Prognosis Associated with Sub-Types of Hyperglycaemia in Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
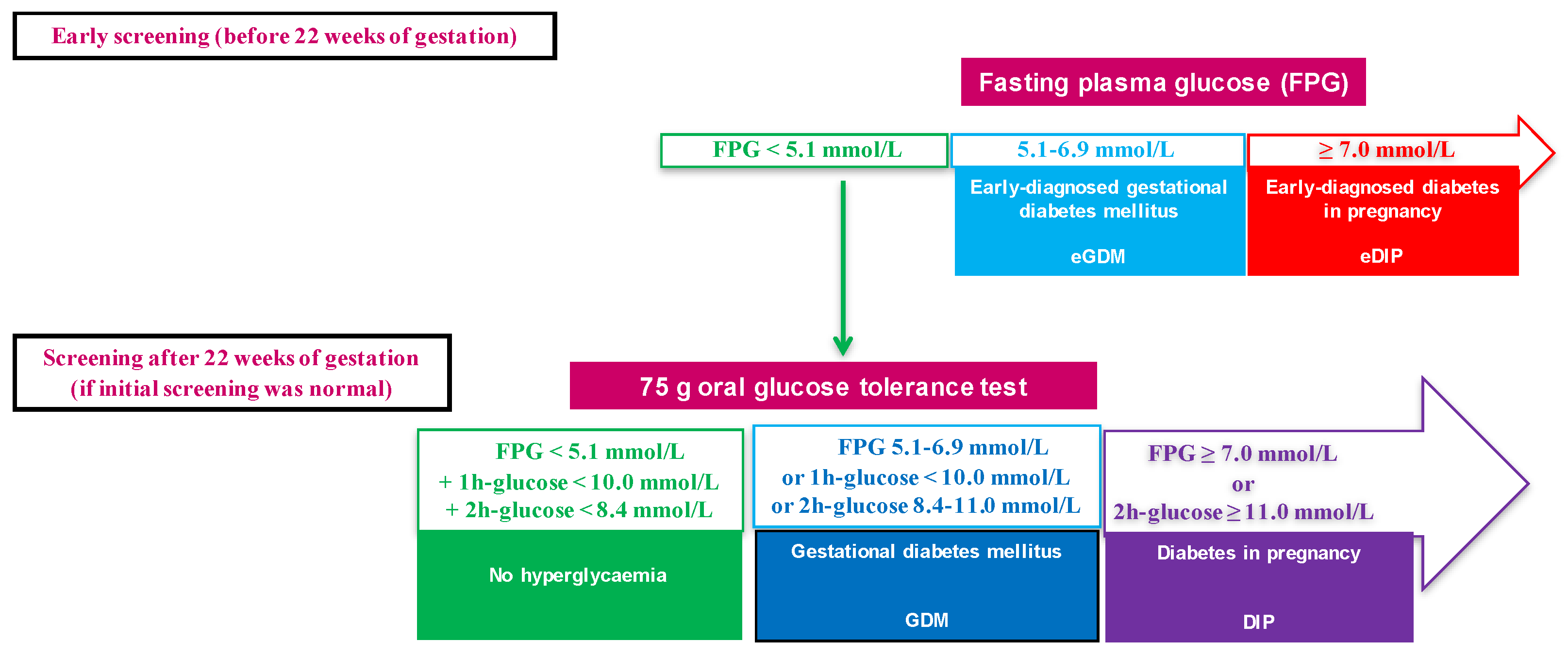
2.2. Definitions of Glycaemic Status during Pregnancy and Management of Hyperglycaemia
2.3. Pregnancy Outcomes
2.4. Statistics
3. Results
3.1. Population Characteristics
3.2. Maternal Outcomes According to HIP Status
3.3. Neonatal Outcomes According to HIP Status
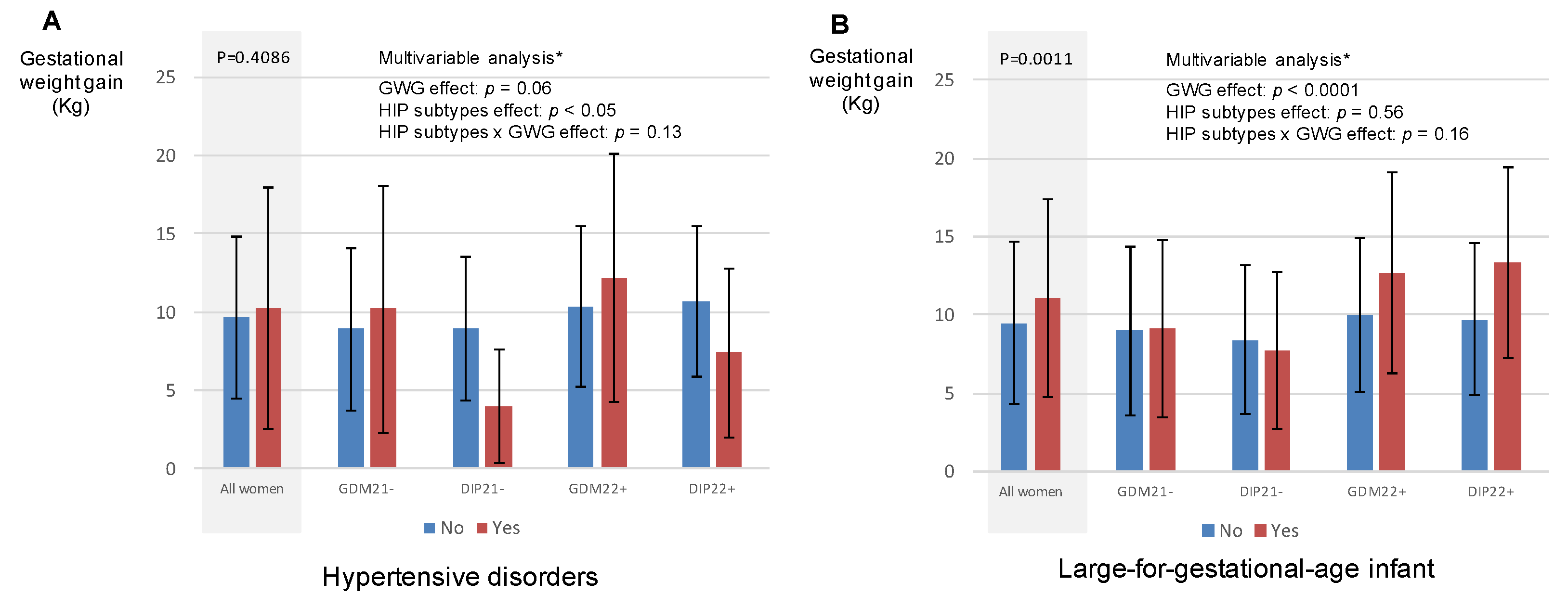
3.4. Hypertensive Disorders during Pregnancy and LGA Infant According to Gestational Weight Gain in Women with HIP
4. Discussion
4.1. Main Results
4.2. Prevalence of HIP Sub-Types
4.3. Is Diabetes in Pregnancy Different between Early and Late Pregnancy?
4.4. Are There Arguments for Screening for HIP in Early Pregnancy?
4.5. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational Diabetes and Adverse Perinatal Outcomes from 716,152 Births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef]
- Gestational Diabetes. Summary of Expert Consensus. Diabetes Metab. 2010, 36, 695–699. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López Stewart, G. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy: A World Health Organization Guideline. Diabet. Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef]
- Catalano, P.M.; Kirwan, J.P.; Haugel-de Mouzon, S.; King, J. Gestational Diabetes and Insulin Resistance: Role in Short- and Long-Term Implications for Mother and Fetus. J. Nutr. 2003, 133, 1674S–1683S. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Sacks, D.A.; Barbour, L.A.; Feig, D.S.; Catalano, P.M.; Damm, P.; McElduff, A. Issues with the Diagnosis and Classification of Hyperglycemia in Early Pregnancy. Diabetes Care 2016, 39, 53–54. [Google Scholar] [CrossRef] [Green Version]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Carpenter, M.W.; Ramin, S.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp, J.M.; et al. A Multicenter, Randomized Trial of Treatment for Mild Gestational Diabetes. N. Engl. J. Med. 2009, 361, 1339–1348. [Google Scholar] [CrossRef]
- Crowther, C.A.; Hiller, J.E.; Moss, J.R.; McPhee, A.J.; Jeffries, W.S.; Robinson, J.S. Effect of Treatment of Gestational Diabetes Mellitus on Pregnancy Outcomes. N. Engl. J. Med. 2005, 352, 2477–2486. [Google Scholar] [CrossRef] [Green Version]
- Cosson, E.; Carbillon, L.; Valensi, P. High Fasting Plasma Glucose during Early Pregnancy: A Review about Early Gestational Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 8921712. [Google Scholar] [CrossRef] [PubMed]
- Cosson, E.; Vicaut, E.; Sandre-Banon, D.; Gary, F.; Pharisien, I.; Portal, J.-J.; Baudry, C.; Cussac-Pillegand, C.; Valensi, P.; Carbillon, L. Initially Untreated Fasting Hyperglycaemia in Early Pregnancy: Prognosis According to Occurrence of Gestational Diabetes Mellitus after 22 Weeks’ Gestation: A Case–Control Study. Diabet. Med. 2020, 37, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Corrado, F.; D’Anna, R.; Cannata, M.L.; Interdonato, M.L.; Pintaudi, B.; Di Benedetto, A. Correspondence between First-Trimester Fasting Glycaemia, and Oral Glucose Tolerance Test in Gestational Diabetes Diagnosis. Diabetes Metab. 2012, 38, 458–461. [Google Scholar] [CrossRef]
- Zhu, W.W.; Yang, H.X.; Wei, Y.M.; Yan, J.; Wang, Z.L.; Li, X.L.; Wu, H.R.; Li, N.; Zhang, M.H.; Liu, X.H.; et al. Evaluation of the Value of Fasting Plasma Glucose in the First Prenatal Visit to Diagnose Gestational Diabetes Mellitus in China. Diabetes Care 2013, 36, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López del Val, T.; Alcázar Lázaro, V.; García Lacalle, C.; Torres Moreno, B.; Castillo Carbajal, G.; Alameda Fernandez, B. Glucemia basal en el primer trimestre como acercamiento inicial al diagnóstico de la diabetes en el embarazo. Endocrinol. Diabetes Nutr. 2019, 66, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, J.; Gebuehr, A.; Wintour, J.; Woods, A.; Luu, J.; Wynne, K. Significance of Hyperglycaemia in First Trimester Pregnancy (SHIFT): A Pilot Study and Literature Review. Aust. N. Z. J. Obstet. Gynaecol. 2021, 61, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Cosson, E.; Vicaut, E.; Sandre-Banon, D.; Gary, F.; Pharisien, I.; Portal, J.-J.; Banu, I.; Bianchi, L.; Cussac-Pillegand, C.; Dina, R.; et al. Early Screening for Gestational Diabetes Mellitus Is Not Associated with Improved Pregnancy Outcomes: An Observational Study Including 9795 Women. Diabetes Metab. 2019, 45, 465–472. [Google Scholar] [CrossRef]
- Cosson, E.; Vicaut, E.; Sandre-Banon, D.; Gary, F.; Pharisien, I.; Portal, J.-J.; Baudry, C.; Cussac-Pillegand, C.; Costeniuc, D.; Valensi, P.; et al. Performance of a Selective Screening Strategy for Diagnosis of Hyperglycaemia in Pregnancy as Defined by IADPSG/WHO Criteria. Diabetes Metab. 2020, 46, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Cosson, E.; Cussac-Pillegand, C.; Benbara, A.; Pharisien, I.; Nguyen, M.T.; Chiheb, S.; Valensi, P.; Carbillon, L. Pregnancy Adverse Outcomes Related to Pregravid Body Mass Index and Gestational Weight Gain, according to the Presence or Not of Gestational Diabetes Mellitus: A Retrospective Observational Study. Diabetes Metab. 2016, 42, 38–46. [Google Scholar] [CrossRef]
- Metzger, B.E.; Contreras, M.; Sacks, D.A.; Watson, W.; Dooley, S.L.; Foderaro, M.; Niznik, C.; Bjaloncik, J.; Catalano, P.M.; Dierker, L.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [Green Version]
- The INSPIRED Research Group; Egan, A.M.; Bogdanet, D.; Griffin, T.P.; Kgosidialwa, O.; Cervar-Zivkovic, M.; Dempsey, E.; Allotey, J.; Alvarado, F.; Clarson, C.; et al. A Core Outcome Set for Studies of Gestational Diabetes Mellitus Prevention and Treatment. Diabetologia 2020, 63, 1120–1127. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis & Management Recommendations for International Practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- Nachtergaele, C.; Vicaut, E.; Pinto, S.; Tatulashvili, S.; Bihan, H.; Sal, M.; Berkane, N.; Allard, L.; Baudry, C.; Carbillon, L.; et al. COVID-19 Pandemic: Can Fasting Plasma Glucose and HbA1c Replace the Oral Glucose Tolerance Test to Screen for Hyperglycaemia in Pregnancy? Diabetes Res. Clin. Pract. 2021, 172, 108640. [Google Scholar] [CrossRef] [PubMed]
- Nachtergaele, C.; Vicaut, E.; Tatulashvili, S.; Pinto, S.; Bihan, H.; Sal, M.; Berkane, N.; Allard, L.; Baudry, C.; Portal, J.-J.; et al. Limiting the Use of Oral Glucose Tolerance Tests to Screen for Hyperglycemia in Pregnancy during Pandemics. JCM 2021, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Riskin-Mashiah, S.; Younes, G.; Damti, A.; Auslender, R. First-Trimester Fasting Hyperglycemia and Adverse Pregnancy Outcomes. Diabetes Care 2009, 32, 1639–1643. [Google Scholar] [CrossRef] [Green Version]
- Wali, A.S.; Rafique, R.; Iftikhar, S.; Ambreen, R.; Yakoob, M.Y. High Proportion of Overt Diabetes Mellitus in Pregnancy and Missed Opportunity for Early Detection of Diabetes at a Tertiary Care Centre in Pakistan. Pak. J. Med. Sci. 2019, 36, S38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampaio, Y.; Porto, L.B.; Lauand, T.C.G.; Marcon, L.P.; Pedrosa, H.C. Gestational Diabetes and Overt Diabetes First Diagnosed in Pregnancy: Characteristics, Therapeutic Approach and Perinatal Outcomes in a Public Healthcare Referral Center in Brazil. Arch. Endocrinol. Metab. 2020, 65, 79–84. [Google Scholar] [CrossRef]
- Paulweber, B.; Valensi, P.; Lindström, J.; Lalic, N.; Greaves, C.; McKee, M.; Kissimova-Skarbek, K.; Liatis, S.; Cosson, E.; Szendroedi, J.; et al. A European Evidence-Based Guideline for the Prevention of Type 2 Diabetes. Horm. Metab. Res. 2010, 42, S3–S36. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.; Ross, G.P.; Jalaludin, B.B.; Flack, J.R. The Clinical Significance of Overt Diabetes in Pregnancy. Diabet. Med. 2013, 30, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, S.-H. Women with Rigorously Managed Overt Diabetes during Pregnancy Do Not Experience Adverse Infant Outcomes but Do Remain at Serious Risk of Postpartum Diabetes. Endocr. J. 2015, 62, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Mañé, L.; Flores-Le Roux, J.A.; Pedro-Botet, J.; Gortazar, L.; Chillarón, J.J.; Llauradó, G.; Payà, A.; Benaiges, D. Is Fasting Plasma Glucose in Early Pregnancy a Better Predictor of Adverse Obstetric Outcomes than Glycated Haemoglobin? Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 79–84. [Google Scholar] [CrossRef]
- Sugiyama, T.; Metoki, H.; Hamada, H.; Nishigori, H.; Saito, M.; Yaegashi, N.; Kusaka, H.; Kawano, R.; Ichihara, K.; Yasuhi, I.; et al. A Retrospective Multi-Institutional Study of Treatment for Mild Gestational Diabetes in Japan. Diabetes Res. Clin. Pract. 2014, 103, 412–418. [Google Scholar] [CrossRef]
- Liu, B.; Cai, J.; Xu, Y.; Long, Y.; Deng, L.; Lin, S.; Zhang, J.; Yang, J.; Zhong, L.; Luo, Y.; et al. Early Diagnosed Gestational Diabetes Mellitus Is Associated with Adverse Pregnancy Outcomes: A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2020, 105, e4264–e4274. [Google Scholar] [CrossRef]
- Sweeting, A.N.; Ross, G.P.; Hyett, J.; Molyneaux, L.; Constantino, M.; Harding, A.J.; Wong, J. Gestational Diabetes Mellitus in Early Pregnancy: Evidence for Poor Pregnancy Outcomes Despite Treatment. Diabetes Care 2016, 39, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, W.; Biggio, J.; Tita, A.; Harper, L. Impact of Early Screening for Gestational Diabetes on Perinatal Outcomes in High-Risk Women. Am. J. Perinatol. 2016, 33, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Alunni, M.L.; Roeder, H.A.; Moore, T.R.; Ramos, G.A. First Trimester Gestational Diabetes Screening—Change in Incidence and Pharmacotherapy Need. Diabetes Res. Clin. Pract. 2015, 109, 135–140. [Google Scholar] [CrossRef]
- Mustafa, M.; Bogdanet, D.; Khattak, A.; Carmody, L.A.; Kirwan, B.; Gaffney, G.; O’Shea, P.M.; Dunne, F. Early Gestational Diabetes Mellitus (GDM) Is Associated with Worse Pregnancy Outcomes Compared with GDM Diagnosed at 24–28 Weeks Gestation despite Early Treatment. QJM Int. J. Med. 2021, 114, 17–24. [Google Scholar] [CrossRef]
- Clarke, E.; Cade, T.J.; Brennecke, S. Early Pregnancy Screening for Women at High-Risk of GDM Results in Reduced Neonatal Morbidity and Similar Maternal Outcomes to Routine Screening. J. Pregnancy 2020, 2020, 9083264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, L.M.; Jauk, V.; Longo, S.; Biggio, J.R.; Szychowski, J.M.; Tita, A.T. Early Gestational Diabetes Screening in Obese Women: A Randomized Controlled Trial. Am. J. Obstet. Gynecol. 2020, 222, 495.e1–495.e8. [Google Scholar] [CrossRef]
- Vinter, C.A.; Tanvig, M.H.; Christensen, M.H.; Ovesen, P.G.; Jørgensen, J.S.; Andersen, M.S.; McIntyre, H.D.; Jensen, D.M. Lifestyle Intervention in Danish Obese Pregnant Women with Early Gestational Diabetes Mellitus According to WHO 2013 Criteria Does Not Change Pregnancy Outcomes: Results from the LiP (Lifestyle in Pregnancy) Study. Diabetes Care 2018, 41, 2079–2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintaudi, B.; Fresa, R.; Dalfrà, M.; Dodesini, A.R.; Vitacolonna, E.; Tumminia, A.; Sciacca, L.; Lencioni, C.; Marcone, T.; Lucisano, G.; et al. The Risk Stratification of Adverse Neonatal Outcomes in Women with Gestational Diabetes (STRONG) Study. Acta Diabetol. 2018, 55, 1261–1273. [Google Scholar] [CrossRef]
| Available Data | Total | No HIP | eGDM | eDIP | GDM | DIP | Global Comparison p-Value | |
|---|---|---|---|---|---|---|---|---|
| n = 4665 | n = 3563 | n = 502 | n = 26 | n = 545 | n = 29 | |||
| Screening for HIP before 22 WG | ||||||||
| Fasting plasma glucose (mmol/L) | n = 4238 | 4.6 (0.6) | 4.5 (0.3) | 5.4 (0.4) ab | 11.6 (3.4) a | 4.6 (0.3) a | 4.6 (0.3) | <0.0001 |
| Gestational age at HIP screening (WG) | n = 4258 | 12.2 (4.2) | 12.2 (4.2) | 11.7 (4.5) | 11.6 (0.7) | 12.6 (4.1) | 13.7 (4.5) | 0.007 |
| Screening with OGTT at 22 WG or later | ||||||||
| Fasting plasma glucose (mmol/L) | n = 3760 | 4.4 (0.5) | 4.3 (0.4) | - | - | 4.9 (0.6) a | 5.3 (1.0) ac | <0.0001 |
| 1-h plasma glucose (mmol/L) | n = 3728 | 7.0 (1.8) | 6.6 (1.5) | - | - | 9.3 (1.7) a | 11.0 (2.4) ac | <0.0001 |
| 2-h plasma glucose (mmol/L) | n = 3734 | 6.1 (1.5) | 5.8 (1.1) | - | - | 8.0 (1.6) a | 11.6 (1.9) ac | <0.0001 |
| Gestational age at OGTT (WG) | n = 4033 | 27.3 (3.0) | 27.2 (3.0) | - | - | 27.7 (3.3) a | 27.6 (3.6) | <0.0001 |
| Metabolic characteristics | ||||||||
| Age (years) | n = 4665 | 30.7 (5.5) | 30.2 (5.4) | 32.6 (5.3) a | 33.3 (5.4) a | 32.1 (5.5) a | 32.6 (5.2) | <0.0001 |
| Pre-pregnancy body mass index (kg/m2) | n = 4515 | 25.2 (5.1) | 24.7 (4.8) | 28.0 (5.9) ab | 27.7 (6.6) a | 26.2 (5.4) a | 27.7 (3.8) ac | <0.0001 |
| Pre-pregnancy obesity | n = 4515 | 833 (18.5) | 531 (15.4) | 170 (34.9) ab | 8 (32.0) | 115 (21.9) a | 9 (33.3) | <0.0001 |
| Pre-pregnancy hypertension | n = 4665 | 39 (0.9) | 20 (0.6) | 9 (1.8) | 1 (3.9) | 9 (1.7) | 0 (0) | 0.0027 |
| Family history of diabetes | n = 4665 | 1291 (27.7) | 892 (25.0) | 204 (40.6) ab | 12 (46.2) | 175 (32.1) a | 8 (27.6) | <0.0001 |
| Employment at beginning of pregnancy | n = 4656 | 1887 (40.5) | 1483 (41.7) | 167 (33.3) a | 9 (34.6) | 217 (39.9) | 11 (37.9) | 0.0097 |
| Parity | n = 4665 | 2.1 (1.2) | 2.1 (1.2) | 2.3 (1.3) a | 2.5 (1.7) | 2.2 (1.3) | 1.8 (0.8) | 0.0001 |
| Previous pregnancy | ||||||||
| History of HIP | n = 4665 | <0.0001 * | ||||||
| First child | 1814 (38.9) | 1452 (40.8) | 147 (29.3) | 7 (26.9) | 195 (35.8) | 13 (44.8) | ||
| No | 2561 (54.9) | 2005 (56.3) | 260 (51.8) | 10 (38.5) | 273 (50.1) | 13 (44.8) | ||
| Yes | 290 (6.2) | 106 (3.00) | 95 (18.9) | 9 (34.6) | 77 (14.1) | 3 (10.3) | ||
| History of large for gestational age infant | n = 4665 | <0.0001 * | ||||||
| First child | 1814 (38.9) | 1452 (40.8) | 147 (29.3) | 7 (26.9) | 195 (35.8) | 13 (44.8) | ||
| No | 2688 (57.6) | 2012 (56.5) | 326 (64.9) | 15 (57.7) | 322 (59.1) | 13 (44.8) | ||
| Yes | 163 (3.5) | 99 (2.8) | 29 (5.8) a | 4 (15.4) a | 28 (5.1) a | 3 (10.3) | ||
| History of hypertensive disorders | n = 4665 | 0.0104 * | ||||||
| First pregnancy | 1265 (27.1) | 1018 (28.6) | 96 (19.1) | 6 (23.1) | 139 (25.5) | 6 (20.7) | ||
| No | 3281 (70.3) | 2462 (69.1) | 383 (76.3) | 18 (69.2) | 395 (72.5) | 23 (79.3) | ||
| Yes | 119 (2.6) | 83 (2.3) | 23 (4.6) a | 2 (7.7) | 11 (2.0) | 0 (0) | ||
| History of foetal death | n = 4665 | 0.0418 * | ||||||
| First pregnancy | 1265 (27.1) | 1018 (28.6) | 96 (19.1) | 6 (23.1) | 139 (25.5) | 6 (20.7) | ||
| No | 3307 (70.9) | 2485 (69.7) | 391 (77.9) | 19 (73.1) | 391 (71.7) | 21 (72.4) | ||
| Yes | 93 (2.0) | 60 (1.7) | 15 (3.0) | 1 (3.9) | 15 (2.8) | 2 (6.9) | ||
| Smoking during pregnancy | n = 4665 | 284 (6.1) | 245 (6.9) | 22 (4.4) | 1 (3.9) | 16 (2.9) a | 0 (0) | 0.0012 |
| Ethnicity | n = 4656 | <0.0001 | ||||||
| European | 1269 (27.3) | 1042 (29.3) | 94 (18.8) | 3 (11.5) | 121 (22.2) | 9 (31.0) | ||
| African | 881 (18.9) | 709 (19.9) | 80 (16.0) | 5 (19.2) | 83 (15.3) | 4 (13.8) | ||
| North African | 1378 (29.6) | 970 (27.3) | 192 (38.3) | 10 (38.5) | 196 (36.0) | 10 (34.5) | ||
| Asian | 96 (2.1) | 70 (2.00) | 10 (2.00) | 1 (3.9) | 14 (2.6) | 1 (3.5) | ||
| Caribbean | 270 (5.8) | 222 (6.2) | 23 (4.6) | 1 (3.9) | 24 (4.4) | 0 (0) | ||
| Indian-Pakistan-Sri Lankan | 519 (11.2) | 346 (9.7) | 82 (16.4) | 6 (23.1) | 80 (14.7) | 5 (17.2) | ||
| Other | 243 (5.2) | 197 (5.5) | 20 (4.0) | 0 (0) | 26 (4.8) | 0 (0) | ||
| Maternal outcomes | ||||||||
| Gestational weight gain (kg) | n = 4316 | 11.1 (5.5) | 11.5 (5.5) | 9.0 (5.4) ab | 8.3 (4.7) a | 10.4 (5.3) a | 10.1 (5.0) | <0.0001 |
| Insulin therapy during pregnancy | n = 1100 | 408 (37.1) | - | 231 (46.0) b | 23 (88.5) | 139 (25.5) | 15 (51.7) c | <0.0001 |
| Caesarean section | n = 4665 | 1038 (22.3) | 750 (21.1) | 139 (27.7) a | 7 (26.9) | 131 (24.0) | 11 (37.9) | 0.0019 |
| Gestational hypertension | n = 4665 | 126 (2.7) | 87 (2.4) | 26 (5.2) ab | 2 (7.7) | 8 (1.5) | 3 (10.3) ac | <0.0001 |
| Preeclampsia | n = 4665 | 90 (1.9) | 66 (1.9) | 9 (1.8) | 1 (3.9) | 11 (2.0) | 3 (10.3) a | 0.0211 |
| Hypertensive disorders during pregnancy | n = 4665 | 214 (4.6) | 151 (4.2) | 35 (7.0) a | 3 (11.5) | 19 (3.5) | 6 (20.7) ac | <0.0001 |
| Neonatal outcomes | ||||||||
| Birthweight (g) | n = 4665 | 3294 (515) | 3285 (508) | 3335 (522) | 3271 (573) | 3321 (541) | 3271 (643) | 0.1984 |
| Large-for-gestational-age infant | n = 4665 | 468 (10.0) | 325 (9.1) | 60 (12.0) | 4 (15.4) | 76 (13.9) a | 3 (10.3) | 0.0039 |
| Small-for-gestational-age infant | n = 4665 | 448 (9.6) | 347 (9.7) | 48 (9.6) | 1 (3.9) | 48 (8.8) | 4 (13.8) | 0.7259 |
| Gestational age at birth (weeks of gestation) | n = 4665 | 39.7 (1.6) | 39.7 (1.6) | 39.5 (1.6) | 38.7 (1.9) a | 39.4 (1.6) a | 39.0 (2.1) | <0.0001 |
| Preterm delivery (<37 weeks of gestation) | n = 4665 | 259 (5.6) | 173 (4.9) | 38 (7.6) | 4 (15.4) a | 39 (7.2) | 5 (17.2) | 0.0002 |
| Neonatal hypoglycaemia | n = 4665 | 29 (0.6) | 12 (0.3) | 6 (1.2) a | 0 (0) | 8 (1.5) a | 3 (10.3) a | <0.0001 |
| Neonatal death and stillbirth | n = 4665 | 18 (0.4) | 14 (0.4) | 2 (0.4) | 0 (0) | 2 (0.4) | 0 (0) | 0.0669 |
| Malformation | n = 4665 | 51 (1.1) | 32 (1.0) | 11 (2.2) a | 2 (7.7) a | 6 (1.1) | 0 (0) | 0.0014 |
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Glycaemic status | |||
| No HIP | REF | ||
| eGDM | 1.29 | 0.86–1.95 | 0.2164 |
| eDIP | 2.16 | 0.61–7.68 | 0.2352 |
| GDM | 0.67 | 0.40–1.11 | 0.1205 |
| DIP | 3.48 | 1.26–9.57 | 0.0159 |
| Pre-pregnancy body mass index (kg/m2) | 1.09 | 1.06–1.11 | <0.0001 |
| Age (year) | 1.07 | 1.04–1.10 | <0.0001 |
| Smoking during pregnancy | 1.37 | 0.76–2.46 | 0.2902 |
| Parity | 0.65 | 0.56–0.76 | <0.0001 |
| Ethnicity | |||
| Europe | REF | ||
| Africa | 1.86 | 1.23–2.84 | 0.0037 |
| North Africa | 0.92 | 0.61–1.39 | 0.6941 |
| Asia | 0.62 | 0.15–2.62 | 0.5166 |
| Caribbean | 1.20 | 0.66–2.20 | 0.5471 |
| Pakistan India Sri Lanka | 1.31 | 0.77–2.24 | 0.3266 |
| Other | 0.36 | 0.11–1.18 | 0.0912 |
| Employment at beginning of pregnancy | 1.08 | 0.79–1.47 | 0.6332 |
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Glycaemic status | |||
| No HIP | REF | ||
| eGDM | 0.96 | 0.70–1.31 | 0.7759 |
| eDIP | 0.95 | 0.27–3.40 | 0.9379 |
| GDM | 1.34 | 1.01–1.78 | 0.0435 |
| DIP | 0.98 | 0.29–3.34 | 0.9694 |
| Pre-pregnancy body mass index (kg/m2) | 1.09 | 1.07–1.11 | <0.0001 |
| Age (year) | 1.00 | 0.98–1.02 | 0.7094 |
| Smoking during pregnancy | 0.44 | 0.25–0.78 | 0.0053 |
| Parity | 1.14 | 1.04–1.24 | 0.0032 |
| Ethnicity | |||
| Europe | REF | ||
| Africa | 0.56 | 0.39–0.79 | 0.0009 |
| North Africa | 1.34 | 1.03–1.75 | 0.0287 |
| Asia | 0.78 | 0.33–1.83 | 0.5616 |
| Caribbean | 0.58 | 0.35–0.98 | 0.0397 |
| Pakistan India Sri Lanka | 0.59 | 0.38–0.92 | 0.0205 |
| Other | 1.48 | 0.95–2.31 | 0.0799 |
| Employment at beginning of pregnancy | 0.98 | 0.78–1.22 | 0.8361 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosson, E.; Bentounes, S.A.; Nachtergaele, C.; Berkane, N.; Pinto, S.; Sal, M.; Bihan, H.; Tatulashvili, S.; Portal, J.-J.; Carbillon, L.; et al. Prognosis Associated with Sub-Types of Hyperglycaemia in Pregnancy. J. Clin. Med. 2021, 10, 3904. https://doi.org/10.3390/jcm10173904
Cosson E, Bentounes SA, Nachtergaele C, Berkane N, Pinto S, Sal M, Bihan H, Tatulashvili S, Portal J-J, Carbillon L, et al. Prognosis Associated with Sub-Types of Hyperglycaemia in Pregnancy. Journal of Clinical Medicine. 2021; 10(17):3904. https://doi.org/10.3390/jcm10173904
Chicago/Turabian StyleCosson, Emmanuel, Sid Ahmed Bentounes, Charlotte Nachtergaele, Narimane Berkane, Sara Pinto, Meriem Sal, Hélène Bihan, Sopio Tatulashvili, Jean-Jacques Portal, Lionel Carbillon, and et al. 2021. "Prognosis Associated with Sub-Types of Hyperglycaemia in Pregnancy" Journal of Clinical Medicine 10, no. 17: 3904. https://doi.org/10.3390/jcm10173904
APA StyleCosson, E., Bentounes, S. A., Nachtergaele, C., Berkane, N., Pinto, S., Sal, M., Bihan, H., Tatulashvili, S., Portal, J.-J., Carbillon, L., & Vicaut, E. (2021). Prognosis Associated with Sub-Types of Hyperglycaemia in Pregnancy. Journal of Clinical Medicine, 10(17), 3904. https://doi.org/10.3390/jcm10173904






