Impact of Reduced-Dose Nonvitamin K Antagonist Oral Anticoagulants on Outcomes Compared to Warfarin in Korean Patients with Atrial Fibrillation: A Nationwide Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
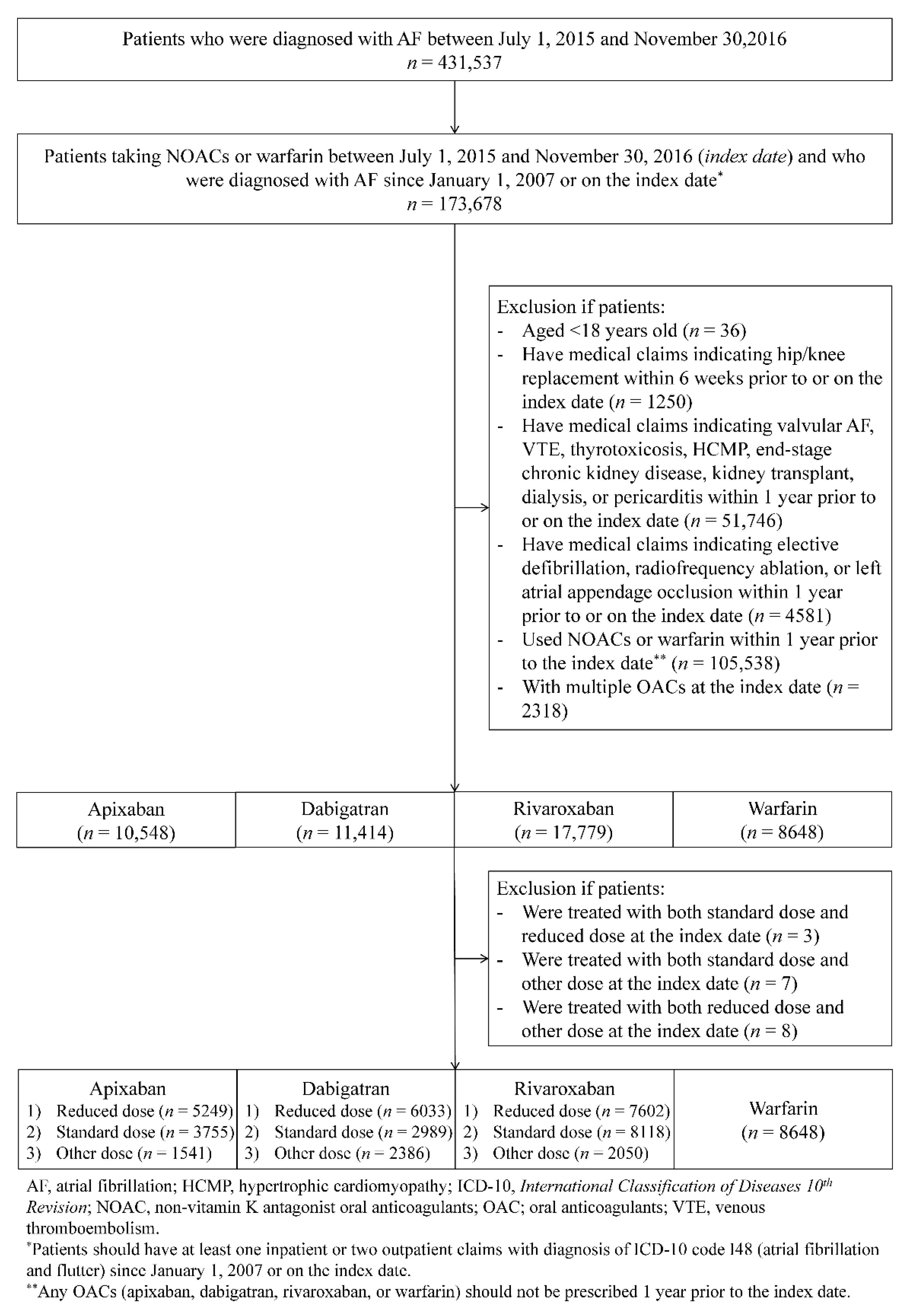
2.2. Study Population
2.3. Study Endpoints
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
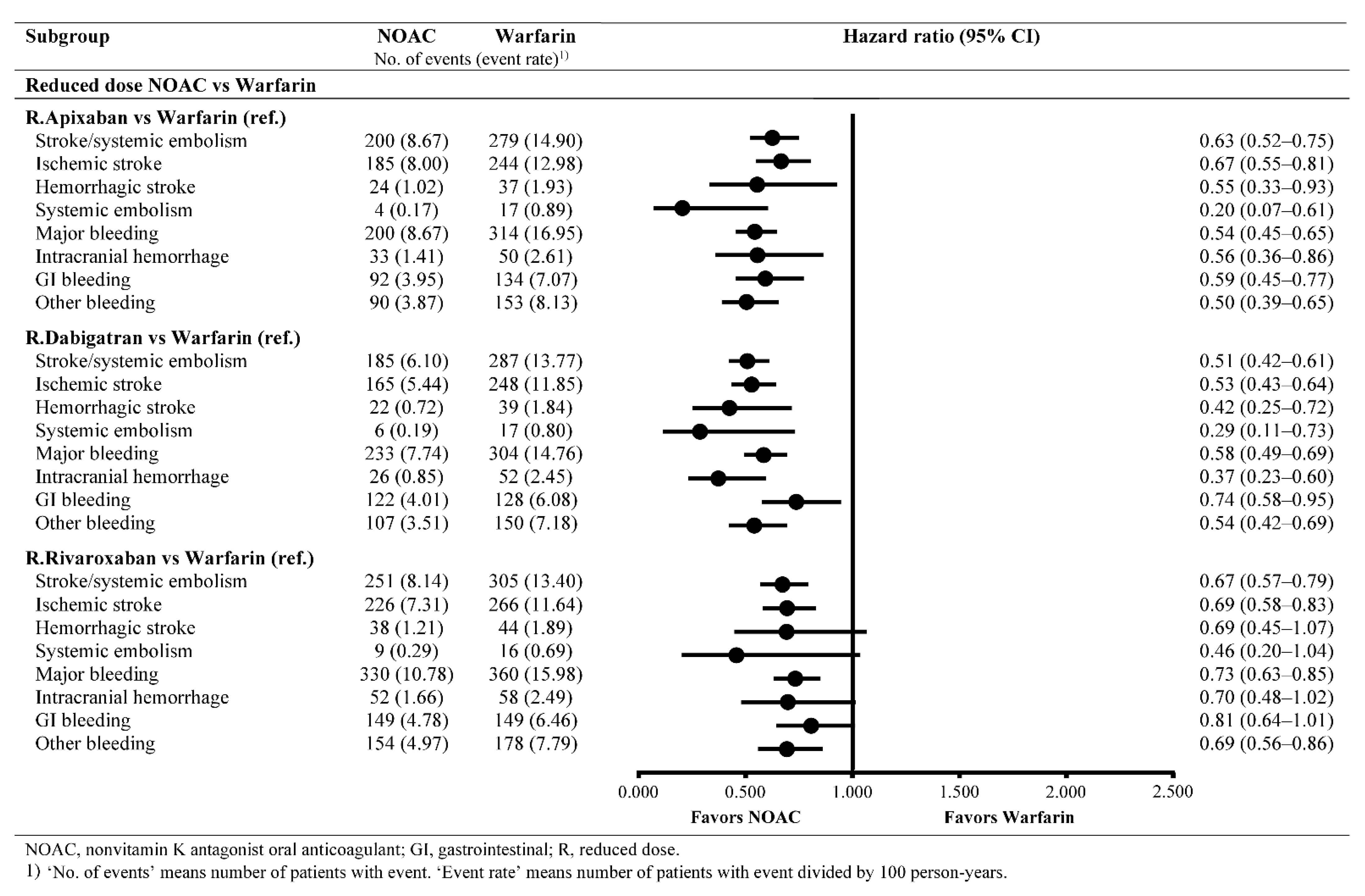
3.2. Effectiveness Outcomes
3.3. Safety Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [Green Version]
- Savelieva, I.; Bajpai, A.; Camm, A.J. Stroke in atrial fibrillation: Update on pathophysiology, new antithrombotic therapies, and evolution of procedures and devices. Ann. Med. 2007, 39, 371–391. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, A.Y.-J.; Yao, J.F.; Brar, S.S.; Jorgensen, M.B.; Chen, W. Racial/Ethnic Differences in the Risk of Intracranial Hemorrhage Among Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2007, 50, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef]
- Chiang, C.-E.; Wang, K.-L.; Lip, G.Y.H. Stroke prevention in atrial fibrillation: An Asian perspective. Thromb. Haemost. 2014, 111, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Inoue, H.; Okumura, K.; Atarashi, H.; Yamashita, T.; Origasa, H.; Kumagai, N.; Sakurai, M.; Kawamura, Y.; Kubota, I.; Matsumoto, K.; et al. Target international normalized ratio values for preventing thromboembolic and hemorrhagic events in Japanese patients with non-valvular atrial fibrillation: Results of the J-RHYTHM Registry. Circ. J. 2013, 77, 2264–2270. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.-M.; Tsoi, T.-H.; Huang, C.-Y. The Lowest Effective Intensity of Prophylactic Anticoagulation for Patients with Atrial Fibrillation. Cerebrovasc. Dis. 2005, 20, 114–119. [Google Scholar] [CrossRef]
- Liu, T.; Hui, J.; Hou, Y.-Y.; Zou, Y.; Jiang, W.-P.; Yang, X.-J.; Wang, X.-H. Meta-Analysis of Efficacy and Safety of Low-Intensity Warfarin Therapy for East Asian Patients with Nonvalvular Atrial Fibrillation. Am. J. Cardiol. 2017, 120, 1562–1567. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Lee, K.-T.; Kao, Y.-W.; Huang, C.-Y.; Chen, Y.-L.; Hang, S.C.-L.; Chu, P.-H. The comparison of non-vitamin K antagonist oral anticoagulants versus well-managed warfarin with a lower INR target of 1.5 to 2.5 in Asians patients with non-valvular atrial fibrillation. PLoS ONE 2019, 14, e0213517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, C.-E.; Okumura, K.; Zhang, S.; Chao, T.-F.; Siu, C.-W.; Lim, T.W.; Saxena, A.; Takahashi, Y.; Siong Teo, W. 2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. J. Arrhythmia 2017, 33, 345–367. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Riva-roxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, B.A.; Shrader, P.; Pieper, K.; Thomas, L.; Allen, L.A.; Ansell, J.; Chan, P.S.; Ezekowitz, M.D.; Fonarow, G.C.; Freeman, J.V.; et al. Frequency and Outcomes of Reduced Dose Non-Vitamin K Antagonist Anticoagulants: Results From ORBIT-AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II). J. Am. Heart Assoc. 2018, 7, e007633. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.S.; Yun, J.E.; Park, J.J.; Kim, Y.J.; Lee, J.; Kim, H.; Park, D.-W.; Nam, G.-B. Outcomes after Use of Standard- and Low-Dose Non–Vitamin K Oral Anticoagulants in Asian Patients With Atrial Fibrillation. Stroke 2019, 50, 110–118. [Google Scholar] [CrossRef]
- Nielsen, P.B.; Skjøth, F.; Søgaard, M.; Kjældgaard, J.N.; Lip, G.Y.; Larsen, T.B. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: Propensity weighted nationwide cohort study. BMJ 2017, 356, j510. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Shah, N.D.; Sangaralingham, L.R.; Gersh, B.J.; Noseworthy, P.A. Non–Vitamin K Antagonist Oral Anticoagulant Dosing in Patients With Atrial Fibrillation and Renal Dysfunction. J. Am. Coll. Cardiol. 2017, 69, 2779–2790. [Google Scholar] [CrossRef]
- Kim, J.-A.; Yoon, S.; Kim, L.-Y.; Kim, D.-S. Towards Actualizing the Value Potential of Korea Health Insurance Review and Assessment (HIRA) Data as a Resource for Health Research: Strengths, Limitations, Applications, and Strategies for Optimal Use of HIRA Data. J. Korean Med. Sci. 2017, 32, 718–728. [Google Scholar] [CrossRef]
- Kim, T.H.; Yang, P.S.; Uhm, J.S.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; Joung, B.; Lip, G.Y.H. CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female) for stroke in Asian patients with atrial fibrillation: A Korean nationwide sample cohort study. Stroke 2017, 48, 1524–1530. [Google Scholar] [PubMed]
- Park, T.H.; Choi, J.C. Validation of Stroke and Thrombolytic Therapy in Korean National Health Insurance Claim Data. J. Clin. Neurol. 2016, 12, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, M.J.; Choi, E.K.; Han, K.D.; Lee, S.R.; Lim, W.H.; Oh, S.; Lip, G.Y.H. Effectiveness and safety of non-vitamin k antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke 2017, 48, 3040–3048. [Google Scholar] [CrossRef]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef] [Green Version]
- Kleinbaum, D.G.; Klein, M. Survival Analysis: A Self-Learning Text, 2nd ed.; Springer Science+Business Media, Inc.: New York, NY, USA, 2005. [Google Scholar]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar]
- Lip, G.Y.H.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef] [Green Version]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021, euab157. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.P.; O’Kane, D.J.; Baudhuin, L.M.; Wiley, C.L.; Fortini, A.; Fisher, P.K.; Dupras, D.M.; Chaudhry, R.; Thapa, P.; Zinsmeister, A.R.; et al. Warfarin Sensitivity Genotyping: A Review of the Literature and Summary of Patient Experience. Mayo Clin. Proc. 2009, 84, 1079–1094. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, B.A.; Shrader, P.; Thomas, L.; Ansell, J.; Fonarow, G.C.; Gersh, B.J.; Kowey, P.R.; Mahaffey, K.W.; Naccarelli, G.; Reiffel, J.; et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: The ORBIT-AF II registry. J. Am. Coll. Cardiol. 2016, 68, 2597–2604. [Google Scholar] [CrossRef]
- Lee, S.-R.; Lee, Y.S.; Park, J.-S.; Cha, M.-J.; Kim, T.-H.; Park, J.; Park, J.-K.; Lee, J.-M.; Kang, K.-W.; Shim, J.; et al. Label Adherence for Non-Vitamin K Antagonist Oral Anticoagulants in a Prospective Cohort of Asian Patients with Atrial Fibrillation. Yonsei Med. J. 2019, 60, 277–284. [Google Scholar] [CrossRef]
- Wang, K.L.; Lopes, R.D.; Patel, M.R.; Büller, H.R.; Tan, D.S.; Chiang, C.E.; Giugliano, R.P. Efficacy and safety of reduced-dose non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: A meta-analysis of randomized controlled trials. Eur. Heart J. 2019, 40, 1492–1500. [Google Scholar] [CrossRef]
- Wang, X.; Fang, L.; Liu, B.; Zheng, Y.; Zeng, J. Real-world comparisons of reduced-dose non-vitamin K antagonist oral anticoagulants versus warfarin in atrial fibrillation: A systematic review and meta-analysis. Heart Fail. Rev. 2020, 25, 973–983. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J.-S.; Oh, Y.-S.; Shin, D.-G.; Pak, H.-N.; Hwang, G.-S.; Choi, K.-J.; Kim, J.-B.; Lee, M.-Y.; Park, H.-W.; et al. Quality of Anticoagulation and Treatment Satisfaction in Patients with Non-Valvular Atrial Fibrillation Treated with Vitamin K Antagonist: Result from the KORean Atrial Fibrillation Investigation II. J. Korean Med Sci. 2018, 33, 323. [Google Scholar] [CrossRef]
- Bang, O.Y.; On, Y.K.; Lee, M.Y.; Jang, S.W.; Han, S.; Han, S.; Won, M.M.; Park, Y.J.; Lee, J.M.; Choi, H.Y.; et al. The risk of stroke/systemic embolism and major bleeding in Asian patients with non-valvular atrial fibrillation treated with non-vitamin K oral anticoagulants compared to warfarin: Results form a real-world data analysis. PLoS ONE 2020, 15, e0242922. [Google Scholar] [CrossRef] [PubMed]
| Groups | Daily Doses (mg) | ||
|---|---|---|---|
| Apixaban | Dabigatran | Rivaroxaban | |
| Standard dose 1 | 10 | 300 | 20 |
| Reduced dose 2 | 5 * | 220 | 15 |
| Other dose 3 | <5 or >10 | <220 or >300 | <15 or >20 |
| Propensity Score Matching | ||||||
|---|---|---|---|---|---|---|
| After 1,2 | ||||||
| R.Apixaban (n = 4774) | Warfarin (n = 4774) | R.Dabigatran (n = 5221) | Warfarin (n = 5221) | R.Rivaroxaban (n = 5746) | Warfarin (n = 5746) | |
| Age (years), mean | 75.50 | 75.45 | 73.94 | 73.85 | 74.21 | 74.15 |
| Female, % | 49.37 | 49.73 | 44.38 | 44.86 | 45.04 | 45.41 |
| CHA2DS2-VASc, mean | 4.97 | 4.99 | 4.67 | 4.68 | 4.71 | 4.73 |
| HAS-BLED, mean | 3.74 | 3.75 | 3.63 | 3.63 | 3.66 | 3.67 |
| CCI, mean | 4.55 | 4.55 | 4.18 | 4.20 | 4.29 | 4.33 |
| Insurance, % | ||||||
| National health insurance | 92.42 | 91.94 | 92.01 | 91.59 | 92.24 | 92.36 |
| Medical aid | 7.58 | 8.06 | 7.99 | 8.41 | 7.76 | 7.64 |
| Medical history, % | ||||||
| Heart failure | 45.35 | 45.92 | 42.56 | 42.56 | 43.84 | 44.43 |
| Hypertension | 87.81 | 88.37 | 88.89 | 89.29 | 88.58 | 89.00 |
| Diabetes | 56.66 | 57.27 | 53.36 | 53.65 | 54.47 | 55.33 |
| Ischemic stroke | 38.04 | 38.77 | 34.09 | 34.17 | 34.08 | 34.16 |
| Vascular disease | 32.45 | 31.57 | 31.68 | 31.95 | 32.02 | 32.09 |
| Renal disease (CKD3/4) | 3.10 | 3.10 | 1.26 | 1.11 | 2.61 | 2.61 |
| Bleeding | 14.62 | 14.73 | 9.84 | 9.83 | 12.63 | 13.00 |
| Medication history, % | ||||||
| NSAIDs | 80.23 | 79.10 | 79.95 | 80.52 | 79.24 | 79.38 |
| Antiplatelets | 75.12 | 75.05 | 74.28 | 74.33 | 75.20 | 75.18 |
| Antiarrhythmics | 48.66 | 47.72 | 46.75 | 46.56 | 45.70 | 45.49 |
| Statins | 58.59 | 59.70 | 56.22 | 57.04 | 54.72 | 55.46 |
| PPI | 46.19 | 45.60 | 43.33 | 43.04 | 44.33 | 43.63 |
| H2RA | 70.34 | 69.92 | 68.86 | 68.51 | 68.53 | 68.97 |
| Digoxin | 27.34 | 27.17 | 27.93 | 27.70 | 28.30 | 28.70 |
| Reduced Dosing | Warfarin (n = 8648) | R.Api (n = 4774) | Warfarin (n = 4774) | R.Dabi (n = 5221) | Warfarin (n = 5221) | R.Riva (n = 5746) | Warfarin (n = 5746) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Apixaban (n = 5249) | Dabigatran (n = 6033) | Rivaroxaban (n = 7602) | ||||||||
| Crude Event Rates 1 | Event Rates in Matched Cohorts 1 | |||||||||
| S/SE | 8.56 | 5.88 | 7.30 | 11.86 | 8.67 | 14.90 | 6.10 | 13.77 | 8.14 | 13.40 |
| IS | 7.92 | 5.28 | 6.52 | 10.33 | 8.00 | 12.98 | 5.44 | 11.85 | 7.31 | 11.64 |
| HS | 0.97 | 0.64 | 1.04 | 1.52 | 1.02 | 1.93 | 0.72 | 1.84 | 1.21 | 1.89 |
| SE | 0.15 | 0.17 | 0.31 | 0.76 | 0.17 | 0.89 | 0.19 | 0.80 | 0.29 | 0.69 |
| MB | 8.40 | 7.60 | 9.94 | 13.53 | 8.67 | 16.95 | 7.74 | 14.76 | 10.78 | 15.98 |
| ICH | 1.35 | 0.81 | 1.47 | 2.06 | 1.41 | 2.61 | 0.85 | 2.45 | 1.66 | 2.49 |
| GI | 3.89 | 4.01 | 4.48 | 5.57 | 3.95 | 7.07 | 4.01 | 6.08 | 4.78 | 6.46 |
| Oth | 3.70 | 3.39 | 4.56 | 6.58 | 3.87 | 8.13 | 3.51 | 7.18 | 4.97 | 7.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Kim, Y.-H.; Lee, M.-Y.; Bang, O.Y.; Jang, S.-W.; Han, S.; Park, Y.-J.; Kang, S.; On, Y.K.; Suh, H.S. Impact of Reduced-Dose Nonvitamin K Antagonist Oral Anticoagulants on Outcomes Compared to Warfarin in Korean Patients with Atrial Fibrillation: A Nationwide Population-Based Study. J. Clin. Med. 2021, 10, 3918. https://doi.org/10.3390/jcm10173918
Han S, Kim Y-H, Lee M-Y, Bang OY, Jang S-W, Han S, Park Y-J, Kang S, On YK, Suh HS. Impact of Reduced-Dose Nonvitamin K Antagonist Oral Anticoagulants on Outcomes Compared to Warfarin in Korean Patients with Atrial Fibrillation: A Nationwide Population-Based Study. Journal of Clinical Medicine. 2021; 10(17):3918. https://doi.org/10.3390/jcm10173918
Chicago/Turabian StyleHan, Sola, Young-Hoon Kim, Myung-Yong Lee, Oh Young Bang, Sung-Won Jang, Seongwook Han, Yoo-Jung Park, Seongsik Kang, Young Keun On, and Hae Sun Suh. 2021. "Impact of Reduced-Dose Nonvitamin K Antagonist Oral Anticoagulants on Outcomes Compared to Warfarin in Korean Patients with Atrial Fibrillation: A Nationwide Population-Based Study" Journal of Clinical Medicine 10, no. 17: 3918. https://doi.org/10.3390/jcm10173918
APA StyleHan, S., Kim, Y.-H., Lee, M.-Y., Bang, O. Y., Jang, S.-W., Han, S., Park, Y.-J., Kang, S., On, Y. K., & Suh, H. S. (2021). Impact of Reduced-Dose Nonvitamin K Antagonist Oral Anticoagulants on Outcomes Compared to Warfarin in Korean Patients with Atrial Fibrillation: A Nationwide Population-Based Study. Journal of Clinical Medicine, 10(17), 3918. https://doi.org/10.3390/jcm10173918






