Review of the 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation—What Has Changed and How Does This Affect Daily Practice
Abstract
:1. Introduction
2. Screening and Diagnosis
2.1. Novel Screening Tools and Their Integration in Clinical Practice
2.2. Management of Atrial High Rate Episodes (AHRE) and Subclinical Atrial Fibrillation in CIED
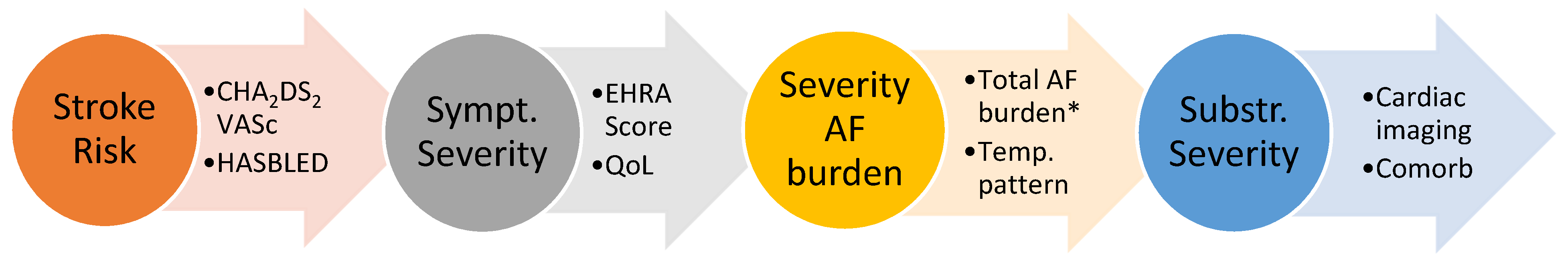
3. Structured Characterization of Atrial Fibrillation
- Stroke Risk: As in previous guidelines, the recommended assessment tool for stroke risk estimation remains the well-known CHA2DS2-VASc Score. The ESC taskforce has refined some of the risk factors by adding precise blood pressure and blood glucose cut-offs as well as including hypertrophic cardiomyopathy, heart failure with preserved ejection fraction and asymptomatic moderate to severe LV dysfunction into the score. Opposed to the original score, angiographically documented significant coronary artery disease (regardless of symptom status) is now included as well. The temporal pattern and total burden of AF are not part of the stroke risk assessment in the current guidelines.
- Symptom Severity: The severity of symptoms should be evaluated in a standardized manner with the EHRA symptom score ranging from 1 to 4 or via quality-of-life questionnaires. Importantly, a symptom-rhythm correlation should be established to differentiate from symptoms due to underlying co-morbidities.
- Severity of AF burden: Assessing the AF burden includes not only the traditionally used classification of the temporal pattern into paroxysmal, persistent and permanent AF but also the total AF burden defined as the percentage of time in AF for a defined time frame. Higher AF burden have been associated with higher stroke risk [19] and mortality rates (if >6–24 h of AF per week) [20], poorer response to rhythm control therapy [21] and may represent progression of advanced atrial remodeling [22]. However, it remains unclear whether progressive AF burden is primarily a marker or a driver or both of progression of the underlying disease and adverse prognosis.
- Substrate Severity: A growing body of evidence showed that the severity and extent of left atrial structural and electrical remodeling has prognostic value for patients with AF. Technological improvements in non-invasive imaging modalities (echocardiography with TDI and strain, cardiac MRI with Late-Gadolinium Enhancement (LGE), cardiac CT) as well as invasive high density electro-anatomical contact mapping has allowed for more detailed assessment of the underlying substrate of AF. Nowadays, it is commonly acknowledged that left atrial size alone is not able to accurately define the disease state. Atrial wall fibrosis [23] and wall thickness [24], epicardial fat infiltration [25], atrial conduction velocities [26] or geometrical assessments such as sphericity [27] are a number of further parameters that have shown prognostic value and may guide treatment decisions, though most are not yet routinely assessed in daily practice. The guidelines suggest the assessment of atrial electrical and mechanical dysfunction and thrombotic risk by means of multimodality imaging and biomarkers as well as comprehensive review of cardiovascular risk factors and comorbidities affecting the atrial substrate. Figure 2 illustrates examples of normal compared to diseased left atrial substrate assessed by electro-anatomical mapping, MRI and TTE.
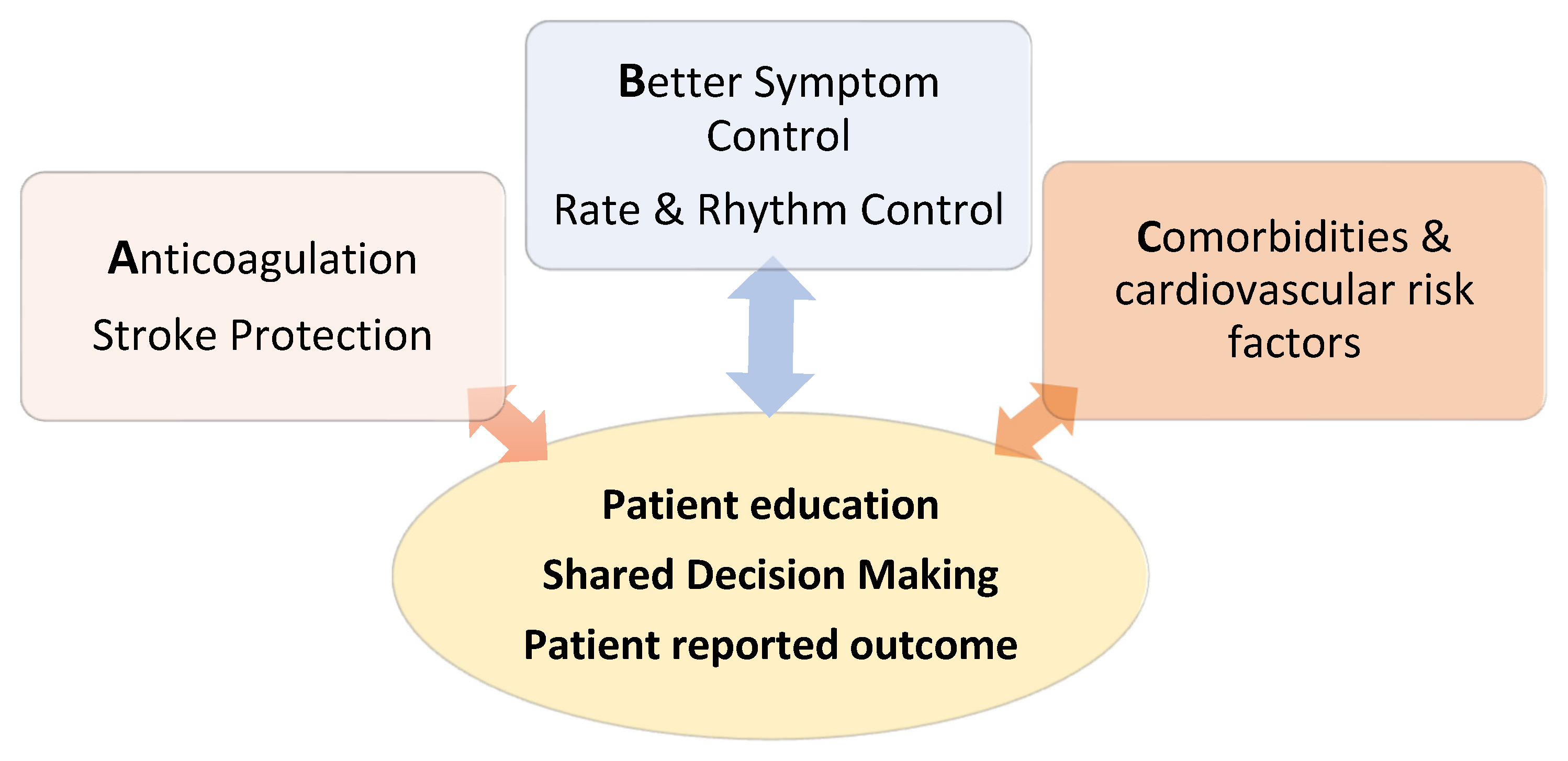
4. Treatment: The ABC Pathway
4.1. Anticoagulation
4.2. Better Symptom Control
4.2.1. Rate Control
4.2.2. Rhythm Control
New Evidence for the Prognostic Benefit of Rhythm Control
Optimal Timing of Rhythm Control
Choice of Rhythm Control Modalities
Focus on AF and Heart Failure
Defining, Measuring and Predicting Rhythm Control Success
5. Cardiovascular Risk Factors and Comorbidities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AHRE | Atrial High Rate Episodes |
| CIED | Cardiac implantable electronic devices |
| HFrEF | Heart failure with reduced ejection fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| CAD | Coronary artery disease |
| VHD | Valvular heart disease |
| ECG | Electrocardiogram |
| NOAC | Novel oral anticoagulants |
| VKA | Vitamin K Antagonists |
| NDCC | Non dihydropyridine calcium channel blocker, |
| TTE | Transthoracic echocardiogram |
| LV | Left Ventricle |
| MRI | Magnetic Resonance Imaging |
| LGE | Late Gadolinium Enhancement |
| CTCA | Computer-tomography Coronary Angiogram |
| AAD | Antiarrhythmic Drugs |
| IST | Inappropriate Sinus Tachycardia |
| RFA | Radiofrequency Ablation |
References
- Staerk, L.; Wang, B.; Preis, S.R.; Larson, M.G.; Lubitz, S.A.; Ellinor, P.T.; McManus, D.D.; Ko, D.; Weng, L.-C.; Lunetta, K.; et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: Cohort study based on longitudinal data from the Framingham Heart Study. BMJ 2018, 361, k1453. [Google Scholar] [CrossRef] [Green Version]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, J.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collabo-ration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Lowres, N.; Neubeck, L.; Salkeld, G.; Krass, I.; McLachlan, A.J.; Redfern, J.; Bennett, A.A.; Briffa, T.; Bauman, A.; Martinez, C.; et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. Thromb. Haemost. 2014, 111, 1167–1176. [Google Scholar] [CrossRef]
- Jacobs, M.S.; Kaasenbrood, F.; Postma, M.J.; Van Hulst, M.; Tieleman, R.G. Cost-effectiveness of screening for atrial fibrillation in primary care with a handheld, single-lead electrocardiogram device in the Netherlands. Eurospace 2016, 20, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, M.; Svennberg, E.; Rosenqvist, M.; Engdahl, J.; Al-Khalili, F.; Friberg, L.; Frykman-Kull, V.; Levin, L.-. Åke Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Eurospace 2015, 17, 1023–1029. [Google Scholar] [CrossRef]
- Svennberg, E.; Engdahl, J.; Al-Khalili, F.; Friberg, L.; Frykman, V.; Rosenqvist, M. Mass screening for untreated atrial fibril-lation: The STROKESTOP study. Circulation 2015, 131, 2176–2184. [Google Scholar] [CrossRef] [Green Version]
- Svennberg, E. The STROKESTOP-STUDY Benefits of Systematic Screening for Atrial fibrillation. In Proceedings of the EHRA Congress 2021, Online, 23–25 April 2021; Available online: https://digital-congress.escardio.org/EHRA-Congress/sessions/511-late-breaking-clinical-trials (accessed on 18 May 2021).
- A Study to Determine If Identification of Undiagnosed Atrial Fibrillation in People at Least 70 Years of Age Reduces the Risk of Stroke (GUARD-AF). Identifier: NCT04126486. Available online: ClinicalTrials.gov (accessed on 18 May 2021).
- Mant, J.; Burt, J.; Griffin, S.; Hobbs, R.; McManus, R.; Williams, K.; Dymond, A.; Lund, J.; Edwards, D.; Massou, L.; et al. Abstract D.11. The SAFER study—Screening for Atrial Fibrillation with ECG to Reduce stroke. In Proceedings of the Society for Academic Primary Care Conference, University of Leeds, Leeds, UK, 15–17 July 2020; p. 49. [Google Scholar]
- Diederichsen, S.Z.; Haugan, K.J.; Køber, L.; Højberg, S.; Brandes, A.; Kronborg, C.; Graff, C.; Holst, A.G.; Nielsen, J.B.; Krieger, D.; et al. Atrial fibrillation detected by continuous electrocardiographic monitoring using implantable loop recorder to prevent stroke in individuals at risk (the LOOP study): Rationale and design of a large randomized controlled trial. Am. Heart J. 2017, 187, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Li, K.H.C.; White, F.A.; Tipoe, T.; Liu, T.; Wong, K.C.; Jesuthasan, A.; Baranchuk, A.; Tse, G.; Yan, B.P. The Current State of Mobile Phone Apps for Monitoring Heart Rate, Heart Rate Variability, and Atrial Fibrillation: Narrative Review. JMIR mHealth uHealth 2019, 7, e11606. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- William, A.D.; Kanbour, M.; Callahan, T.; Bhargava, M.; Varma, N.; Rickard, J.; Saliba, W.; Wolski, K.; Hussein, A.; Lindsay, B.D.; et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: The iREAD Study. Heart Rhythm 2018, 15, 1561–1565. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. Anartificial intelligence-enabled ECG algorithm for the identification of patients with atrial fi-brillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Healey, J.; Connolly, S.; Alings, M.; Lopes, R. Apixaban for the Reduction of Thrombo-Embolism in Patients with Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA). Available online: https://clinicaltrials.gov/ct2/show/NCT01938248 (accessed on 17 August 2021).
- Kirchhof, P.; Blank, B. Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial High Rate Episodes (NOAH). Available online: https://clinicaltrials.gov/ct2/show/NCT02618577 (accessed on 17 August 2021).
- Gorenek, B.C.; Bax, J.; Boriani, G.; Chen, S.-A.; Dagres, N.; Glotzer, T.V.; Healey, J.S.; Israel, C.W.; Kudaiberdieva, G.; Levin, L.-A.; et al. ESC Scientific Document Group. Device- detected subclinical atrial tachyarrhythmias: Definition, implications and management—An European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisio-logia (SOLEACE). Europace 2017, 19, 1556–1578. [Google Scholar]
- Van Gelder, I.C.; Healey, J.S.; Crijns, H.J.; Wang, J.; Hohnloser, H.; Gold, M.R.; Capucci, A.; Lau, C.P.; Morillo, C.A.; Hobbelt, A.H.; et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in AS-SERT. Eur. Heart J. 2017, 38, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Chew, D.; Hartshorne, T.; Selvanayagam, J.B.; Aylward, P.; Sanders, P.; McGavigan, A.D. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: A systematic review and meta-analysis. Eur. Heart J. 2016, 37, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Passman, R.; Turakhia, M.; Connolly, A.T.; Nabutovsky, Y.; Varma, N. Atrial fibrillation burden, progression, and the risk of death: A case-crossover analysis in patients with cardiac implantable electronic devices. Eurospace 2018, 21, 404–413. [Google Scholar] [CrossRef]
- Ecker, V.; Knoery, C.; Rushworth, G.; Rudd, I.; Ortner, A.; Begley, D.; Leslie, S.J. A review of factors associated with maintenance of sinus rhythm after elective electrical cardioversion for atrial fibrillation. Clin. Cardiol. 2018, 41, 862–870. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef]
- Marrouche, N.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibril-lation catheter ablation: The DECAAF study. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef]
- Whitaker, J.; Rajani, R.; Chubb, H.; Gabrawi, M.; Varela, M.; Wright, M.; Niederer, S.; O’Neill, M. The role of myocardial wall thickness in atrial arrhythmogenesis. Eurospace 2016, 18, 1758–1772. [Google Scholar] [CrossRef]
- Wong, C.; Ganesan, A.; Selvanayagam, J.B. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur. Heart J. 2016, 38, 1294–1302. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, K.; Habibi, M.; Ipek, E.; Zahid, S.; Khurram, I.M.; Zimmerman, S.L.; Zipunnikov, V.; Spragg, D.; Ashikaga, H.; Trayanova, N.; et al. Association of Left atrial Local Conduction Velocity with Late Gadolinium En-hancement on Car-diac Magnetic Resonance in Patients with Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2016, 9, e002897. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Lee, M.-H.; Yu, J.; Pak, H.-N.; Ha, J.-W.; Lee, M.; Kim, Y.J.; Joung, B. Prognostic implication of left atrial sphericity in atrial fibrillation patients undergoing radiofrequency catheter ablation. Pacing Clin. Electrophysiol. 2017, 40, 713–720. [Google Scholar] [CrossRef]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have non-valvu-lar atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef]
- Borre, E.; Goode, A.; Raitz, G.; Shah, B.; Lowenstern, A.; Chatterjee, R.; Sharan, L.; Lapointe, N.M.A.; Yapa, R.; Davis, J.K.; et al. Predicting Thromboembolic and Bleeding Event Risk in Patients with Non-Valvular Atrial Fibrillation: A Systematic Review. Thromb. Haemost. 2018, 118, 2171–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulatns with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Boersma, L.V.; Bosschaert, M. Left atrial appendage occlusion for AF patients unable to Use oral Anticoagulation Therapy (COMPARE-LAAO). ClinicalTrials.gov Identifier: NCT04676880. Available online: https://clinicaltrials.gov/ct2/show/NCT04676880 (accessed on 23 May 2021).
- Whitlock, R.P.; Belley-Cote, E.P.; Paparella, D.; Healey, J.S.; Brady, K.; Sharma, M.; Reents, W.; Budera, P.; Baddour, A.J.; Fila, P.; et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N. Engl. J. Med. 2021, 384, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vranckx, P.; Valgimigli, M.; Eckardt, L.; Tijssen, J.; Lewalter, T.; Gargiulo, G.; Batushkin, V.; Campo, G.; Lysak, Z.; Vakaliuk, I.; et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): A randomised, open-label, phase 3b trial. Lancet 2019, 394, 1335–1343. [Google Scholar] [CrossRef]
- Gibson, C.M.; Mehran, R.; Bode, C.; Halperin, J.; Verheugt, F.W.; Wildgoose, P.; Birmingham, M.; Ianus, J.; Burton, P.; Van Eickels, M.; et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N. Engl. J. Med. 2016, 375, 2423–2434. [Google Scholar] [CrossRef] [Green Version]
- Lopes, R.D.; Hong, H.; Harskamp, R.E.; Bhatt, D.L.; Mehran, R.; Cannon, C.P.; Granger, C.B.; Verheugt, F.W.A.; Li, J.; Ten Berg, J.M.; et al. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: A network meta-analysis of randomized controlled trials. JAMA Cardiol. 2019, 4, 747–755. [Google Scholar] [CrossRef]
- Fiedler, K.A.; Maeng, M.; Mehilli, J.; Schulz-Schüpke, S.; Byrne, R.A.; Sibbing, D.; Hoppmann, P.; Schneider, S.; Fusaro, M.; Ott, I.; et al. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implan-tation: The ISAR-TRIPLE trial. J. Am. Coll. Cardiol. 2015, 65, 1619–1629. [Google Scholar] [CrossRef] [Green Version]
- Dewilde, W.J.; Oirbans, T.; Verheugt, F.W.; Kelder, J.C.; De Smet, B.J.; Herrman, J.-P.; Adriaenssens, T.; Vrolix, M.; Heestermans, A.A.; Vis, M.M.; et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013, 381, 1107–1115. [Google Scholar] [CrossRef]
- Cannon, C.P.; Bhatt, D.L.; Oldgren, J.; Lip, G.Y.; Ellis, S.G.; Kimura, T.; Maeng, M.; Merkely, B.; Zeymer, U.; Gropper, S.; et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N. Engl. J. Med. 2017, 377, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.J.; Berriman, T.J.; Diab, I.; Kamdar, R.; Richmond, L.; Baker, V.; Goromonzi, F.; Sawhney, V.; Duncan, E.; Page, S.P.; et al. A Randomized Controlled Trial of Catheter Ablation Versus Medical Treatment of Atrial Fibrillation in Heart Failure (The CAMTAF Trial). Circ. Arrhythm. Electrophysiol. 2014, 7, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.N.; Jaïs, P.; Cummings, J.; Di Biase, L.; Sanders, P.; Martin, D.O.; Kautzner, J.; Hao, S.; Themistoclakis, S.; Fanelli, R.; et al. Pulmonary-Vein Isolation for Atrial Fibrillation in Patients with Heart Failure. N. Engl. J. Med. 2008, 359, 1778–1785. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, S.; Taylor, A.J.; Costello, B.T.; Kaye, D.M.; McLellan, A.J.A.; Voskoboinik, A.; Sugumar, H.; Lockwood, S.M.; Stokes, M.B.; Pathik, B.; et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J. Am. Coll. Cardiol. 2017, 70, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.G.; Haldar, S.K.; Hussain, W.; Sharma, R.; Francis, D.P.; Rahman-Haley, S.L.; McDonagh, T.A.; Underwood, S.R.; Markides, V.; Wong, T. A randomized trial to assess catheter ablation versus rate control in the man-agement of persistent atrial fibrillation in heart failure. J. Am. Coll. Cardiol. 2013, 61, 1894–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, Z.K.; Safi, K.; Adeel, A.; Muhammad, S.Z.; Muhammad, U.K.; Muhammad, S.K.; Edo, K.; Mohamad, A. Meta-Analysis of Catheter Ablation versus Medical Therapy in Patients with AF without Heart Failure. J. Atr. Fibrillation 2020, 12, 2266. [Google Scholar]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; Van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Turakhia, M.P.; Santangeli, P.; Winkelmayer, W.C.; Xu, X.; Ullal, A.J.; Than, C.T.; Schmitt, S.; Holmes, T.H.; Frayne, S.M.; Phibbs, C.S.; et al. Increased mortality associated with Digoxin in contemporary pa-tients with atrial fibrillation: Findings from the TREAT-AF Study. J. Am. Coll. Cardiol. 2014, 64, 660–668. [Google Scholar] [CrossRef] [Green Version]
- Whitbeck, M.G.; Charnigo, R.J.; Khairy, P.; Ziada, K.; Bailey, A.L.; Zegarra, M.M.; Shah, J.; Morales, G.; Macaulay, T.; Sorrell, V.L.; et al. Increased mortality among patients taking digoxin—Analysis from the AFFIRM study. Eur. Heart J. 2012, 34, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Fonarow, G.; Van Veldhuisen, D.J.; Cleland, J.G.; Butler, J.; Epstein, A.E.; Patel, K.; Aban, I.B.; Aronow, W.S.; Anker, S.D.; et al. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: Findings from post hoc propensity-matched analysis of the AFFIRM trial. Eur. Heart J. 2013, 34, 1489–1497. [Google Scholar] [CrossRef] [Green Version]
- Kotecha, D.; Holmes, J.; Krum, H.; Altman, D.G.; Manzano, L.; Cleland, J.G.F.; Lip, G.Y.H.; Coats, A.J.S.; Andersson, B.; Kirchhof, P.; et al. Efficacy of beta blockers in patients with heart failure plus atrial fi-brillation: An indi-vidual-patient data meta-analysis. Lancet 2014, 384, 2235–2243. [Google Scholar] [CrossRef] [Green Version]
- Bavendiek, U.; Berliner, D.; Davila, L.A.; Schwab, J.; Maier, L.; Philipp, S.; Rieth, A.J.; Westenfeld, R.; Pirkowski, C..; Weber, K.; et al. DIGIT-HF Investigators and Committees. Rationale and design of the DIGIT-HF trial (DIGi-toxin to Improve ouTcomes in patients with advanced chronic Heart Failure): A randomized, double-blind, pla-cebo-controlled study. Eur J. Heart Fail. 2019, 21, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Willems, S.; Meyer, C.; De Bono, J.; Brandes, A.; Eckardt, L.; Elvan, A.; Van Gelder, I.; Goette, A.; Gulizia, M.; Haegeli, L.; et al. Cabins, castles, and constant hearts: Rhythm control therapy in patients with atrial fibrillation. Eur. Heart J. 2019, 40, 3793–3799. [Google Scholar] [CrossRef] [Green Version]
- Marijon, E.; Le Heuzey, J.-Y.; Connolly, S.; Yang, S.; Pogue, J.; Brueckmann, M.; Eikelboom, J.; Themeles, E.; Ezekowitz, M.; Wallentin, L.; et al. Response to Letter Regarding Article, “Causes of Death and Influencing Factors in Patients With Atrial Fibrillation: A Competing-Risk Analysis From the Randomized Evaluation of Long-Term Anticoagulant Therapy Study”. Circulation. 2014, 130, e85. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Yang, P.S.; You, S.C.; Sung, J.-H.; Jang, E.; Yu, H.T.; Kim, T.-H.; Pak, H.-N.; Lee, M.-H.; Lip, G.Y.H.; et al. Treatment timing and the effect of rhythm control strategy in patients with atrial fibril-lation: Nationwide cohort study. BMJ 2021, 373, n991. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.-S.; Sung, J.-H.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Pak, H.-N.; Lee, M.-H.; et al. Less dementia after catheter ablation for atrial fibrillation: A nationwide cohort study. Eur. Heart J. 2020, 41, 4483–4493. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Ali, A.A.; Mohamed, A.A.; El-Sherif, L.; Abdelsamed, M.-A.; Mohamed, M.K.; Sayed, M.K.; Mohamed, N.A.; Osman, A.A.; Shaheen, S.M.; et al. Rhythm Versus Rate Control for Atrial Fibrillation: A Meta-analysis of Randomized Controlled Trials. Biomed. Pharmacol. J. 2018, 11, 609–620. [Google Scholar] [CrossRef]
- Roy, D.; Talajic, M.; Nattel, S.; Wyse, D.G.; Dorian, P.; Lee, K.L.; Bourassa, M.G.; Arnold, J.M.O.; Buxton, A.E.; Camm, A.J.; et al. Rhythm Control versus Rate Control for Atrial Fibrillation and Heart Failure. N. Engl. J. Med. 2008, 358, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Corley, S.; Epstein, A.; DiMarco, J.P.; Domanski, M.J.; Geller, N.; Greene, H.L.; Josephson, R.A.; Kellen, J.C.; Klein, R.C.; Krahn, A.D.; et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004, 109, 1509–1513. [Google Scholar]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Wells, G.A.; Deyell, M.W.; Bennet, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; Khaykin, Y.; et al. Cryoablation or drug therapy for initial treatment of AF. N. Engl. J. Med. 2021, 384, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.M.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Biebauer, M.; Makati, K.; Halperon, B.; Gauri, A.; et al. Cryoballoon Ablation as initial Theapy for atrial Fibrillation. N. Engl. J. Med. 2021, 384, 316–324. [Google Scholar] [CrossRef]
- Velagic, V.; Pavlovic, N.; Chierchia, G.-B.; Hermida, J.-S.; Healey, S.; Arena, G.; Badenco, N.; Meyer, C.; Chen, J.; Iacopino, S.; et al. Abstract 13915: Cryoballoon Catheter Ablation versus Antiarrhythmic Drug as a First-Line Therapy for Patients With Paroxysmal Atrial Fibrillation: Results of the Cryo-FIRST Study. Circle 2020, 142, 13915. [Google Scholar] [CrossRef]
- Chew, D.S.; Black-Maier, E.; Loring, Z.; Noseworthy, P.A.; Packer, D.L.; Exner, D.V.; Mark, D.B.; Piccini, J.P. Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: A systematic review and meta-analysis of observational studies. Circ. Arrhythm. Electrophysiol. 2020, 13, e008128. [Google Scholar] [CrossRef]
- Pallisgaard, J.L.; Gislason, G.; Hansen, J.; Johannessen, A.; Torp-Pedersen, C.; Rasmussen, P.V.; Hansen, M.L. Temporal trends in atrial fibrillation recurrence rates after ablation between 2005 and 2014: A nationwide Danish cohort study. Eur. Heart J. 2018, 39, 442–449. [Google Scholar] [CrossRef]
- Walters, T.E.; Nisbet, A.; Morris, G.M.; Tan, G.; Mearns, M.; Teo, E.; Lewis, N.; Ng, A.; Gould, P.; Lee, G.; et al. Progression of atrial remodeling in patients with high-burden atrial fibrillation: Implications for early ablative therapy. Heart Rhythm 2016, 13, 331. [Google Scholar] [CrossRef] [Green Version]
- Pluymaekers, N.; Dudink, E.; Luermans, J.; Meeder, J.G.; Lenderink, T.; Widdershoven, J.; Bucx, J.J.J.; Rienstra, M.; Kamp, O.; Opstal, J.M.V.; et al. RACE ACWAS Investigators. Early or delayed cardioversion in recent-onset atrial fibrilla-tion. N. Engl. J. Med. 2019, 380, 1499–1508. [Google Scholar] [CrossRef]
- Jaïs, P.; Cauchemez, B.; Macle, L.; Daoud, E.; Khairy, P.; Subbiah, R.; Hocini, M.; Extramiana, F.; Sacher, F.; Bordachar, P.; et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: The A4 study. Circulation 2008, 118, 2498–2505. [Google Scholar] [CrossRef] [Green Version]
- Pappone, C.; Augello, G.; Sala, S.; Gugliotta, F.; Vicedomini, G.; Gulleta, S.; Paglino, G.; Mazzone, P.; Sora, N.; Greiss, I.; et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: The APAF Study. J. Am. Coll. Cardiol. 2006, 48, 2340–2347. [Google Scholar] [CrossRef] [Green Version]
- Packer, D.L.; Kowal, R.C.; Wheelan, K.R.; Irwin, J.M.; Champagne, J.; Guerra, P.G.; Dubuc, M.; Reddy, V.; Nelson, L.; Holcomb, R.G.; et al. STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for parox-ysmal atrial fibrillation: First results of the North American Arctic Front (STOP AF) pivotal trial. J. Am. Coll. Cardiol. 2013, 61, 1713–1723. [Google Scholar] [CrossRef] [Green Version]
- Wilber, D.J.; Pappone, C.; Neuzil, P.; Paola, A.D.; Marchlinski, F.; Natale, A.; Macle, L.; Daoud, E.G.; Calkins, H.; Hall, B.; et al. ThermoCool AF Trial Investigators. Comparison of antiarrhythmic drug therapy and radiofre-quency catheter ablation in patients with paroxysmal atrial fibrillation: A randomized controlled trial. JAMA 2010, 303, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Mont, L.; Bisbal, F.; Hernandez-Madrid, A.; Pérez-Castellano, N.; Viñolas, V.; Arenal, A.; Arribas, F.; Fernández-Lozano, I.; Bodegas, A.; Cobos, A.; et al. SARA investigators. Catheter ablation vs antiarrhythmic drug treatment of persistent atrial fibrillation: A multicentre, randomized, controlled trial (SARA study). Eur. Heart J. 2014, 35, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Delurgio, D.B.; Crossen, K.J.; Gill, J.; Blauth, C.; Oza, S.R.; Magnano, A.R.; Mostovych, M.A.; Halkos, M.E.; Tschopp, D.R.; Kerendi, F.; et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2020, 13, 009288. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Marrouche, N.F.; Khaykin, Y.; Gillinov, A.M.; Wazni, O.; Martin, D.O.; Rossillo, A.; Verma, A.; Cummings, J.; Ercyyes, D.; et al. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired sys-tolic function. J. Am. Coll. Cardiol. 2004, 43, 1004–1009. [Google Scholar] [CrossRef] [Green Version]
- Di Biase, L.; Mohanty, P.; Mohanty, S.; Santangeli, P.; Lakkireddy, D.; Reddy, M.; Jais, P.; Themistocklais, S.; Russo, A.D. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with conges-tive heart failure and an implanted device: Results from the AATAC multicenter randomized trial. Circulation 2016, 133, 1637–1644. [Google Scholar] [CrossRef] [Green Version]
- Packer, D.L.; Piccini, J.P.; Monahan, K.H.; Al-Khalidi, H.R.; Silverstein, A.P.; Noseworthy, P.A.; Poole, J.E.; Bahnson, T.D.; Lee, K.L.; Mark, D.B.; et al. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure. Circulation 2021, 143, 1377–1390. [Google Scholar] [CrossRef]
- Kuck, K.-H.; Merkley, B.; Zahn, R.; Arentz, T.; Seidl, K.; Schlüter, M.; Tilz, R.R.; Piorkowski, C.; Gellér, L.; Kleemann, T.; et al. Catheter Ablation versus best medical therapy in Patients with Persistent Atrial Fibrillation and Congestive Heart Failure: The randomized AMICA trial. Circ. Arrhythm. Electrophysiol. 2019, 12, e007731. [Google Scholar] [CrossRef]
- Cochrane Central Register of Controlled Trials. CAMERA-MRI II trial: Catheter Ablation versus Medical Rate Control of Atrial Fibrillation with Systolic Heart Failure and Myocardial Fibrosis—An MRI Guided Multi-Centre Randomised Controlled Clinical Trial. CN-02165295. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02165295/full (accessed on 20 May 2021).
- Black-Maier, E.; Ren, X.; Steinberg, B.A.; Green, C.L.; Barnett, A.S.; Rosa, N.S.; Al-Khatib, S.M.; Atwater, B.D.; Daubert, J.P.; Frazier-Mills, C.; et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm 2018, 15, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; DeVore, A.D.; Wu, J.J.; Hammill, B.G.; Sharma, A.; Cooper, L.B.; Felker, G.M.; Piccini, J.P.; Allen, L.A.; Heidenreich, P.A.; et al. Rhythm Control Versus Rate Control in Patients with Atrial Fibrillation and Heart Failure with Pre-served Ejection Fraction: Insights from Get with The Guidelines—Heart Failure. JAHA 2019, 8, e011560. [Google Scholar] [CrossRef]
- Terricabras, M.; Verma, A.; Morillo, C.A. Measuring Success in Ablation of Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2018, 11, e006582. [Google Scholar] [CrossRef]
- Camm, J.; Reiffel, J. Defining endpoints in clinical trials on atrial fibrillation. Eur. Heart J. Suppl. 2008, 10, H55–H78. [Google Scholar] [CrossRef] [Green Version]
- Jackson, N.; Mahmoodi, E.; Leitch, J.; Varlow, M.; Davies, A.; Collins, N.; Leigh, L.; Pldmeadow, C.; Boyle, A. Effect of Outcome Measures on the Apparent Efficacy of Ablation for Atrial fi-brillation: Why “Success” is an Inappropriate Term. Heart Lung Circ. 2021, 30, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Dretzke, J.; Chuchu, N.; Agarwal, R.; Herd, C.; Chua, W.; Fabritz, L.; Bayliss, S.; Kotecha, D.; Deeks, J.; Kirchhof, P.; et al. Predicting recurrent atrial fibrillation after catheter ablation: A systematic review of prognostic models. Eurpace 2020, 22, 748–760. [Google Scholar] [CrossRef]

| Rate Control | Medical Rhythm Control + | |||
|---|---|---|---|---|
| No significant SHD | CAD, VAD, HFpEF | HFrEF | ||
| 1. Line | Betablocker NDCC * (IB) | Flecainide Propafenone Dronedarone (IA) | Dronedarone Amiodarone (IA) | Amiodarone (IA) |
| 2. Line | Digoxin (IB) Combinations (IIa) | Sotalol (IIbA) | Sotalol (IIbA) | - |
| 3. Line | Pace&Ablate (IIaB) Amiodarone (IIbB) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonko, J.B.; Wright, M.J. Review of the 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation—What Has Changed and How Does This Affect Daily Practice. J. Clin. Med. 2021, 10, 3922. https://doi.org/10.3390/jcm10173922
Tonko JB, Wright MJ. Review of the 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation—What Has Changed and How Does This Affect Daily Practice. Journal of Clinical Medicine. 2021; 10(17):3922. https://doi.org/10.3390/jcm10173922
Chicago/Turabian StyleTonko, Johanna B., and Matthew J. Wright. 2021. "Review of the 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation—What Has Changed and How Does This Affect Daily Practice" Journal of Clinical Medicine 10, no. 17: 3922. https://doi.org/10.3390/jcm10173922
APA StyleTonko, J. B., & Wright, M. J. (2021). Review of the 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation—What Has Changed and How Does This Affect Daily Practice. Journal of Clinical Medicine, 10(17), 3922. https://doi.org/10.3390/jcm10173922






