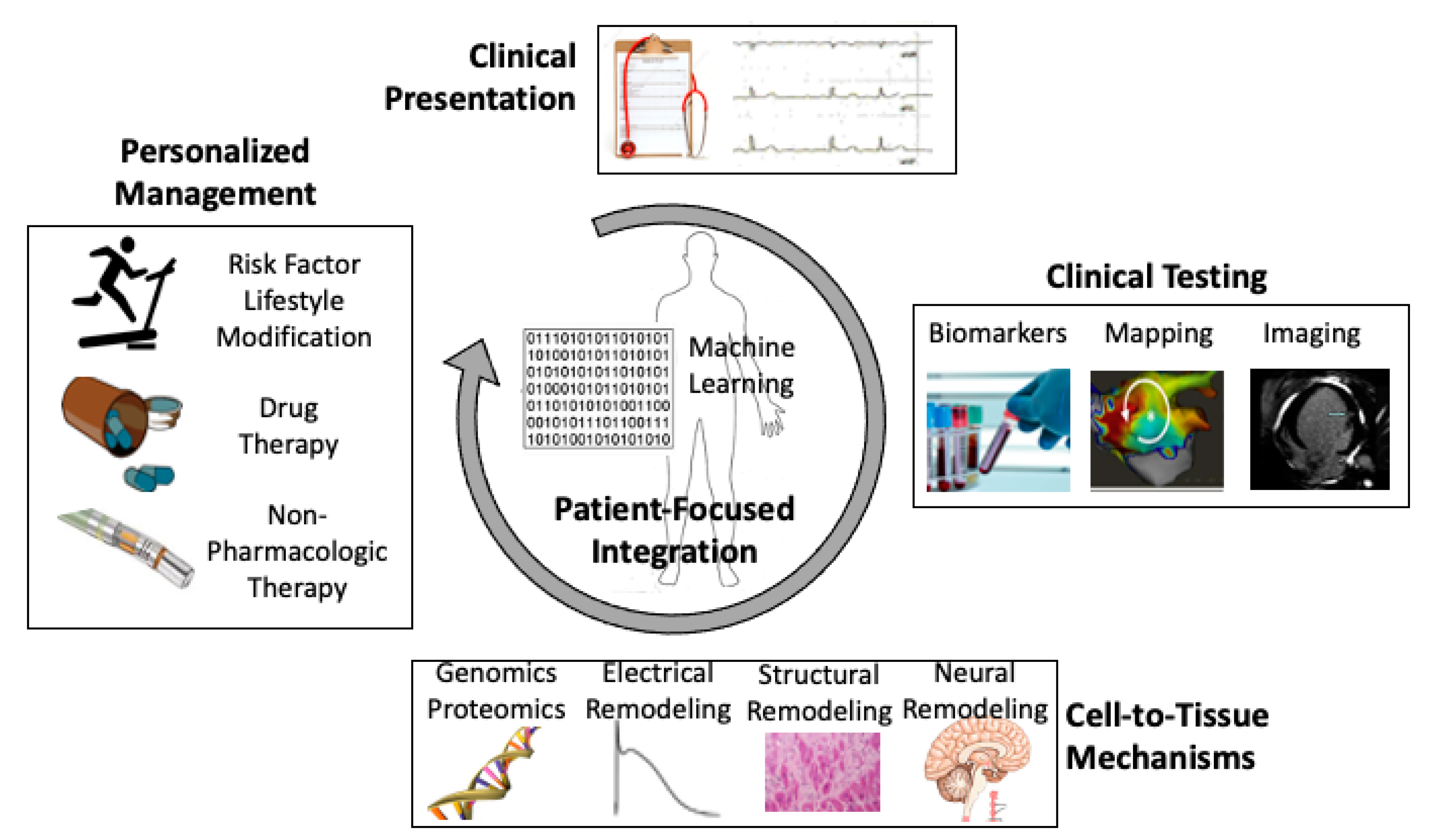
Identifying Atrial Fibrillation Mechanisms for Personalized Medicine
Abstract
:1. Introduction
2. Risk Factors Provide Mechanistic Clues
3. Pathophysiology of AF at the Genetic Level
4. Pathophysiology for AF at the Cellular Level
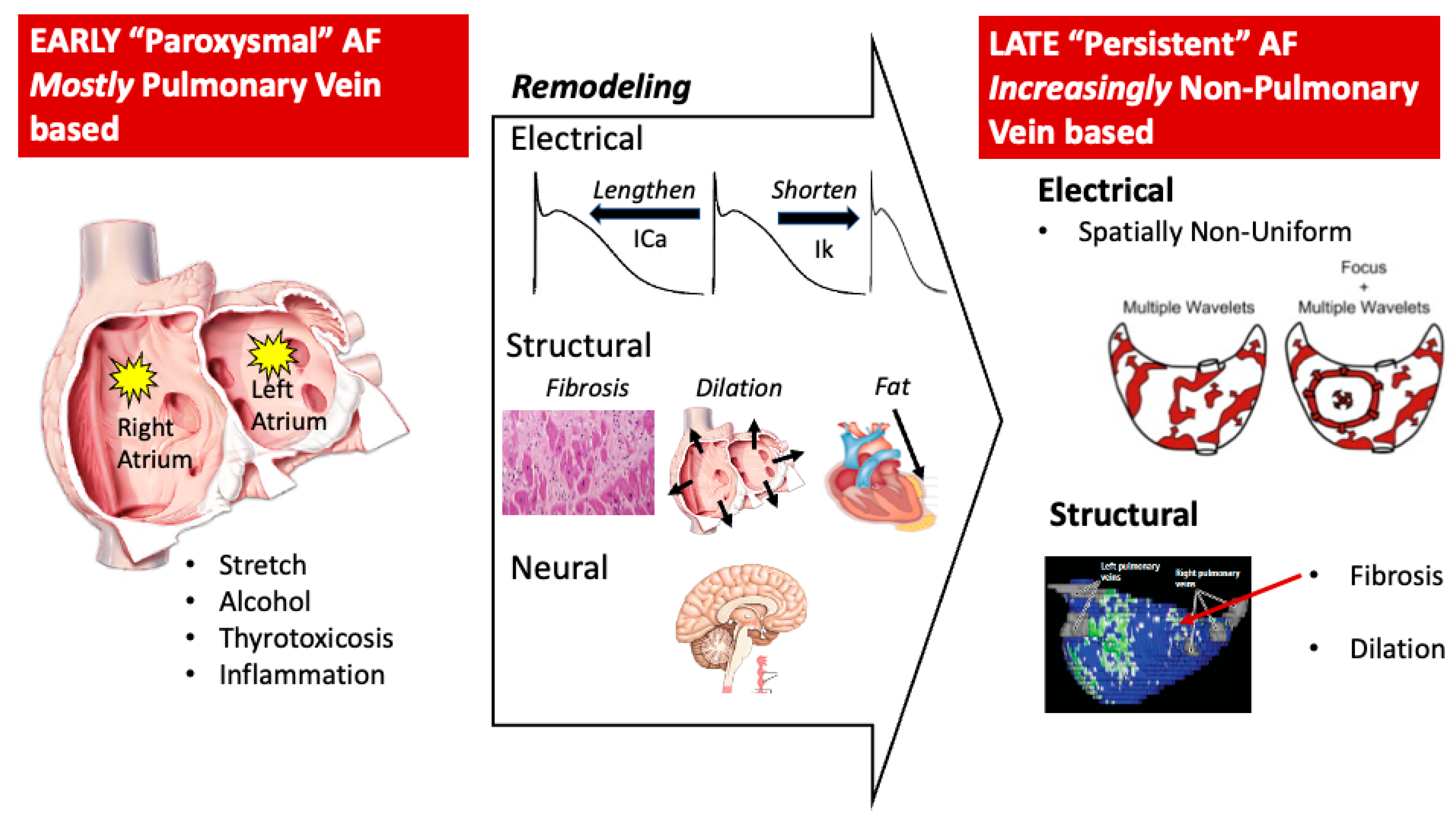
4.1. Electrical Modeling
4.2. Structural Remodeling
4.3. Autonomic Remodeling
5. AF Pathophysiology within the Heart
5.1. Triggers
5.2. Which Triggers Initiate AF?
5.3. Mechanisms for the Maintenance of AF Once Initiated (Substrate)
5.4. Clinical Mapping of Driver Regions
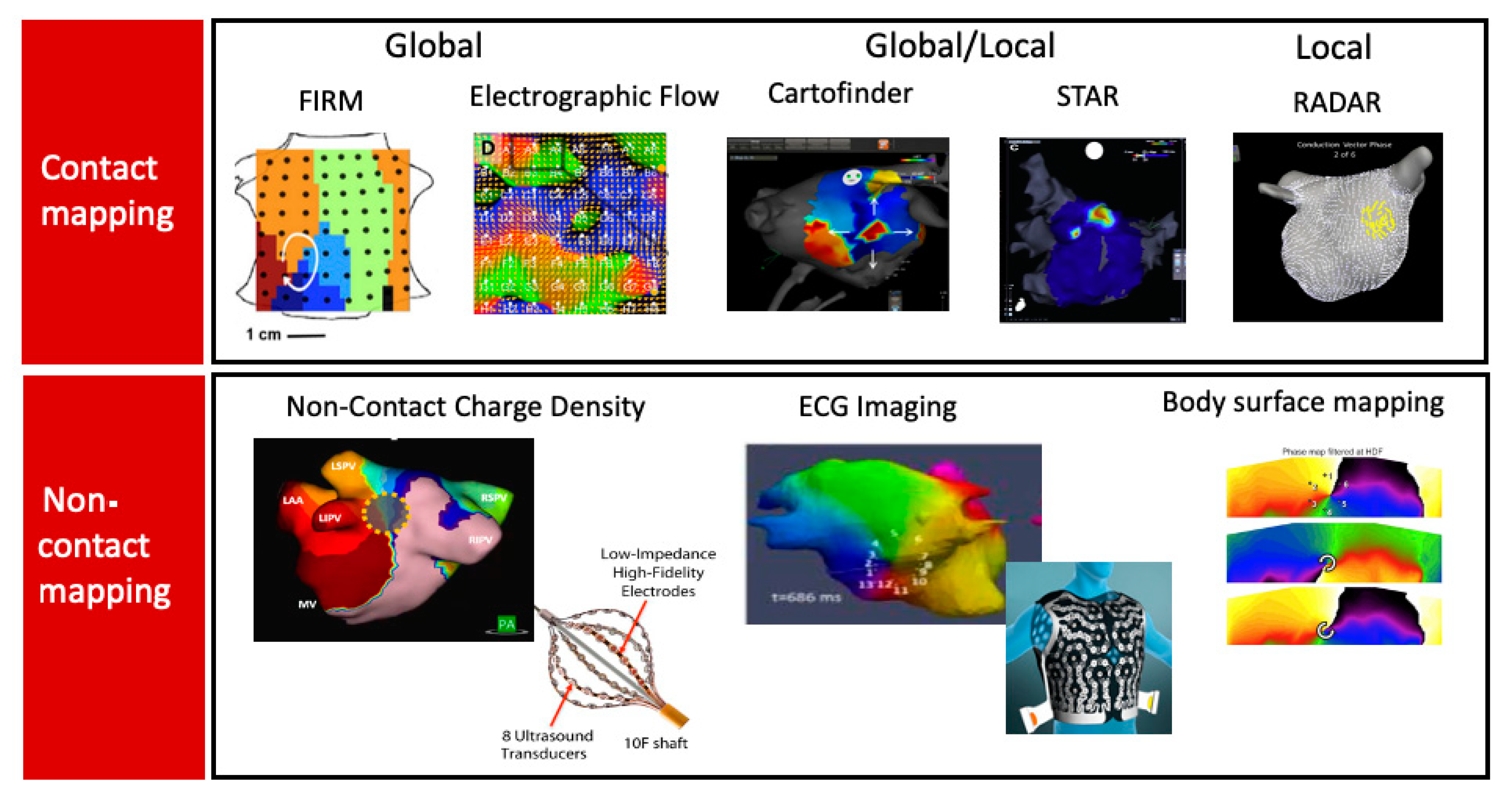
5.5. Contact Mapping
5.5.1. Global or Panoramic Mapping
5.5.2. Local Contact Mapping, i.e., Small Regions Mapped Sequentially
5.5.3. Mixed (Both Local and Global)
5.5.4. Non-Contact Mapping
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, D.A.; Skjøth, F.; Lip, G.Y.H.; Larsen, T.B.; Kotecha, D. Temporal Trends in Incidence, Prevalence, and Mortality of Atrial Fibrillation in Primary Care. J. Am. Heart Assoc. 2017, 6, e005155. [Google Scholar] [CrossRef] [PubMed]
- Savelieva, I.; Camm, A.J. Clinical relevance of silent atrial fibrillation: Prevalence, prognosis, quality of life, and management. J. Interv. Card. Electrophysiol. 2000, 4, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Olshansky, B. The electrocardiogram in atrial fibrillation. In UpToDate; Knight, B.P., Yeon, S.B., Eds.; UpToDate: Waltham, MA, USA, 2021. [Google Scholar]
- Krittanawong, C.; Johnson, K.W.; Rosenson, R.S.; Wang, Z.; Aydar, M.; Baber, U.; Min, J.K.; Tang, W.W.; Halperin, J.L.; Narayan, S.M. Deep learning for cardiovascular medicine: A practical primer. Eur. Heart J. 2019, 40, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.J.; Selvalingam, A.; Alhusseini, M.I.; Krummen, D.E.; Corrado, C.; Abuzaid, F.; Baykaner, T.; Meyer, C.; Clopton, P.; Giles, W.; et al. Machine Learned Cellular Phenotypes in Cardiomyopathy Predict Sudden Death. Circ. Res. 2021, 128, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Dobrev, D. Deciphering the fundamental mechanisms of atrial fibrillation: A quest for over a century. Cardiovasc. Res. 2016, 109, 465–466. [Google Scholar] [CrossRef] [Green Version]
- Peirlinck, M.; Costabal, F.S.; Yao, J.; Guccione, J.M.; Tripathy, S.; Wang, Y.; Ozturk, D.; Segars, P.; Morrison, T.M.; Levine, S.; et al. Precision medicine in human heart modeling: Perspectives, challenges, and opportunities. Biomech. Model. Mechanobiol. 2021, 20, 803–831. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2017, 14, e275–e444. [Google Scholar] [CrossRef] [Green Version]
- Charitos, E.I.; Pürerfellner, H.; Glotzer, T.V.; Ziegler, P.D. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: Insights from 1195 patients continuously monitored with implantable devices. J. Am. Coll. Cardiol. 2014, 63, 2840–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, J.E.; Bahnson, T.D.; Monahan, K.H.; Johnson, G.; Rostami, H.; Silverstein, A.P.; Al-Khalidi, H.R.; Rosenberg, Y.; Mark, D.B.; Lee, K.L.; et al. Recurrence of Atrial Fibrillation After Catheter Ablation or Antiarrhythmic Drug Therapy in the CABANA Trial. J. Am. Coll. Cardiol. 2020, 75, 3105–3118. [Google Scholar] [CrossRef]
- Andrade, J.G.; Yao, R.R.; Deyell, M.W.; Hawkins, N.M.; Rizkallah, J.; Jolly, U.; Khoo, C.; Raymond, J.M.; McKinney, J.; Cheung, C.; et al. Clinical assessment of AF pattern is poorly correlated with AF burden and post ablation outcomes: A CIRCA-DOSE sub-study. J. Electrocardiol. 2020, 60, 159–164. [Google Scholar] [CrossRef]
- Jons, C.; Hansen, P.S.; Johannessen, A.; Hindricks, G.; Raatikainen, P.; Kongstad, O.; Walfridsson, H.; Pehrson, S.; Almroth, H.; Hartikainen, J.; et al. The Medical ANtiarrhythmic Treatment or Radiofrequency Ablation in Paroxysmal Atrial Fibrillation (MANTRA-PAF) trial: Clinical rationale, study design, and implementation. Europace 2009, 11, 917–923. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Wazni, O.M.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Niebauer, M.; Makati, K.; Halperin, B.; Gauri, A.; et al. Cryoballoon Ablation as Initial Therapy for Atrial Fibrillation. N. Engl. J. Med. 2020, 384, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Tucker, N.R.; Clauss, S.; Ellinor, P.T. Common variation in atrial fibrillation: Navigating the path from genetic association to mechanism. Cardiovasc. Res. 2016, 109, 493–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, M. Characterization, Pathogenesis, and Clinical Implications of Inflammation-Related Atrial Myopathy as an Important Cause of Atrial Fibrillation. J. Am. Heart Assoc. 2020, 9, e015343. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; McEvoy, R.D.; Cowie, M.R.; Somers, V.K.; Nattel, S.; Lévy, P.; Kalman, J.M.; Sanders, P. Associations of Obstructive Sleep Apnea With Atrial Fibrillation and Continuous Positive Airway Pressure Treatment: A Review. JAMA Cardiol. 2018, 3, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Voskoboinik, A.; Kalman, J.M.; De Silva, A.; Nicholls, T.; Costello, B.; Nanayakkara, S.; Prabhu, S.; Stub, D.; Azzopardi, S.; Vizi, D.; et al. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N. Engl. J. Med. 2020, 382, 20–28. [Google Scholar] [CrossRef]
- Guasch, E.; Benito, B.; Qi, X.; Cifelli, C.; Naud, P.; Shi, Y.; Mighiu, A.; Tardif, J.C.; Tadevosyan, A.; Chen, Y.; et al. Atrial fibrillation promotion by endurance exercise: Demonstration and mechanistic exploration in an animal model. J. Am. Coll. Cardiol. 2013, 62, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Sun, Y.; Zhang, H.; Wang, B.; Chen, C.; Wang, Y.; Chen, J.; Tan, X.; Zhang, J.; Xia, F.; et al. Long-term night shift work is associated with the risk of atrial fibrillation and coronary heart disease. Eur. Heart J. 2021, 42, 4180–4188. [Google Scholar] [CrossRef]
- Wang, T.J.; Parise, H.; Levy, D.; D’Agostino, R.B.; Wolf, P.A.; Vasan, R.S.; Benjamin, E.J. Obesity and the Risk of New-Onset Atrial Fibrillation. JAMA 2004, 292, 2471–2477. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agostino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J.; et al. Inter-Relationship between AF and CHF in the Framingham Heart Study. Circulation 2003, 107, 2920–2925. [Google Scholar] [CrossRef] [Green Version]
- Barrett, T.W.; Self, W.H.; Wasserman, B.S.; McNaughton, C.D.; Darbar, D. Evaluating the HATCH score for predicting progression to sustained atrial fibrillation in ED patients with new atrial fibrillation. Am. J. Emerg. Med. 2013, 31, 792–797. [Google Scholar] [CrossRef] [Green Version]
- Potpara, T.S.; Polovina, M.M.; Marinkovic, J.M.; Lip, G.Y. A comparison of clinical characteristics and long-term prognosis in asymptomatic and symptomatic patients with first-diagnosed atrial fibrillation: The Belgrade Atrial Fibrillation Study. Int. J. Cardiol. 2013, 168, 4744–4749. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Skjoth, F.; Nielsen, P.B.; Larsen, T.B. Evaluation of the C2HEST Risk Score as a Possible Opportunistic Screening Tool for Incident Atrial Fibrillation in a Healthy Population (From a Nationwide Danish Cohort Study). Am. J. Cardiol. 2020, 125, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Khurshid, S.; Kartoun, U.; Ashburner, J.M.; Trinquart, L.; Philippakis, A.; Khera, A.V.; Ellinor, P.T.; Ng, K.; Lubitz, S.A. Performance of Atrial Fibrillation Risk Prediction Models in Over 4 Million Individuals. Circ. Arrhythm. Electrophysiol. 2021, 14, e008997. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Hindricks, G.; Kosiuk, J.; Arya, A.; Sommer, P.; Husser, D.; Rolf, S.; Richter, S.; Huo, Y.; Piorkowski, C.; et al. Comparison of CHADS2, R2CHADS2, and CHA2DS2-VASc scores for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation: The Leipzig Heart Center AF Ablation Registry. Circ. Arrhythm. Electrophysiol. 2014, 7, 281–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornej, J.; Hindricks, G.; Shoemaker, M.B.; Husser, D.; Arya, A.; Sommer, P.; Rolf, S.; Saavedra, P.; Kanagasundram, A.; Whalen, S.P.; et al. The APPLE score: A novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin. Res. Cardiol. 2015, 104, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Ham, H.A.; Klungel, O.H.; Singer, D.E.; Leufkens, H.G.; van Staa, T.P. Comparative Performance of ATRIA, CHADS2, and CHA2DS2-VASc Risk Scores Predicting Stroke in Patients With Atrial Fibrillation: Results From a National Primary Care Database. J. Am. Coll. Cardiol. 2015, 66, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Abed, H.S.; Wittert, G.A.; Leong, D.P.; Shirazi, M.G.; Bahrami, B.; Middeldorp, M.E.; Lorimer, M.F.; Lau, D.H.; Antic, N.A.; Brooks, A.G.; et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: A randomized clinical trial. JAMA 2013, 310, 2050–2060. [Google Scholar] [CrossRef]
- Alonso, A.; Bahnson, J.L.; Gaussoin, S.A.; Bertoni, A.; Johnson, K.C.; Lewis, C.E.; Vetter, M.; Mantzoros, C.S.; Jeffery, R.W.; Soliman, E.Z. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: The Look AHEAD randomized trial. Am. Heart J. 2015, 170, 770–777.e5. [Google Scholar] [CrossRef] [Green Version]
- Gessler, N.; Willems, S.; Steven, D.; Aberle, J.; Akbulak, R.O.; Gosau, N.; Hoffmann, B.A.; Meyer, C.; Sultan, A.; Tilz, R.; et al. Supervised Obesity Reduction Trial for AF Ablation Patients: Results from the SORT-AF trial. Europace 2021, 23, 1548–1558. [Google Scholar] [CrossRef]
- Mahida, S.; Lubitz, S.A.; Rienstra, M.; Milan, D.J.; Ellinor, P.T. Monogenic atrial fibrillation as pathophysiological paradigms. Cardiovasc. Res. 2011, 89, 692–700. [Google Scholar] [CrossRef]
- Yoneda, Z.T.; Anderson, K.C.; Quintana, J.A.; O’Neill, M.J.; Sims, R.A.; Glazer, A.M.; Shaffer, C.M.; Crawford, D.M.; Stricker, T.; Ye, F.; et al. Early-Onset Atrial Fibrillation and the Prevalence of Rare Variants in Cardiomyopathy and Arrhythmia Genes. JAMA Cardiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Thorolfsdottir, R.B.; Fritsche, L.G.; Zhou, W.; Skov, M.W.; Graham, S.E.; Herron, T.J.; McCarthy, S.; Schmidt, E.; Sveinbjornsson, G.; et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat. Genet. 2018, 50, 1234–1239. [Google Scholar] [CrossRef]
- Shoemaker, M.B.; Husser, D.; Roselli, C.; Al Jazairi, M.; Chrispin, J.; Kühne, M.; Neumann, B.; Knight, S.; Sun, H.; Mohanty, S.; et al. Genetic Susceptibility for Atrial Fibrillation in Patients Undergoing Atrial Fibrillation Ablation. Circ. Arrhythm. Electrophysiol. 2020, 13, e007676. [Google Scholar] [CrossRef]
- Unterhuber, M.; Kresoja, K.-P.; Rommel, K.-P.; Besler, C.; Baragetti, A.; Klöting, N.; Ceglarek, U.; Blüher, M.; Scholz, M.; Catapano, A.L.; et al. Proteomics-Enabled Deep Learning Machine Algorithms Can Enhance Prediction of Mortality. J. Am. Coll. Cardiol. 2021, 78, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Voigt, N.; Nattel, S.; Dobrev, D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 2014, 114, 1483–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Benardeau, A.; Nattel, S. Contrasting efficacy of dofetilide in different experimental models of atrial fibrillatoin. Circulation 2000, 102, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Chaldoupi, S.M.; Loh, P.; Hauer, R.N.; de Bakker, J.M.; van Rijen, H.V. The role of connexin40 in atrial fibrillation. Cardiovasc. Res. 2009, 84, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karck, M.; Wehrens, X.H.T.; Nattel, S.; Dobrev, D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Zlochiver, S.; Johnson, C.; Tolkacheva, E.G. Constant DI pacing suppresses cardiac alternans formation in numerical cable models. Chaos 2017, 27, 093903. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Surget, E.; Michel, C.; Benoist, D.; Dubes, V.; Walton, R.D.; Martinez, M.E.; Guillot, B.; Constantin, M.; Hocini, M.; et al. B-AB01-01 local repolarization heterogeneity is more arrhythmogenic than structural and functional conduction heterogeneities in an ex vivo porcine model. Heart Rhythm. 2021, 18, S1. [Google Scholar] [CrossRef]
- Roux, J.F.; Zado, E.; Callans, D.J.; Garcia, F.; Lin, D.; Marchlinski, F.; Bala, R.; Dixit, S.; Riley, M.; Russo, A.M.; et al. Antiarrhythmics After Ablation of Atrial Fibrillation (5A Study). Circulation 2009, 120, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Mesubi, O.O.; Anderson, M.E. Atrial remodelling in atrial fibrillation: CaMKII as a nodal proarrhythmic signal. Cardiovasc. Res. 2016, 109, 542–557. [Google Scholar] [CrossRef] [Green Version]
- Purohit, A.; Rokita, A.G.; Guan, X.; Chen, B.; Koval, O.; Voigt, N.; Neef, S.; Sowa, T.; Gao, Z.; Luczak, E.D.; et al. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 2013, 128, 1748–1757. [Google Scholar] [CrossRef] [Green Version]
- Reilly, S.N.; Liu, X.; Carnicer, R.; Recalde, A.; Muszkiewicz, A.; Jayaram, R.; Carena, M.C.; Wijesurendra, R.; Stefanini, M.; Surdo, N.C.; et al. Up-regulation of miR-31 in human atrial fibrillation begets the arrhythmia by depleting dystrophin and neuronal nitric oxide synthase. Sci. Transl. Med. 2016, 8, 340ra74. [Google Scholar] [CrossRef] [Green Version]
- Violi, F.; Pastori, D.; Pignatelli, P.; Loffredo, L. Antioxidants for prevention of atrial fibrillation: A potentially useful future therapeutic approach? A review of the literature and meta-analysis. Europace 2014, 16, 1107–1116. [Google Scholar] [CrossRef]
- Abed, H.S.; Samuel, C.S.; Lau, D.H.; Kelly, D.; Royce, S.G.; Alasady, M.; Mahajan, R.; Kuklik, P.; Zhang, Y.; Brooks, A.G.; et al. Obesity results in progressive atrial structural and electrical remodeling: Implications for atrial fibrillation. Heart Rhythm. 2013, 10, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Opacic, D.; van Bragt, K.A.; Nasrallah, H.M.; Schotten, U.; Verheule, S. Atrial metabolism and tissue perfusion as determinants of electrical and structural remodelling in atrial fibrillation. Cardiovasc. Res. 2016, 109, 527–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olshansky, B.; Heller, E.N.; Mitchell, L.B.; Chandler, M.; Slater, W.; Green, M.; Brodsky, M.; Barrell, P.; Greene, H.L. Are Transthoracic Echocardiographic Parameters Associated With Atrial Fibrillation Recurrence or Stroke?: Results From the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. J. Am. Coll. Cardiol. 2005, 45, 2026–2033. [Google Scholar] [CrossRef] [Green Version]
- Bunch, T.J.; Munger, T.M.; Friedman, P.A.; Asirvatham, S.J.; Brady, P.A.; Cha, Y.-M.; Rea, R.F.; Shen, W.-K.; Powell, B.D.; Ommen, S.R.; et al. Substrate and procedural predictors of outcomes after catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Electrophysiol. 2008, 19, 1009–1014. [Google Scholar] [CrossRef]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D.; Dominic, P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: A meta-analysis. Europace 2018, 20, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Demarchi, A.; Neumann, L.; Rordorf, R.; Conte, G.; Sanzo, A.; Özkartal, T.; Savastano, S.; Regoli, F.; Vicentini, A.; Caputo, M.L.; et al. Long-term outcome of catheter ablation for atrial fibrillation in patients with severe left atrial enlargement and reduced left ventricular ejection fraction. Europace 2021, 23, 1751–1756. [Google Scholar] [CrossRef]
- Ahlberg, G.; Andreasen, L.; Ghouse, J.; Bertelsen, L.; Bundgaard, H.; Haunsø, S.; Svendsen, J.H.; Olesen, M.S. Genome-wide association study identifies 18 novel loci associated with left atrial volume and function. Eur. Heart J. 2021, 42, 4523–4534. [Google Scholar] [CrossRef] [PubMed]
- Rettmann, M.E.; Holmes, D.R., III; Monahan, K.H.; Breen, J.F.; Bahnson, T.D.; Mark, D.B.; Poole, J.E.; Ellis, A.M.; Silverstein, A.P.; Al-Khalidi, H.R.; et al. Treatment-Related Changes in Left Atrial Structure in Atrial Fibrillation: Findings From the CABANA Imaging Substudy. Circ. Arrhythm. Electrophysiol. 2021, 14, e008540. [Google Scholar] [CrossRef]
- Luong, C.; Thompson, D.J.; Bennett, M.; Gin, K.; Jue, J.; Barnes, M.E.; Colley, P.; Tsang, T.S. Right atrial volume is superior to left atrial volume for prediction of atrial fibrillation recurrence after direct current cardioversion. Can. J. Cardiol. 2015, 31, 29–35. [Google Scholar] [CrossRef]
- Johner, N.; Namdar, M.; Shah, D.C. Right Atrial Complexity Evolves With Stepwise Left-Sided Persistent Atrial Fibrillation Substrate Ablation and Predicts Outcomes. JACC Clin. Electrophysiol. 2020, 6, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Platonov, P.G.; Mitrofanova, L.B.; Orshanskaya, V.; Ho, S.Y. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J. Am. Coll. Cardiol. 2011, 58, 2225–2232. [Google Scholar] [CrossRef] [Green Version]
- Lau, C.P.; Gbadebo, T.D.; Connolly, S.J.; Van Gelder, I.C.; Capucci, A.; Gold, M.R.; Israel, C.W.; Morillo, C.; Siu, C.-W.; Abe, H.; et al. Ethnic differences in atrial fibrillation identified using implanted cardiac devices. J. Cardiovasc. Electrophysiol. 2013, 24, 381–387. [Google Scholar] [CrossRef]
- Zahid, S.; Whyte, K.N.; Schwarz, E.L.; Blake, R.C.; Boyle, P.M.; Chrispin, J.; Prakosa, A.; Ipek, E.G.; Pashakhanloo, F.; Halperin, H.R.; et al. Feasibility of using patient-specific models and the “minimum cut” algorithm to predict optimal ablation targets for left atrial flutter. Heart Rhythm. 2016, 13, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Greene, T.; Dean, J.M.; Kholmovski, E.G.; Boer, L.M.; Mansour, M.; Calkins, H.; Marchlinski, F.; Wilber, D.; Hindricks, G.; et al. Efficacy of LGE-MRI-guided fibrosis ablation versus conventional catheter ablation of atrial fibrillation: The DECAAF II trial: Study design. J. Cardiovasc. Electrophysiol. 2021, 32, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Zghaib, T.; Keramati, A.; Chrispin, J.; Huang, D.; Balouch, M.A.; Ciuffo, L.; Berger, R.D.; Marine, J.E.; Ashikaga, H.; Calkins, H.; et al. Multimodal Examination of Atrial Fibrillation Substrate: Correlation of Left Atrial Bipolar Voltage Using Multi-Electrode Fast Automated Mapping, Point-by-Point Mapping, and Magnetic Resonance Image Intensity Ratio. JACC Clin. Electrophysiol. 2018, 4, 59–68. [Google Scholar] [CrossRef]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2013, 36, 795–805. [Google Scholar] [CrossRef] [Green Version]
- Stojanovska, J.; Kazerooni, E.A.; Sinno, M.; Gross, B.H.; Watcharotone, K.; Patel, S.; Jacobson, J.A.; Oral, H. Increased epicardial fat is independently associated with the presence and chronicity of atrial fibrillation and radiofrequency ablation outcome. Eur. Radiol. 2015, 25, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.N.; Redheuil, A.; Gandjbakhch, E. Cardiac adipose tissue and atrial fibrillation: The perils of adiposity. Cardiovasc. Res. 2016, 109, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, R.; Lau, D.H.; Brooks, A.G.; Shipp, N.J.; Wood, J.P.; Manavis, J.; Samuel, C.S.; Patel, K.P.; Finnie, J.W.; Alasady, M.; et al. Atrial Fibrillation and Obesity: Reverse Remodeling of Atrial Substrate With Weight Reduction. JACC Clin. Electrophysiol. 2021, 7, 630–641. [Google Scholar] [CrossRef]
- El Mahdiui, M.; Simon, J.; Smit, J.M.; Kuneman, J.H.; van Rosendael, A.R.; Steyerberg, E.W.; van der Geest, R.J.; Száraz, L.; Herczeg, S.; Szegedi, N.; et al. Posterior Left Atrial Adipose Tissue Attenuation Assessed by Computed Tomography and Recurrence of Atrial Fibrillation after Catheter Ablation. Circ. Arrhythm. Electrophysiol. 2021, 4, e009135. [Google Scholar] [CrossRef]
- Katritsis, D.G.; Pokushalov, E.; Romanov, A.; Giazitzoglou, E.; Siontis, G.C.; Po, S.S.; Camm, A.J.; Ioannidis, J.P. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: A randomized clinical trial. J. Am. Coll. Cardiol. 2013, 62, 2318–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, J.S.; Shabanov, V.; Ponomarev, D.; Losik, D.; Ivanickiy, E.; Kropotkin, E.; Polyakov, K.; Ptaszynski, P.; Keweloh, B.; Yao, C.J.; et al. Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial. JAMA 2020, 323, 248–255. [Google Scholar] [CrossRef]
- Stavrakis, S.; Stoner, J.A.; Humphrey, M.B.; Morris, L.; Filiberti, A.; Reynolds, J.C.; Elkholey, K.; Javed, I.; Twidale, N.; Riha, P.; et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. JACC Clin. Electrophysiol. 2020, 6, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.; Singh, J.P.; Parks, K.A.; Katritsis, D.G.; Stavrakis, S.; Armoundas, A.A. Low-Level Tragus Stimulation Modulates Atrial Alternans and Fibrillation Burden in Patients With Paroxysmal Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e020865. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.H.; Mitchell, L.B.; Ploquin, N.; Faruqi, S.; Kuriachan, V.P. Review of Stereotactic Arrhythmia Radioablation Therapy for Cardiac Tachydysrhythmias. CJC Open 2021, 3, 236–247. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; Van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Clarnette, J.A.; Brooks, A.G.; Mahajan, R.; Elliott, A.D.; Twomey, D.; Pathak, R.K.; Kumar, S.; Munawar, D.A.; Young, G.D.; Kalman, J.M.; et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: A systematic review and meta-analysis. Europace 2018, 20, f366–f376. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Wells, G.A.; Deyell, M.W.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; Khaykin, Y.; et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N. Engl. J. Med. 2020, 384, 305–315. [Google Scholar] [CrossRef]
- Khan, R. Identifying and understanding the role of pulmonary vein activity in atrial fibrillation. Cardiovasc. Res. 2004, 64, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Nery, P.B.; Belliveau, D.; Nair, G.M.; Bernick, J.; Redpath, C.J.; Szczotka, A.; Sadek, M.M.; Green, M.S.; Wells, G.; Birnie, D.H. Relationship Between Pulmonary Vein Reconnection and Atrial Fibrillation Recurrence: A Systematic Review and Meta-Analysis. JACC Clin. Electrophysiol. 2016, 2, 474–483. [Google Scholar] [CrossRef]
- Pratola, C.; Baldo, E.; Notarstefano, P.; Toselli, T.; Ferrari, R. Radiofrequency atrial fibrillation ablation based on pathophysiology: A diversified protocol with long-term follow-up. J. Cardiovasc. Med. 2008, 9, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H.; Hoffmann, B.A.; Ernst, S.; Wegscheider, K.; Treszl, A.; Metzner, A.; Eckardt, L.; Lewalter, T.; Breithardt, G.; Willems, S. Impact of Complete Versus Incomplete Circumferential Lines Around the Pulmonary Veins During Catheter Ablation of Paroxysmal Atrial Fibrillation: Results From the Gap-Atrial Fibrillation-German Atrial Fibrillation Competence Network 1 Trial. Circ. Arrhythm. Electrophysiol. 2016, 9, e003337. [Google Scholar] [CrossRef]
- Prabhu, S.; Kalla, M.; Peck, K.Y.; Voskoboinik, A.; McLellan, A.J.; Pathik, B.; Nalliah, C.J.; Wong, G.R.; Sugumar, H.; Azzopardi, S.M.; et al. Pulmonary vein activity does not predict the outcome of catheter ablation for persistent atrial fibrillation: A long-term multicenter prospective study. Heart Rhythm. 2018, 15, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Haissaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-A.; Hsieh, M.H.; Tai, C.T.; Tsai, C.F.; Prakash, V.S.; Yu, W.C.; Hsu, T.L.; Ding, Y.A.; Chang, M.S. Initiation of Atrial Fibrillation by Ectopic Beats Originating From the Pulmonary Veins: Electrophysiological Characteristics, Pharmacological Responses, and Effects of Radiofrequency Ablation. Circulation 1999, 100, 1879–1886. [Google Scholar] [CrossRef] [Green Version]
- Hocini, M.; Ho, S.Y.; Kawara, T.; Linnenbank, A.C.; Potse, M.; Shah, D.; Jaïs, P.; Janse, M.J.; Haïssaguerre, M.; De Bakker, J.M. Electrical conduction in canine pulmonary veins: Electrophysiological and anatomic correlation. Circulation 2002, 105, 2442–2448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, T.; Shah, D.C.; Jaïs, P.; Hocini, M.; Deisenhofer, I.; Choi, K.-J.; Macle, L.; Clémenty, J.; Haïssaguerre, M. Electrogram polarity reversal as an additional indicator of breakthroughs from the left atrium to the pulmonary veins. J. Am. Coll. Cardiol. 2002, 39, 1337–1344. [Google Scholar] [CrossRef] [Green Version]
- Patterson, E.; Lazzara, R.; Szabo, B.; Liu, H.; Tang, D.; Li, Y.-H.; Scherlag, B.J.; Po, S.S. Sodium-calcium exchange initiated by the Ca2+ transient: An arrhythmia trigger within pulmonary veins. J. Am. Coll. Cardiol. 2006, 47, 1196–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, M.; Vaquero, L.M.; Hou, L.; Campbell, K.; Zlochiver, S.; Klos, M.; Mironov, S.; Berenfeld, O.; Honjo, H.; Kodama, I.; et al. Mechanisms of stretch-induced atrial fibrillation in the presence and the absence of adrenocholinergic stimulation: Interplay between rotors and focal discharges. Heart Rhythm. 2009, 6, 1009–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijesurendra, R.S.; Casadei, B. Mechanisms of atrial fibrillation. Heart 2019, 105, 1860–1867. [Google Scholar] [CrossRef]
- Jais, P.; Hocini, M.; Macle, L.; Choi, K.-J.; Deisenhofer, I.; Weerasooriya, R.; Shah, D.C.; Garrigue, S.; Raybaud, F.; Scavee, C.; et al. Distinctive Electrophysiological Properties of Pulmonary Veins in Patients With Atrial Fibrillation. Circulation 2002, 106, 2479–2485. [Google Scholar] [CrossRef] [Green Version]
- Wit, A.L.; Cranefield, P.F. Triggered and automatic activity in the canine coronary sinus. Circ. Res. 1977, 41, 434–445. [Google Scholar] [CrossRef]
- Schmitt, C.; Ndrepepa, G.; Weber, S.; Schmieder, S.; Weyerbrock, S.; Schneider, M.; Karch, M.R.; Deisenhofer, I.; Schreieck, J.; Zrenner, B.; et al. Biatrial multisite mapping of atrial premature complexes triggering onset of atrial fibrillation. Am. J. Cardiol. 2002, 89, 1381–1387. [Google Scholar] [CrossRef]
- Lin, W.-S.; Tai, C.-T.; Hsieh, M.-H.; Tsai, C.-F.; Lin, Y.-K.; Tsao, H.-M.; Huang, J.-L.; Yu, W.-C.; Yang, S.-P.; Ding, Y.-A.; et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation 2003, 107, 3176–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santangeli, P.; Marchlinski, F.E. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm. 2017, 14, 1087–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Sun, J.-Y.; Wu, L.-D.; Hao, J.-F.; Wang, R.-X. The long-term efficacy and safety of combining ablation and left atrial appendage closure: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2021, 32, 3068–3081. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.M.; Kazi, D.; Krummen, D.E.; Rappel, W.-J. Repolarization and Activation Restitution Near Human Pulmonary Veins and Atrial Fibrillation Initiation: A Mechanism for the Initiation of Atrial Fibrillation by Premature Beats. J. Am. Coll. Cardiol. 2008, 52, 1222–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, S.M.; Franz, M.R.; Clopton, P.; Pruvot, E.J.; Krummen, D.E. Repolarization Alternans Reveals Vulnerability to Human Atrial Fibrillation. Circulation 2011, 123, 2922–2930. [Google Scholar] [CrossRef] [Green Version]
- Krummen, D.E.; Bayer, J.D.; Ho, J.; Ho, G.; Smetak, M.R.; Clopton, P.; Trayanova, N.A.; Narayan, S.M. Mechanisms of human atrial fibrillation initiation: Clinical and computational studies of repolarization restitution and activation latency. Circ. Arrhythm. Electrophysiol. 2012, 5, 1149–1159. [Google Scholar] [CrossRef] [Green Version]
- Lalani, G.; Schricker, A.; Gibson, M.; Rostamian, A.; Krummen, D.E.; Narayan, S.M. Atrial Conduction Slows Immediately Before the Onset of Human Atrial Fibrillation: A Bi-Atrial Contact Mapping Study of Transitions to Atrial Fibrillation. J. Am. Coll. Cardiol. 2012, 59, 595–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostock, T.; Steven, D.; Lutomsky, B.; Servatius, H.; Drewitz, I.; Klemm, H.; Müllerleile, K.; Ventura, R.; Meinertz, T.; Willems, S. Atrial fibrillation begets atrial fibrillation in the pulmonary veins on the impact of atrial fibrillation on the electrophysiological properties of the pulmonary veins in humans. J. Am. Coll. Cardiol. 2008, 51, 2153–2160. [Google Scholar] [CrossRef] [Green Version]
- Hao, S.C.; Christini, D.J.; Stein, K.M.; Jordan, P.N.; Iwai, S.; Bramwell, O.; Markowitz, S.M.; Mittal, S.; Lerman, B.B. Effect of beta-adrenergic blockade on dynamic electrical restitution in vivo. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H390–H394. [Google Scholar] [CrossRef] [Green Version]
- Karwath, A.; Bunting, K.V.; Gill, S.K.; Tica, O.; Pendleton, S.; Aziz, F.; Barsky, A.D.; Chernbumroong, S.; Duan, J.; Mobley, A.R.; et al. Redefining β-blocker response in heart failure patients with sinus rhythm and atrial fibrillation: A machine learning cluster analysis. Lancet 2021, 398, 1427–1435. [Google Scholar] [CrossRef]
- Shinagawa, K.; Shiroshita-Takeshita, A.; Schram, G.; Nattel, S. Effects of Antiarrhythmic Drugs on Fibrillation in the Remodeled Atrium: Insights Into the Mechanism of the Superior Efficacy of Amiodarone. Circulation 2003, 107, 1440–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schricker, A.A.; Lalani, G.G.; Krummen, D.E.; Rappel, W.-J.; Narayan, S.M. Human atrial fibrillation initiates via organized rather than disorganized mechanisms. Circ. Arrhythm. Electrophysiol. 2014, 7, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Vidmar, D.; Narayan, S.M.; Rappel, W.J. Phase synchrony reveals organization in human atrial fibrillation. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H2118–H2126. [Google Scholar] [CrossRef] [Green Version]
- Kowalewski, C.A.B.; Shenasa, F.; Rodrigo, M.; Clopton, P.; Meckler, G.; Alhusseini, M.I.; Swerdlow, M.A.; Joshi, V.; Hossainy, S.; Zaman, J.A.B.; et al. Interaction of Localized Drivers and Disorganized Activation in Persistent Atrial Fibrillation: Reconciling Putative Mechanisms Using Multiple Mapping Techniques. Circ. Arrhythm. Electrophysiol. 2018, 11, e005846. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; O’Neill, M.D.; Hocini, M.; Dubois, R.; Matsuo, S.; Knecht, S.; Mahapatra, S.; Lim, K.-T.; Jaïs, P.; Jonsson, A.; et al. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J. Am. Coll. Cardiol. 2008, 51, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Stiles, M.K.; Brooks, A.G.; Kuklik, P.; John, B.; Dimitri, H.; Lau, D.H.; Wilson, L.; Dhar, S.; Roberts-Thomson, R.L.; Mackenzie, L.; et al. High-density mapping of atrial fibrillation in humans: Relationship between high-frequency activation and electrogram fractionation. J. Cardiovasc. Electrophysiol. 2008, 19, 1245–1253. [Google Scholar] [CrossRef]
- Lazar, S.; Dixit, S.; Callans, D.J.; Lin, D.; Marchlinski, F.; Gerstenfeld, E.P. Effect of pulmonary vein isolation on the left-to-right atrial dominant frequency gradient in human atrial fibrillation. Heart Rhythm. 2006, 3, 889–895. [Google Scholar] [CrossRef]
- Krummen, D.E.; Peng, K.A.; Bullinga, J.R.; Narayan, S.M. Centrifugal Gradients of Rate and Organization in Human Atrial Fibrillation. Pacing Clin. Electrophysiol. 2009, 32, 1366–1378. [Google Scholar] [CrossRef] [Green Version]
- Coveney, S.; Corrado, C.; Roney, C.H.; O’Hare, D.; Williams, S.E.; O’Neill, M.D.; Niederer, S.A.; Clayton, R.H.; Oakley, J.E.; Wilkinson, R.D. Gaussian process manifold interpolation for probabilistic atrial activation maps and uncertain conduction velocity. Philos. Trans. A Math. Phys. Eng. Sci. 2020, 378, 20190345. [Google Scholar] [CrossRef]
- Sanders, P.; Nalliah, C.J.; Dubois, R.; Takahashi, Y.; Hocini, M.; Rotter, M.; Rostock, T.; Sacher, F.; Hsu, L.-F.; Jonsson, A.; et al. Frequency mapping of the pulmonary veins in paroxysmal versus permanent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2006, 17, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Petrutiu, S.; Sahakian, A.V.; Fisher, W.; Swiryn, S. Manifestation of Left Atrial Events and Interatrial Frequency Gradients in the Surface Electrocardiogram During Atrial Fibrillation: Contributions from Posterior Leads. J. Cardiovasc. Electrophysiol. 2009, 20, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Ulphani, J.S.; Ng, J.; Aggarwal, R.; Cain, J.H.; Gordon, D.; Yang, E.; Morris, A.R.; Arora, R.; Goldberger, J.J.; Kadish, A.H. Frequency gradients during two different forms of fibrillation in the canine atria. Heart Rhythm. 2007, 4, 1315–1323. [Google Scholar] [CrossRef]
- Lim, P.B.; Malcolme-Lawes, L.C.; Stuber, T.; Kojodjojo, P.; Wright, I.J.; Francis, D.P.; Davies, D.W.; Peters, N.S.; Kanagaratnam, P. Stimulation of the Intrinsic Cardiac Autonomic Nervous System Results in a Gradient of Fibrillatory Cycle Length Shortening Across the Atria During Atrial Fibrillation in Humans. J. Cardiovasc. Electrophysiol. 2011, 22, 1224–1231. [Google Scholar] [CrossRef]
- Sarmast, F.; Kolli, A.; Zaitsev, A.; Parisian, K.; Dhamoon, A.S.; Guha, P.K.; Warren, M.; Anumonwo, J.M.; Taffet, S.M.; Berenfeld, O.; et al. Cholinergic atrial fibrillation: IK,ACh gradients determine unequal left/right atrial frequencies and rotor dynamics. Cardiovasc. Res. 2003, 59, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M.; Mandapati, R.; Berenfeld, O.; Chen, J.; Samie, F.H.; Jalife, J. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation 2001, 103, 2631–2636. [Google Scholar] [CrossRef] [Green Version]
- Schuessler, R.B.; Kawamoto, T.; Hand, D.E.; Mitsuno, M.; Bromberg, B.I.; Cox, J.L.; Boineau, J.P. Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium. Circulation 1993, 88, 250–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allessie, M.A.; de Groot, N.M.S.; Houben, R.P.M.; Schotten, U.; Boersma, E.; Smeets, J.L.; Crijns, H.J. Electropathological Substrate of Long-standing Persistent Atrial Fibrillation in Patients with Structural Heart Disease: Longitudinal Dissociation. Circ. Arrhythm. Electrophysiol. 2010, 3, 606–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigmond, E.; Pashaei, A.; Amraoui, S.; Cochet, H.; Hassaguerre, M. Percolation as a mechanism to explain atrial fractionated electrograms and reentry in a fibrosis model based on imaging data. Heart Rhythm 2016, 13, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Moe, G.K.; Rheinboldt, W.C.; Abildskov, J.A. A computer model of atrial fibrillation. Am. Heart J. 1964, 67, 200–220. [Google Scholar] [CrossRef]
- Allessie, M.A.; Lammers, W.; Smeets, J.; Bonke, F.I.M.; Hollen, J. Experimental evaluation of Moe’s multiple wavelet hypothesis of atrial fibrillation. In Cardiac Arrythmias 1985; Grune & Stratton: New York, NY, USA, 1985; pp. 265–276. [Google Scholar]
- Davidenko, J.M.; Pertsov, A.M.; Salomonsz, R.; Baxter, W.P.; Jalife, J. Spatiotemporal irregularities of spiral wave activity in isolated ventricular muscle. J. Electrocardiol. 1992, 24, 113–122. [Google Scholar] [CrossRef]
- Gray, R.A.; Pertsov, A.M.; Jalife, J. Spatial and temporal organization during cardiac fibrillation. Nature 1998, 392, 75–78. [Google Scholar] [CrossRef]
- Pandit, S.V.; Jalife, J. Rotors and the dynamics of cardiac fibrillation. Circ. Res. 2013, 112, 849–862. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.J.; Briggs, C.; Moore, B.T.; Csepe, T.A.; Li, N.; Zhao, J.; Garikipati, N.V.; Janssen, P.M.; Mohler, P.J.; Hummel, J.D.; et al. Human Atrial Fibrillation Drivers Seen Simultaneously by Focal Impulse and Rotor Mapping and High-resolution Optical Mapping. Circulation 2015, 132, A18402. [Google Scholar] [CrossRef]
- Baykaner, T.; Rogers, A.J.; Meckler, G.L.; Zaman, J.; Navara, R.; Rodrigo, M.; Alhusseini, M.; Kowalewski, C.A.; Viswanathan, M.N.; Narayan, S.M.; et al. Clinical Implications of Ablation of Drivers for Atrial Fibrillation: A Systematic Review and Meta-Analysis. Circ. Arrhythm. Electrophysiol. 2018, 11, e006119. [Google Scholar] [CrossRef] [PubMed]
- Honarbakhsh, S.; Schilling, R.J.; Providência, R.; Keating, E.; Chow, A.; Sporton, S.; Lowe, M.; Earley, M.J.; Lambiase, P.D.; Hunter, R.J. Characterization of drivers maintaining atrial fibrillation: Correlation with markers of rapidity and organization on spectral analysis. Heart Rhythm 2018, 15, 1296–1303. [Google Scholar] [CrossRef]
- Hansen, B.J.; Zhao, J.; Li, N.; Zolotarev, A.; Zakharkin, S.; Wang, Y.; Atwal, J.; Kalyanasundaram, A.; Abudulwahed, S.H.; Helfrich, K.M.; et al. Human Atrial Fibrillation Drivers Resolved With Integrated Functional and Structural Imaging to Benefit Clinical Mapping. JACC Clin. Electrophysiol. 2018, 4, 1501–1515. [Google Scholar] [CrossRef]
- Anter, E.; Duytschaever, M.; Shen, C.; Strisciuglio, T.; Leshem, E.; Contreras-Valdes, F.M.; Waks, J.W.; Zimetbaum, P.J.; Kumar, K.; Spector, P.S.; et al. Activation Mapping With Integration of Vector and Velocity Information Improves the Ability to Identify the Mechanism and Location of Complex Scar-Related Atrial Tachycardias. Circ. Arrhythm. Electrophysiol. 2018, 11, e006536. [Google Scholar] [CrossRef] [PubMed]
- Spector, P.S.; De Sa, D.D.C.; Tischler, E.S.; Thompson, N.C.; Habel, N.; Stinnett-Donnelly, J.; Benson, B.E.; Bielau, P.; Bates, J.H. Ablation of multi-wavelet re-entry: General principles and in silico analyses. Europace 2012, 14 (Suppl. 5), v106–v111. [Google Scholar] [CrossRef] [Green Version]
- Baykaner, T.; Zografos, T.; Zaman, J.; Pantos, I.; Alhusseini, M.; Navara, R.; Krummen, D.E.; Narayan, S.M.; Katritsis, D.G. Spatial relationship of organized rotational and focal sources in human atrial fibrillation to autonomic ganglionated plexi. Int. J. Cardiol. 2017, 240, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, F.D.; Birnie, D.H.; Nair, G.M.; Szczotka, A.; Redpath, C.J.; Sadek, M.M.; Nery, P.B. Efficacy and safety of driver-guided catheter ablation for atrial fibrillation: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2017, 28, 1371–1378. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, Y.-J.; Narayan, S.M.; Baykaner, T.; Lo, M.-T.; Chung, F.-P.; Chen, Y.-Y.; Chang, S.-L.; Lo, L.-W.; Hu, Y.-F.; et al. Comparison of phase mapping and electrogram-based driver mapping for catheter ablation in atrial fibrillation. Pacing Clin. Electrophysiol. 2019, 42, 216–223. [Google Scholar] [CrossRef]
- Bellmann, B.; Lin, T.; Ruppersberg, P.; Zettwitz, M.; Guttmann, S.; Tscholl, V.; Nagel, P.; Roser, M.; Landmesser, U.; Rillig, A. Identification of active atrial fibrillation sources and their discrimination from passive rotors using electrographical flow mapping. Clin. Res. Cardiol. 2018, 107, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, M.; Tamboli, M.; Ms, M.A.; Moosvi, N.; Rogers, A.; Leef, G.; Wang, P.; Rillig, A.; Brachmann, J.; Sauer, W.; et al. Comparing phase and electrographic flow mapping for persistent atrial fibrillation. Pacing Clin. Electrophysiol. 2019, 42, 499–507. [Google Scholar] [CrossRef]
- Seitz, J.; Bars, C.; Théodore, G.; Beurtheret, S.; Lellouche, N.; Bremondy, M.; Ferracci, A.; Faure, J.; Penaranda, G.; Yamazaki, M.; et al. AF Ablation Guided by Spatiotemporal Electrogram Dispersion Without Pulmonary Vein Isolation: A Wholly Patient-Tailored Approach. J. Am. Coll. Cardiol. 2017, 69, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Jiang, W.-F.; Wu, S.-H.; Xu, K.; Liu, X. Electrogram dispersion-guided driver ablation adjunctive to high-quality pulmonary vein isolation in atrial fibrillation of varying durations. J. Cardiovasc. Electrophysiol. 2020, 31, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Honarbakhsh, S.; Hunter, R.J.; Ullah, W.; Keating, E.; Finlay, M.; Schilling, R.J. Ablation in Persistent Atrial Fibrillation Using Stochastic Trajectory Analysis of Ranked Signals (STAR) Mapping Method. JACC Clin. Electrophysiol. 2019, 5, 817–829. [Google Scholar] [CrossRef]
- Choudry, S.; Mansour, M.; Sundaram, S.; Nguyen, D.T.; Dukkipati, S.R.; Whang, W.; Kessman, P.; Reddy, V.Y. RADAR: A Multicenter Food and Drug Administration Investigational Device Exemption Clinical Trial of Persistent Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2020, 13, e007825. [Google Scholar] [CrossRef] [PubMed]
- Daoud, E.G.; Zeidan, Z.; Hummel, J.D.; Weiss, R.; Houmsse, M.; Augostini, R.; Kalbfleisch, S.J. Identification of Repetitive Activation Patterns Using Novel Computational Analysis of Multielectrode Recordings During Atrial Fibrillation and Flutter in Humans. JACC Clin. Electrophysiol. 2017, 3, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Sarkozy, A.; Skanes, A.; Duytschaever, M.; Bulava, A.; Urman, R.; Amos, Y.A.; De Potter, T. Characterization and significance of localized sources identified by a novel automated algorithm during mapping of human persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 1480–1488. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yamammoto, T.; Sekigawa, M.; Yamaguchi, J.; Shirai, Y.; Tao, S.; Hayashi, T.; Takigawa, M.; Goya, M.; Sasano, T. Mapping After Pulmonary Vein Isolation in Persistent Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2020, 13, e008511. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.; Willems, S.; Meyer, C.; Verma, A.; Heck, P.; Zhu, M.; Shi, X.; Chou, D.; Dang, L.; Scharf, C.; et al. High-resolution noncontact charge-density mapping of endocardial activation. JCI Insight 2019, 4, e126422. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Parikh, P.; Chen, Z.; Angel, N.; Norman, M.; Hussain, W.; Butcher, C.; Haldar, S.; Jones, D.G.; Riad, O.; et al. Validation of Dipole Density Mapping during Atrial Fibrillation and Sinus Rhythm in Human Left Atrium. JACC Clin. Electrophysiol. 2019, 6, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Narayan, S.M. Getting in Contact With Atrial Fibrillation or Not. JACC Clin. Electrophysiol. 2020, 6, 182–184. [Google Scholar] [CrossRef]
- Willems, S.; Verma, A.; Betts, T.; Murray, S.; Neuzil, P.; Ince, H.; Steven, D.; Sultan, A.; Heck, P.M.; Hall, M.C.; et al. Targeting Nonpulmonary Vein Sources in Persistent Atrial Fibrillation Identified by Noncontact Charge Density Mapping. Circ. Arrhythm. Electrophysiol. 2019, 12, e007233. [Google Scholar] [CrossRef] [PubMed]
- Knecht, S.; Sohal, M.; Deisenhofer, I.; Albenque, J.-P.; Arentz, T.; Neumann, T.; Cauchemez, B.; Duytschaever, M.; Ramoul, K.; Verbeet, T.; et al. Multicentre evaluation of non-invasive biatrial mapping for persistent atrial fibrillation ablation: The AFACART study. Europace 2017, 19, 1302–1309. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Hocini, M.; Denis, A.; Shah, A.J.; Komatsu, Y.; Yamashita, S.; Daly, M.; Amraoui, S.; Zellerhoff, S.; Picat, M.-Q.; et al. Driver domains in persistent atrial fibrillation. Circulation 2014, 130, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deb, B.; Ganesan, P.; Feng, R.; Narayan, S.M. Identifying Atrial Fibrillation Mechanisms for Personalized Medicine. J. Clin. Med. 2021, 10, 5679. https://doi.org/10.3390/jcm10235679
Deb B, Ganesan P, Feng R, Narayan SM. Identifying Atrial Fibrillation Mechanisms for Personalized Medicine. Journal of Clinical Medicine. 2021; 10(23):5679. https://doi.org/10.3390/jcm10235679
Chicago/Turabian StyleDeb, Brototo, Prasanth Ganesan, Ruibin Feng, and Sanjiv M. Narayan. 2021. "Identifying Atrial Fibrillation Mechanisms for Personalized Medicine" Journal of Clinical Medicine 10, no. 23: 5679. https://doi.org/10.3390/jcm10235679
APA StyleDeb, B., Ganesan, P., Feng, R., & Narayan, S. M. (2021). Identifying Atrial Fibrillation Mechanisms for Personalized Medicine. Journal of Clinical Medicine, 10(23), 5679. https://doi.org/10.3390/jcm10235679





