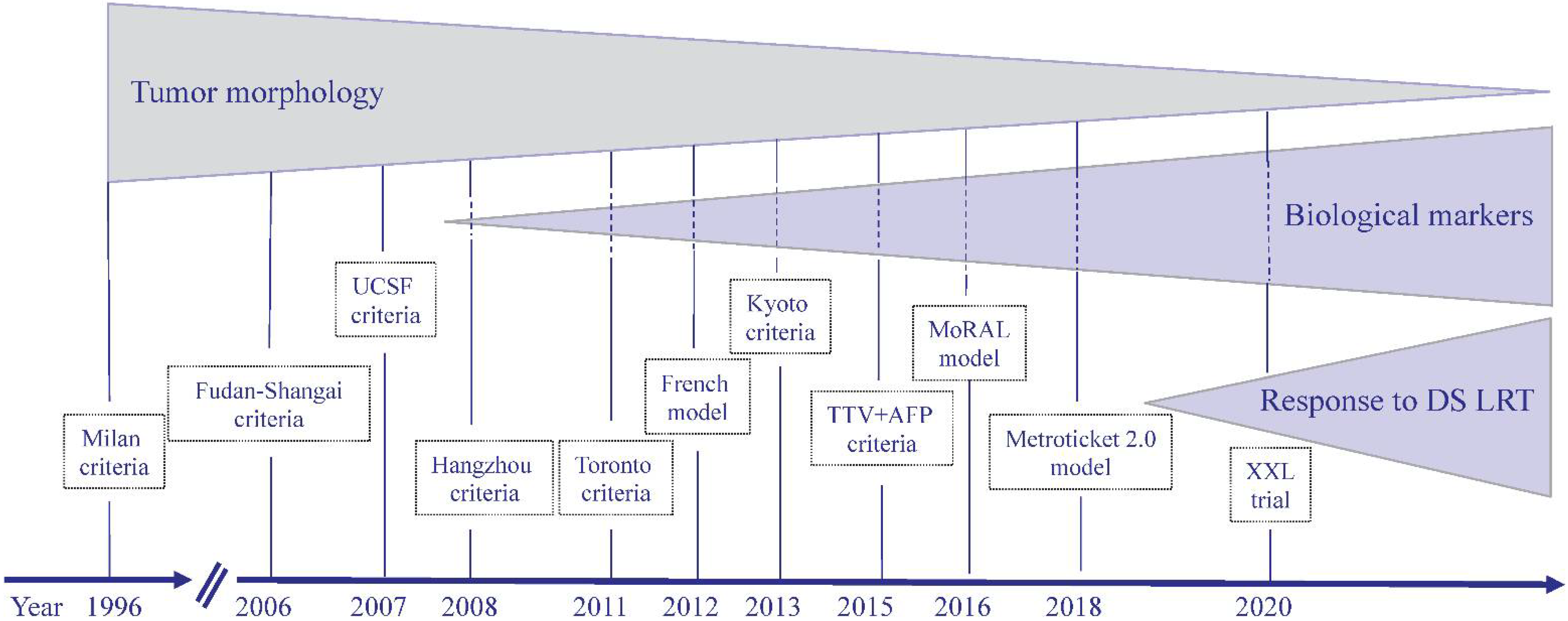
1. Introduction
2. Selection Criteria for LT Based on HCC Morphological Characteristics
3. Selection Criteria for LT Based on HCC Histological Characteristics
4. Selection Criteria Based on Serum Biological Marker Measurements
5. Selection Criteria Based on the Addition of AFP and/or DCP Serum Level Measurements to HCC Morphology
6. Selection Criteria Based on the Response of HCC to Bridging and Downstaging Treatments
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- White, D.L.; Thrift, A.P.; Kanwal, F.; Davila, J.; El-Serag, H.B. Incidence of Hepatocellular Carcinoma in All 50 United States, from 2000 through 2012. Gastroenterology 2017, 152, 812–820.e5. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Vibert, E.; Schwartz, M.; Olthoff, K.M. Advances in resection and transplantation for hepatocellular carcinoma. J. Hepatol. 2020, 72, 262–276. [Google Scholar] [CrossRef]
- Starzl, T.E.; Marchioro, T.L.; Vonkaulla, K.N.; Hermann, G.; Brittain RSWaddell, W.R. Homotransplantation of the Liver in Humans. Surg. Gynecol. Obs. 1963, 117, 659–676. [Google Scholar]
- Starzl, T.E.; Groth, C.G.; Brettschneider, L.; Penn, I.; Fulginiti, V.A.; Moon, J.B.; Blanchard, H.; Martin, A.J., Jr.; Porter, K.A. Orthotopic homotransplantation of the human liver. Ann. Surg. 1968, 168, 392–415. [Google Scholar] [CrossRef]
- Iwatsuki, S.; Gordon, R.D.; Shaw, B.W., Jr.; Starzl, T.E. Role of liver transplantation in cancer therapy. Ann. Surg. 1985, 202, 401–407. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef]
- Lingiah, V.A.; Niazi, M.; Olivo, R.; Paterno, F.; Guarrera, J.V.; Pyrsopoulos, N.T. Liver Transplantation Beyond Milan Criteria. J. Clin. Transl. Hepatol. 2020, 8, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Plessier, A.; Codes, L.; Consigny, Y.; Sommacale, D.; Dondero, F.; Cortes, A.; Degos, F.; Brillet, P.Y.; Vilgrain, V.; Paradis, V.; et al. Underestimation of the influence of satellite nodules as a risk factor for post-transplantation recurrence in patients with small hepatocellular carcinoma. Liver Transplant. 2004, 10, S86–S90. [Google Scholar] [CrossRef] [PubMed]
- Escartin, A.; Sapisochin, G.; Bilbao, I.; Vilallonga, R.; Bueno, J.; Castells, L.; Dopazo, C.; Castro, E.; Caralt, M.; Balsells, J. Recurrence of hepatocellular carcinoma after liver transplantation. Transplant. Proc. 2007, 39, 2308–2310. [Google Scholar] [CrossRef] [PubMed]
- Roayaie, S.; Schwartz, J.D.; Sung, M.W.; Emre, S.H.; Miller, C.M.; Gondolesi, G.E.; Krieger, N.R.; Schwartz, M.E. Recurrence of hepatocellular carcinoma after liver transplant: Patterns and prognosis. Liver Transplant. 2004, 10, 534–540. [Google Scholar] [CrossRef]
- Bruix, J.; Fuster, J.; Llovet, J.M. Liver transplantation for hepatocellular carcinoma: Foucault pendulum versus evidence-based decision. Liver Transplant. 2003, 9, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Xiao, L.; Bass, N.M.; Kerlan, R.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Validation of the UCSF-expanded criteria based on preoperative imaging. Am. J. Transplant. 2007, 7, 2587–2596. [Google Scholar] [CrossRef]
- Herrero, J.I.; Sangro, B.; Pardo, F.; Quiroga, J.; Inarrairaegui, M.; Rotellar, F.; Montiel, C.; Alegre, F.; Prieto, J. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transplant. 2008, 14, 272–278. [Google Scholar] [CrossRef]
- Silva, M.; Moya, A.; Berenguer, M.; Sanjuan, F.; Lopez-Andujar, R.; Pareja, E.; Torres-Quevedo, R.; Aguilera, V.; Montalva, E.; De Juan, M.; et al. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transplant. 2008, 14, 1449–1460. [Google Scholar] [CrossRef]
- Guiteau, J.J.; Cotton, R.T.; Washburn, W.K.; Harper, A.; O’Mahony, C.A.; Sebastian, A.; Cheng, S.; Klintmalm, G.; Ghobrial, M.; Halff, G.; et al. An early regional experience with expansion of Milan Criteria for liver transplant recipients. Am. J. Transplant. 2010, 10, 2092–2098. [Google Scholar] [CrossRef]
- Costentin, C.E.; Bababekov, Y.J.; Zhu, A.X.; Yeh, H. Is It Time to Reconsider the Milan Criteria for Selecting Patients With Hepatocellular Carcinoma for Deceased-Donor Liver Transplantation? Hepatology 2019, 69, 1324–1336. [Google Scholar] [CrossRef]
- Toniutto, P.; Fornasiere, E.; Fumolo, E.; Bitetto, D. Risk factors for hepatocellular carcinoma recurrence after liver transplantation. Hepatoma Res. 2020, 6, 50–71. [Google Scholar] [CrossRef]
- Fan, J.; Zhou, J.; Xu, Y.; Qiu, S.J.; Wu, Z.Q.; Yu, Y.; Huang, X.W.; Tang, Z.Y.; Wang, Y.Q. Indication of liver transplantation for hepatocellular carcinoma: Shanghai Fudan Criteria. Zhonghua Yi Xue Za Zhi 2006, 86, 1227–1231. [Google Scholar]
- DuBay, D.; Sandroussi, C.; Sandhu, L.; Cleary, S.; Guba, M.; Cattral, M.S.; McGilvray, I.; Ghanekar, A.; Selzner, M.; Greig, P.D.; et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann. Surg. 2011, 253, 166–172. [Google Scholar] [CrossRef]
- Zheng, S.S.; Xu, X.; Wu, J.; Chen, J.; Wang, W.L.; Zhang, M.; Liang, T.B.; Wu, L.M. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008, 85, 1726–1732. [Google Scholar] [CrossRef]
- Toso, C.; Meeberg, G.; Hernandez-Alejandro, R.; Dufour, J.F.; Marotta, P.; Majno, P.; Kneteman, N.M. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015, 62, 158–165. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver transplantation for hepatocellular carcinoma: A model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012, 143, 986–994 e3. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef]
- Kaido, T.; Ogawa, K.; Mori, A.; Fujimoto, Y.; Ito, T.; Tomiyama, K.; Takada, Y.; Uemoto, S. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery 2013, 154, 1053–1060. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, Y.; Kim, H.Y.; Cho, E.J.; Lee, D.H.; Yu, S.J.; Lee, J.W.; Yi, N.J.; Lee, K.W.; Kim, S.H.; et al. Serum Tumor Markers Provide Refined Prognostication in Selecting Liver Transplantation Candidate for Hepatocellular Carcinoma Patients Beyond the Milan Criteria. Ann. Surg. 2016, 263, 842–850. [Google Scholar] [CrossRef]
- Shah, S.A.; Tan, J.C.; McGilvray, I.D.; Cattral, M.S.; Levy, G.A.; Greig, P.D.; Grant, D.R. Does microvascular invasion affect outcomes after liver transplantation for HCC? A histopathological analysis of 155 consecutive explants. J. Gastrointest. Surg. 2007, 11, 464–471. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Shen, F.; Lau, W.Y. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2018, 33, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Bechstein, W.O.; Steinmuller, T.; Herrmann, M.; Radke, C.; Berg, T.; Settmacher, U.; Neuhaus, P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001, 33, 1080–1086. [Google Scholar] [CrossRef]
- Fan, J.; Yang, G.S.; Fu, Z.R.; Peng, Z.H.; Xia, Q.; Peng, C.H.; Qian, J.M.; Zhou, J.; Xu, Y.; Qiu, S.J.; et al. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: A multi-center experience in Shanghai, China. J. Cancer Res. Clin. Oncol. 2009, 135, 1403–1412. [Google Scholar] [CrossRef]
- Sotiropoulos, G.C.; Malago, M.; Molmenti, E.; Paul, A.; Nadalin, S.; Brokalaki, E.; Kuhl, H.; Dirsch, O.; Lang, H.; Broelsch, C.E. Liver transplantation for hepatocellular carcinoma in cirrhosis: Is clinical tumor classification before transplantation realistic? Transplantation 2005, 79, 483–487. [Google Scholar] [CrossRef]
- Shah, S.A.; Tan, J.C.; McGilvray, I.D.; Cattral, M.S.; Cleary, S.P.; Levy, G.A.; Greig, P.D.; Grant, D.R. Accuracy of staging as a predictor for recurrence after liver transplantation for hepatocellular carcinoma. Transplantation 2006, 81, 1633–1639. [Google Scholar] [CrossRef]
- Kierans, A.S.; Song, C.; Gavlin, A.; Roudenko, A.; Lu, L.; Askin, G.; Hecht, E.M. Diagnostic Performance of LI-RADS Version 2018, LI-RADS Version 2017, and OPTN Criteria for Hepatocellular Carcinoma. AJR Am. J. Roentgenol. 2020, 215, 1085–1092. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.S.; Roh, Y.H.; Choi, J.Y.; Park, M.S.; Kim, M.J. Diagnostic Performance of CT/MRI Liver Imaging Reporting and Data System v2017 for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Int. 2020, 40, 1488–1497. [Google Scholar] [CrossRef]
- Rudnick, S.R.; Russo, M.W. Liver transplantation beyond or downstaging within the Milan criteria for hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 265–275. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Cillo, U.; Vitale, A.; Bassanello, M.; Boccagni, P.; Brolese, A.; Zanus, G.; Burra, P.; Fagiuoli, S.; Farinati, F.; Rugge, M.; et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann. Surg. 2004, 239, 150–159. [Google Scholar] [CrossRef]
- Cuccurullo, V.; Di Stasio, G.D.; Mazzarella, G.; Cascini, G.L. Microvascular Invasion in HCC: The Molecular Imaging Perspective. Contrast Media Mol. Imaging 2018, 2018, 9487938. [Google Scholar] [CrossRef]
- Yaprak, O.; Acar, S.; Ertugrul, G.; Dayangac, M. Role of pre-transplant 18F-FDG PET/CT in predicting hepatocellular carcinoma recurrence after liver transplantation. World J. Gastrointest. Oncol. 2018, 10, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Byun, J.H.; Kwon, H.J.; Ha, H.I.; Lee, S.J.; Kim, S.Y.; Won, H.J.; Kim, P.N. The usefulness of gadoxetic acid-enhanced dynamic magnetic resonance imaging in hepatocellular carcinoma: Toward improved staging. Ann. Surg. Oncol. 2015, 22, 819–825. [Google Scholar] [CrossRef]
- Sweeney, M.J.; Ashmore, J.; Morris, H.P.; Weber, G. Comparative Biochemistry Hepatomas. Iv. Isotope Studies of Glucose and Fructose Metabolism in Liver Tumors of Different Growth Rates. Cancer Res. 1963, 23, 995–1002. [Google Scholar] [PubMed]
- Torizuka, T.; Tamaki, N.; Inokuma, T.; Magata, Y.; Sasayama, S.; Yonekura, Y.; Tanaka, A.; Yamaoka, Y.; Yamamoto, K.; Konishi, J. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J. Nucl. Med. 1995, 36, 1811–1817. [Google Scholar] [PubMed]
- Lin, C.Y.; Liao, C.W.; Chu, L.Y.; Yen, K.Y.; Jeng, L.B.; Hsu, C.N.; Lin, C.L.; Kao, C.H. Predictive Value of 18F-FDG PET/CT for Vascular Invasion in Patients With Hepatocellular Carcinoma Before Liver Transplantation. Clin. Nucl. Med. 2017, 42, e183–e187. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Lee, J.M.; Joo, I.; Lee, E.S.; Lee, S.J.; Cheon, G.J.; Han, J.K.; Choi, B.I. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom. Imaging 2015, 40, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Kaido, T.; Shirabe, K.; Nagano, H.; Egawa, H.; Sugawara, Y.; Taketomi, A.; Takahara, T.; Wakabayashi, G.; Nakanishi, C.; et al. Significance of preoperative fluorodeoxyglucose-positron emission tomography in prediction of tumor recurrence after liver transplantation for hepatocellular carcinoma patients: A Japanese multicenter study. J. Hepatobiliary Pancreat. Sci. 2017, 24, 49–57. [Google Scholar] [CrossRef]
- Lee, S.D.; Kim, S.H.; Kim, S.K.; Kim, Y.K.; Park, S.J. Clinical Impact of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Living Donor Liver Transplantation for Advanced Hepatocellular Carcinoma. Transplantation 2015, 99, 2142–2149. [Google Scholar] [CrossRef]
- Hakeem, A.R.; Young, R.S.; Marangoni, G.; Lodge, J.P.; Prasad, K.R. Systematic review: The prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2012, 35, 987–999. [Google Scholar] [CrossRef]
- Lai, Q.; Avolio, A.W.; Graziadei, I.; Otto, G.; Rossi, M.; Tisone, G.; Goffette, P.; Vogel, W.; Pitton, M.B.; Lerut, J.; et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transplant. 2013, 19, 1108–1118. [Google Scholar] [CrossRef]
- Dumitra, T.C.; Dumitra, S.; Metrakos, P.P.; Barkun, J.S.; Chaudhury, P.; Deschenes, M.; Paraskevas, S.; Hassanain, M.; Tchervenkov, J.I. Pretransplantation alpha-fetoprotein slope and milan criteria: Strong predictors of hepatocellular carcinoma recurrence after transplantation. Transplantation 2013, 95, 228–233. [Google Scholar] [CrossRef]
- Burra, P.; Giannini, E.G.; Caraceni, P.; Ginanni Corradini, S.; Rendina, M.; Volpes, R.; Toniutto, P. Specific issues concerning the management of patients on the waiting list and after liver transplantation. Liver Int. 2018, 38, 1338–1362. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Goldaracena, N.; Laurence, J.M.; Dib, M.; Barbas, A.; Ghanekar, A.; Cleary, S.P.; Lilly, L.; Cattral, M.S.; Marquez, M.; et al. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology 2016, 64, 2077–2088. [Google Scholar] [CrossRef]
- Toso, C.; Asthana, S.; Bigam, D.L.; Shapiro, A.M.; Kneteman, N.M. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology 2009, 49, 832–838. [Google Scholar] [CrossRef]
- Notarpaolo, A.; Layese, R.; Magistri, P.; Gambato, M.; Colledan, M.; Magini, G.; Miglioresi, L.; Vitale, A.; Vennarecci, G.; Ambrosio, C.D.; et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J. Hepatol. 2017, 66, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Varona, M.A.; Soriano, A.; Aguirre-Jaime, A.; Garrido, S.; Oton, E.; Diaz, D.; Portero, J.; Bravo, P.; Barrera, M.A.; Perera, A. Risk factors of hepatocellular carcinoma recurrence after liver transplantation: Accuracy of the alpha-fetoprotein model in a single-center experience. Transplant. Proc. 2015, 47, 84–89. [Google Scholar] [CrossRef]
- Pinero, F.; Tisi Bana, M.; de Ataide, E.C.; Hoyos Duque, S.; Marciano, S.; Varon, A.; Anders, M.; Zerega, A.; Menendez, J.; Zapata, R.; et al. Liver transplantation for hepatocellular carcinoma: Evaluation of the alpha-fetoprotein model in a multicenter cohort from Latin America. Liver Int. 2016, 36, 1657–1667. [Google Scholar] [CrossRef]
- Rhu, J.; Kim, J.M.; Choi, G.S.; Kwon, C.H.D.; Joh, J.W. Validation of the alpha-fetoprotein Model for Hepatocellular Carcinoma Recurrence After Transplantation in an Asian Population. Transplantation 2018, 102, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Okuda, H.; Nakanishi, T.; Takatsu, K.; Saito, A.; Hayashi, N.; Yamamoto, M.; Takasaki, K.; Nakano, M. Comparison of clinicopathological features of patients with hepatocellular carcinoma seropositive for alpha-fetoprotein alone and those seropositive for des-gamma-carboxy prothrombin alone. J. Gastroenterol. Hepatol. 2001, 16, 1290–1296. [Google Scholar] [CrossRef]
- Hong, Y.M.; Cho, M.; Yoon, K.T.; Chu, C.W.; Yang, K.H.; Park, Y.M.; Rhu, J.H. Risk factors of early recurrence after curative hepatectomy in hepatocellular carcinoma. Tumour Biol. 2017, 39, 1010428317720863. [Google Scholar] [CrossRef]
- Takada, Y.; Ito, T.; Ueda, M.; Sakamoto, S.; Haga, H.; Maetani, Y.; Ogawa, K.; Ogura, Y.; Oike, F.; Egawa, H.; et al. Living donor liver transplantation for patients with HCC exceeding the Milan criteria: A proposal of expanded criteria. Dig. Dis. 2007, 25, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, M.; Takada, Y.; Ogura, Y.; Oike, F.; Kaido, T.; Teramukai, S.; Uemoto, S. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am. J. Transplant. 2009, 9, 2362–2371. [Google Scholar] [CrossRef]
- Soejima, Y.; Taketomi, A.; Yoshizumi, T.; Uchiyama, H.; Aishima, S.; Terashi, T.; Shimada, M.; Maehara, Y. Extended indication for living donor liver transplantation in patients with hepatocellular carcinoma. Transplantation 2007, 83, 893–899. [Google Scholar] [CrossRef]
- Shirabe, K.; Itoh, S.; Yoshizumi, T.; Soejima, Y.; Taketomi, A.; Aishima, S.; Maehara, Y. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J. Surg. Oncol. 2007, 95, 235–240. [Google Scholar] [CrossRef]
- Shirabe, K.; Taketomi, A.; Morita, K.; Soejima, Y.; Uchiyama, H.; Kayashima, H.; Ninomiya, M.; Toshima, T.; Maehara, Y. Comparative evaluation of expanded criteria for patients with hepatocellular carcinoma beyond the Milan criteria undergoing living-related donor liver transplantation. Clin. Transplant. 2011, 25, E491–E498. [Google Scholar] [CrossRef]
- Chaiteerakij, R.; Zhang, X.; Addissie, B.D.; Mohamed, E.A.; Harmsen, W.S.; Theobald, P.J.; Peters, B.E.; Balsanek, J.G.; Ward, M.M.; Giama, N.H.; et al. Combinations of biomarkers and Milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transplant. 2015, 21, 599–606. [Google Scholar] [CrossRef]
- Lai, Q.; Iesari, S.; Levi Sandri, G.B.; Lerut, J. Des-gamma-carboxy prothrombin in hepatocellular cancer patients waiting for liver transplant: A systematic review and meta-analysis. Int. J. Biol. Markers 2017, 32, e370–e374. [Google Scholar] [CrossRef]
- Clavien, P.A.; Lesurtel, M.; Bossuyt, P.M.; Gores, G.J.; Langer, B.; Perrier, A.; The OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol. 2012, 13, e11–e22. [Google Scholar] [CrossRef]
- Otto, G.; Herber, S.; Heise, M.; Lohse, A.W.; Monch, C.; Bittinger, F.; Hoppe-Lotichius, M.; Schuchmann, M.; Victor, A.; Pitton, M. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transplant. 2006, 12, 1260–1267. [Google Scholar] [CrossRef]
- Millonig, G.; Graziadei, I.W.; Freund, M.C.; Jaschke, W.; Stadlmann, S.; Ladurner, R.; Margreiter, R.; Vogel, W. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transplant. 2007, 13, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Mehta, N.; Flemming, J.; Dodge, J.; Hameed, B.; Fix, O.; Hirose, R.; Fidelman, N.; Kerlan, R.K., Jr.; Roberts, J.P. Downstaging of hepatocellular cancer before liver transplant: Long-term outcome compared to tumors within Milan criteria. Hepatology 2015, 61, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Grazi, G.L.; Piscaglia, F.; Trevisani, F.; Cescon, M.; Ercolani, G.; Vivarelli, M.; Golfieri, R.; D’Errico Grigioni, A.; Panzini, I.; et al. Liver transplantation for hepatocellular carcinoma: Results of down-staging in patients initially outside the Milan selection criteria. Am. J. Transplant. 2008, 8, 2547–2557. [Google Scholar] [CrossRef]
- Mehta, N.; Guy, J.; Frenette, C.T.; Dodge, J.L.; Osorio, R.W.; Minteer, W.B.; Roberts, J.P.; Yao, F.Y. Excellent Outcomes of Liver Transplantation Following Down-Staging of Hepatocellular Carcinoma to Within Milan Criteria: A Multicenter Study. Clin. Gastroenterol. Hepatol. 2018, 16, 955–964. [Google Scholar] [CrossRef]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Yao, F.Y.; Fidelman, N. Reassessing the boundaries of liver transplantation for hepatocellular carcinoma: Where do we stand with tumor down-staging? Hepatology 2016, 63, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.D.; Waljee, A.K.; Singal, A.G. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transplant. 2015, 21, 1142–1152. [Google Scholar] [CrossRef]
- Mehta, N.; Bhangui, P.; Yao, F.Y.; Mazzaferro, V.; Toso, C.; Akamatsu, N.; Durand, F.; Ijzermans, J.; Polak, W.; Zheng, S.; et al. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1136–1142. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Kulik, L.M.; Riaz, A.; Senthilnathan, S.; Mulcahy, M.F.; Ryu, R.K.; Ibrahim, S.M.; Sato, K.T.; Baker, T.; Miller, F.H.; et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: Chemoembolization versus radioembolization. Am. J. Transplant. 2009, 9, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, M.B.; Riaz, I.B.; Naqvi, S.A.A.; Almquist, D.R.; Mina, S.; Almasri, J.; Shah, S.; Almader-Douglas, D.; Uson Junior, P.L.S.; Mahipal, A.; et al. Systemic Therapy and Sequencing Options in Advanced Hepatocellular Carcinoma: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2020, 6, e204930. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Bargellini, I.; Vignali, C.; Cioni, R.; Petruzzi, P.; Cicorelli, A.; Campani, D.; De Simone, P.; Filipponi, F.; Bartolozzi, C. Hepatocellular carcinoma: CT for tumor response after transarterial chemoembolization in patients exceeding Milan criteria--selection parameter for liver transplantation. Radiology 2010, 255, 289–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.J.; Clark, P.J.; Heimbach, J.; Rosen, C.; Sanchez, W.; Watt, K.; Charlton, M.R. Recurrence of hepatocellular carcinoma: Importance of mRECIST response to chemoembolization and tumor size. Am. J. Transplant. 2014, 14, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.P.; Venook, A.; Kerlan, R.; Yao, F. Hepatocellular carcinoma: Ablate and wait versus rapid transplantation. Liver Transplant. 2010, 16, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Halazun, K.J.; Patzer, R.E.; Rana, A.A.; Verna, E.C.; Griesemer, A.D.; Parsons, R.F.; Samstein, B.; Guarrera, J.V.; Kato, T.; Brown, R.S., Jr.; et al. Standing the test of time: Outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology 2014, 60, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Dodge, J.L.; Grab, J.D.; Yao, F.Y. National Experience on Down-Staging of Hepatocellular Carcinoma Before Liver Transplant: Influence of Tumor Burden, Alpha-Fetoprotein, and Wait Time. Hepatology 2020, 71, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Citterio, D.; Bhoori, S.; Bongini, M.; Miceli, R.; De Carlis, L.; Colledan, M.; Salizzoni, M.; Romagnoli, R.; Antonelli, B.; et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): A randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020, 21, 947–956. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Volk, M.L.; Vijan, S.; Marrero, J.A. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am. J. Transplant. 2008, 8, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V. Squaring the circle of selection and allocation in liver transplantation for HCC: An adaptive approach. Hepatology 2016, 63, 1707–1717. [Google Scholar] [CrossRef]
| Authors | Criterion Name | Country | No. of Patients | HCC Morphology | Post-LT Survival | Post-LT RFS |
|---|---|---|---|---|---|---|
| Mazzaferro et al. [8] | Milan | Italy | 48 | Up to 3 nodules <3 cm in diameter or up to 5 cm in diameter in the case of a single nodule | 75% at 4 years | 83% at 4 years |
| Fan et al. [20] | Shanghai Fudan | China | 1078 | Single nodule ≤ 9 cm in diameter, no more than 3 nodules with the largest ≤5 cm, a total tumor diameter ≤ 9 cm, without MVI or EHS | 80% at 3 years | 88% at 3 years |
| Yao et al. [14] | UCSF | US | 168 | Single nodule ≤ 6 cm in diameter or 2–3 nodules ≤ 4.5 cm, with a total tumor diameter ≤ 8 cm | - | 80.7% at 5 years |
| Authors | Criterion Name | Country | No. of Patients | HCC Characteristics | Post-LT Survival | Post-LT RFS |
|---|---|---|---|---|---|---|
| Du Bay et al. [21] | Toronto | Canada | 294 | HCC confined to the liver, AFP serum levels <400 ng/mL, no poor histologic differentiation | 70% at 5years | 66% at 5 years |
| Zheng et al. [22] | Hangzhou | China | 195 | HCC ≤ 8 cm in diameter or >8 cm if associated with AFP serum levels < 400 ng/mL and histological grade I–II | 70.7% at 5 years | 62.4% at 5 years |
| Toso et al. [23] | Toso | Canada Swiss UK | 233 | Total tumor volume ≤ 115 cm3 and AFP serum levels ≤ 400 ng/mL | 74.6% at 4 years | 68% at 4 years |
| Duvoux et al. [24] | French | France | 972 | Nodule diameters ≤ 3 cm, between 3–6 cm, or ≥6 cm and AFP serum levels ≤ 100, between 100–1000, or >1000 ng/mL | 69.9% at 5 years | 66.6% at 5 years |
| Mazzaferro et al. [25] | Metroticket 2.0 | Italy China | 1359 | The sum of the size (in cm) of the larger HCC and the number of nodules not exceeding 7, without MVI | 74.9% at 5 years | 77.9% at 5 years |
| * Kaido et al. [26] | Kyoto | Japan | 198 | Up to 10 HCCs with a diameter ≤ 5 cm and DCP serum levels ≤400 mAU/mL | 82% at 5 years | - |
| * Lee et al. [27] | MoRAL | Korea | 566 | Simultaneous evaluation of DCP and AFP serum levels | 86% at 5 years | 66.3% at 5 years |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toniutto, P.; Fumolo, E.; Fornasiere, E.; Bitetto, D. Liver Transplantation in Patients with Hepatocellular Carcinoma beyond the Milan Criteria: A Comprehensive Review. J. Clin. Med. 2021, 10, 3932. https://doi.org/10.3390/jcm10173932
Toniutto P, Fumolo E, Fornasiere E, Bitetto D. Liver Transplantation in Patients with Hepatocellular Carcinoma beyond the Milan Criteria: A Comprehensive Review. Journal of Clinical Medicine. 2021; 10(17):3932. https://doi.org/10.3390/jcm10173932
Chicago/Turabian StyleToniutto, Pierluigi, Elisa Fumolo, Ezio Fornasiere, and Davide Bitetto. 2021. "Liver Transplantation in Patients with Hepatocellular Carcinoma beyond the Milan Criteria: A Comprehensive Review" Journal of Clinical Medicine 10, no. 17: 3932. https://doi.org/10.3390/jcm10173932
APA StyleToniutto, P., Fumolo, E., Fornasiere, E., & Bitetto, D. (2021). Liver Transplantation in Patients with Hepatocellular Carcinoma beyond the Milan Criteria: A Comprehensive Review. Journal of Clinical Medicine, 10(17), 3932. https://doi.org/10.3390/jcm10173932





