Combined Neutrophil-to-Lymphocyte and Platelet-Volume-to-Platelet Ratio (NLR and PVPR Score) Represents a Novel Prognostic Factor in Advanced Gastric Cancer Patients
Abstract
:1. Introduction
2. Patients and Methods
Statistical Analysis
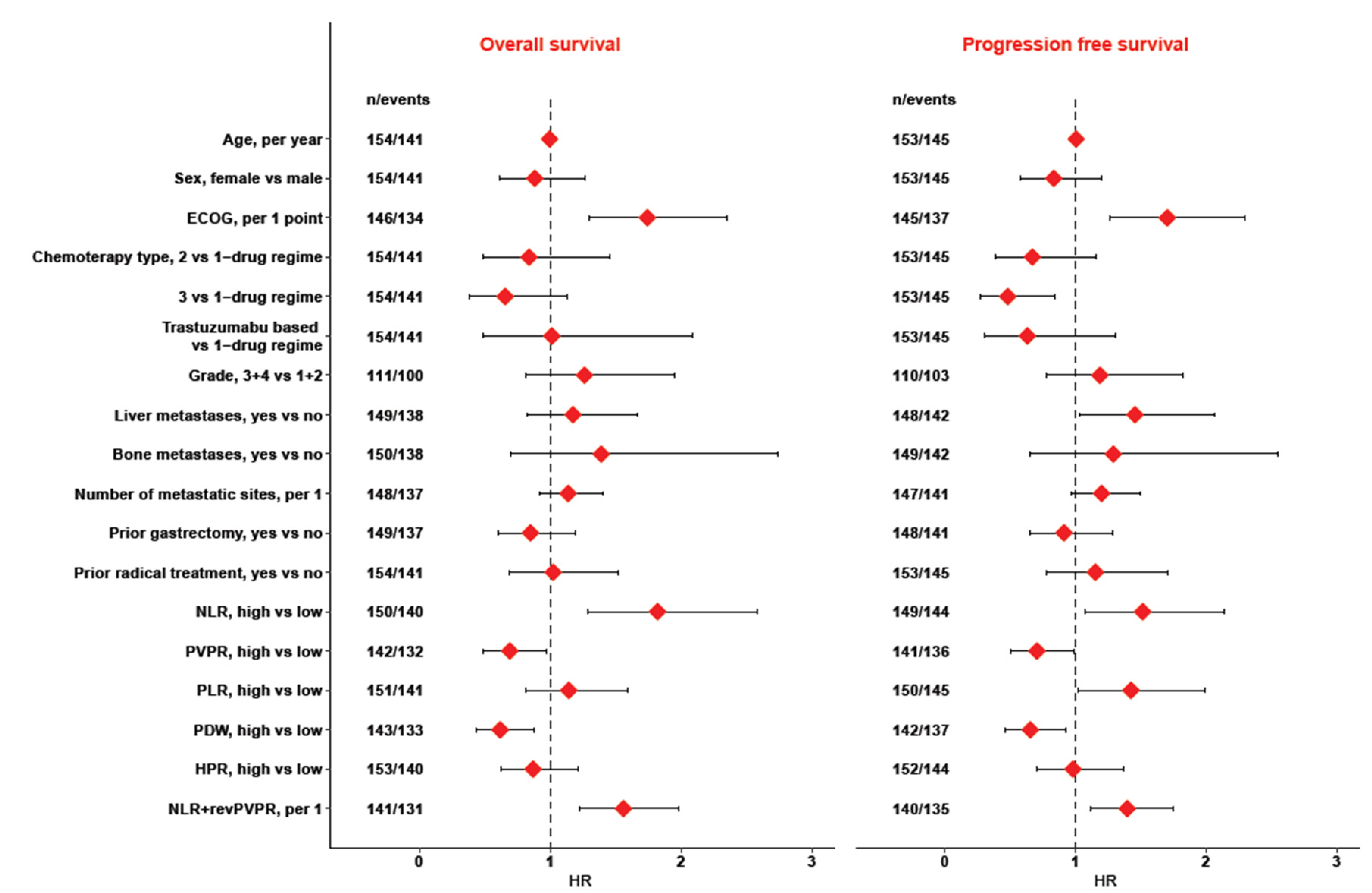
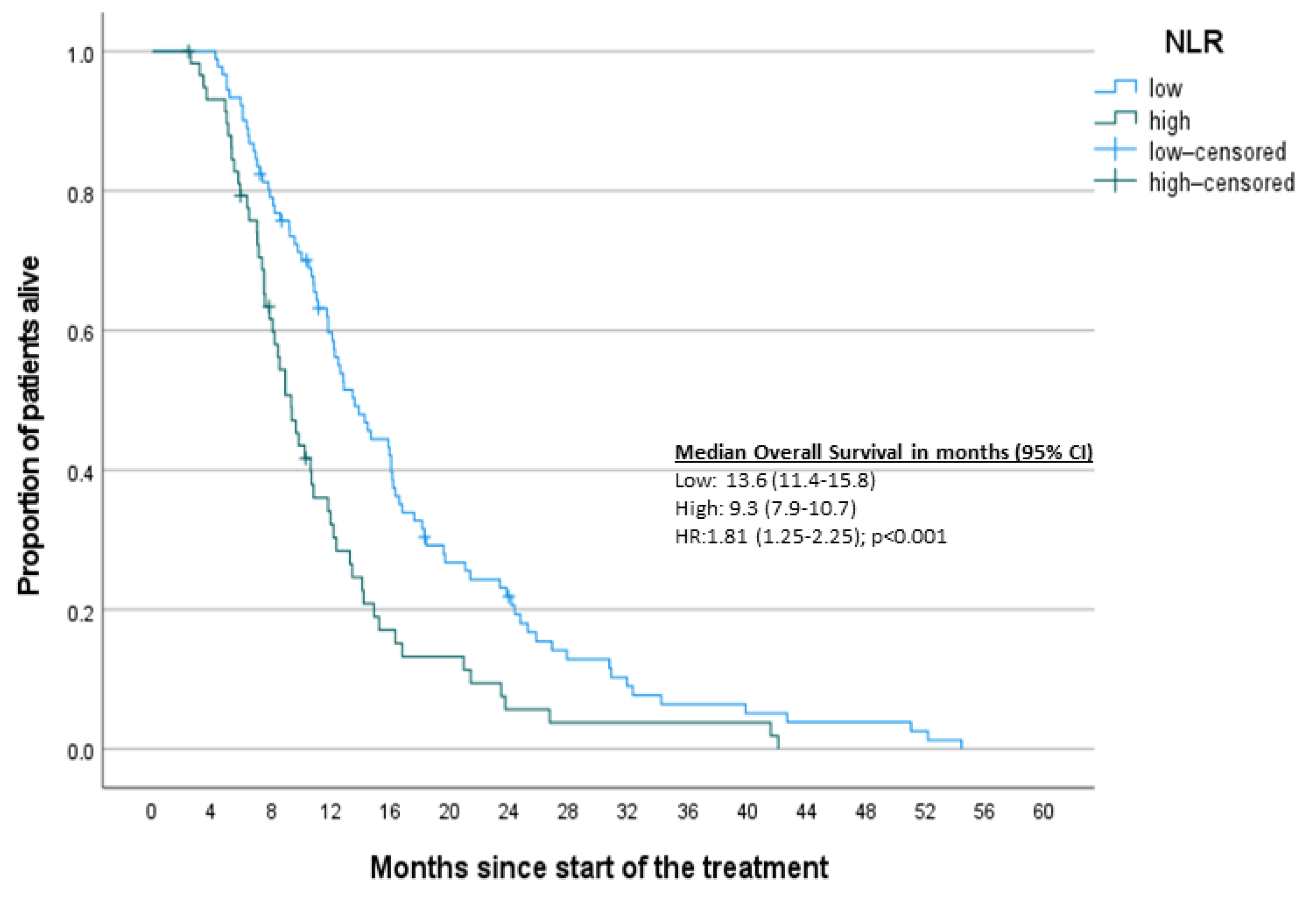
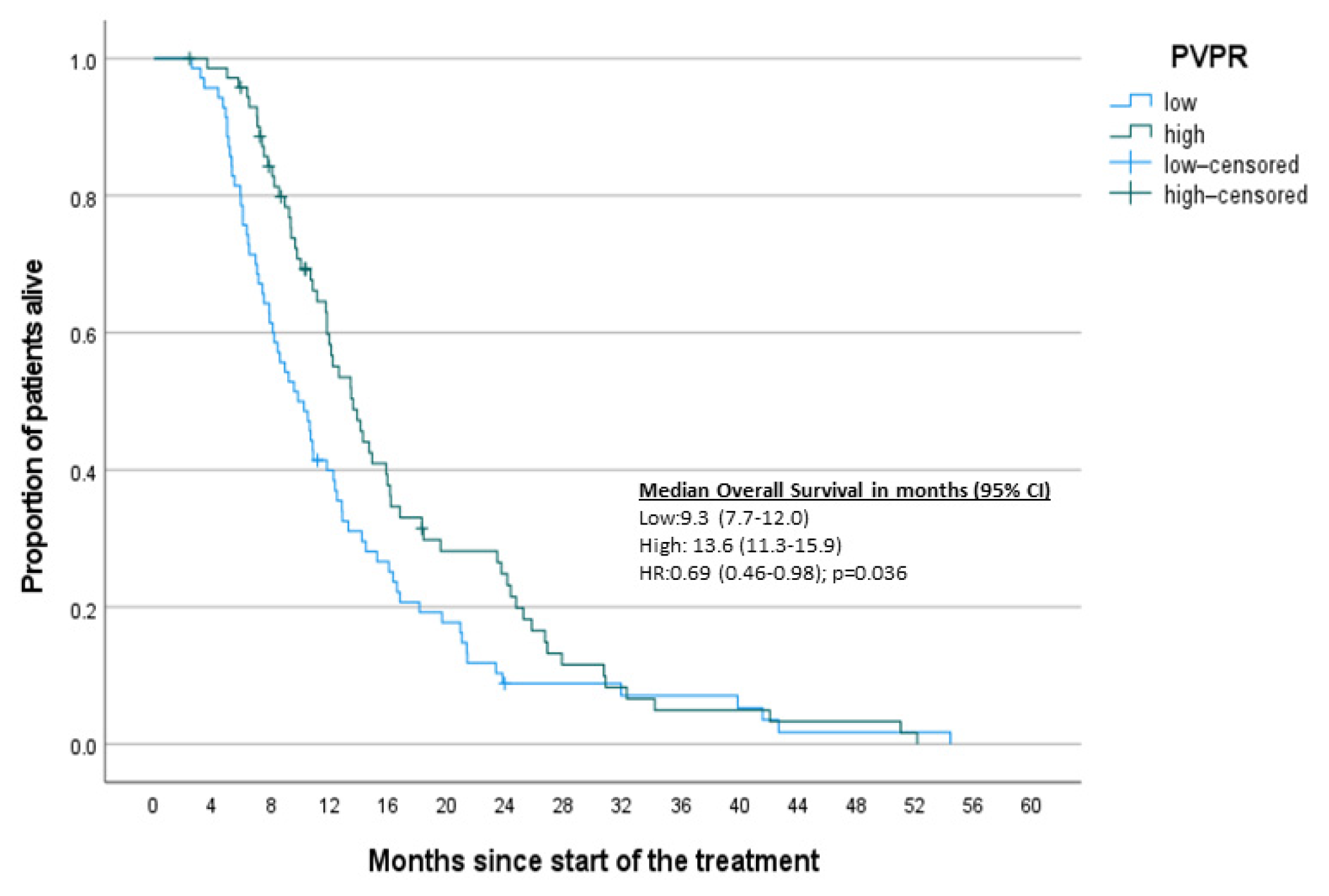
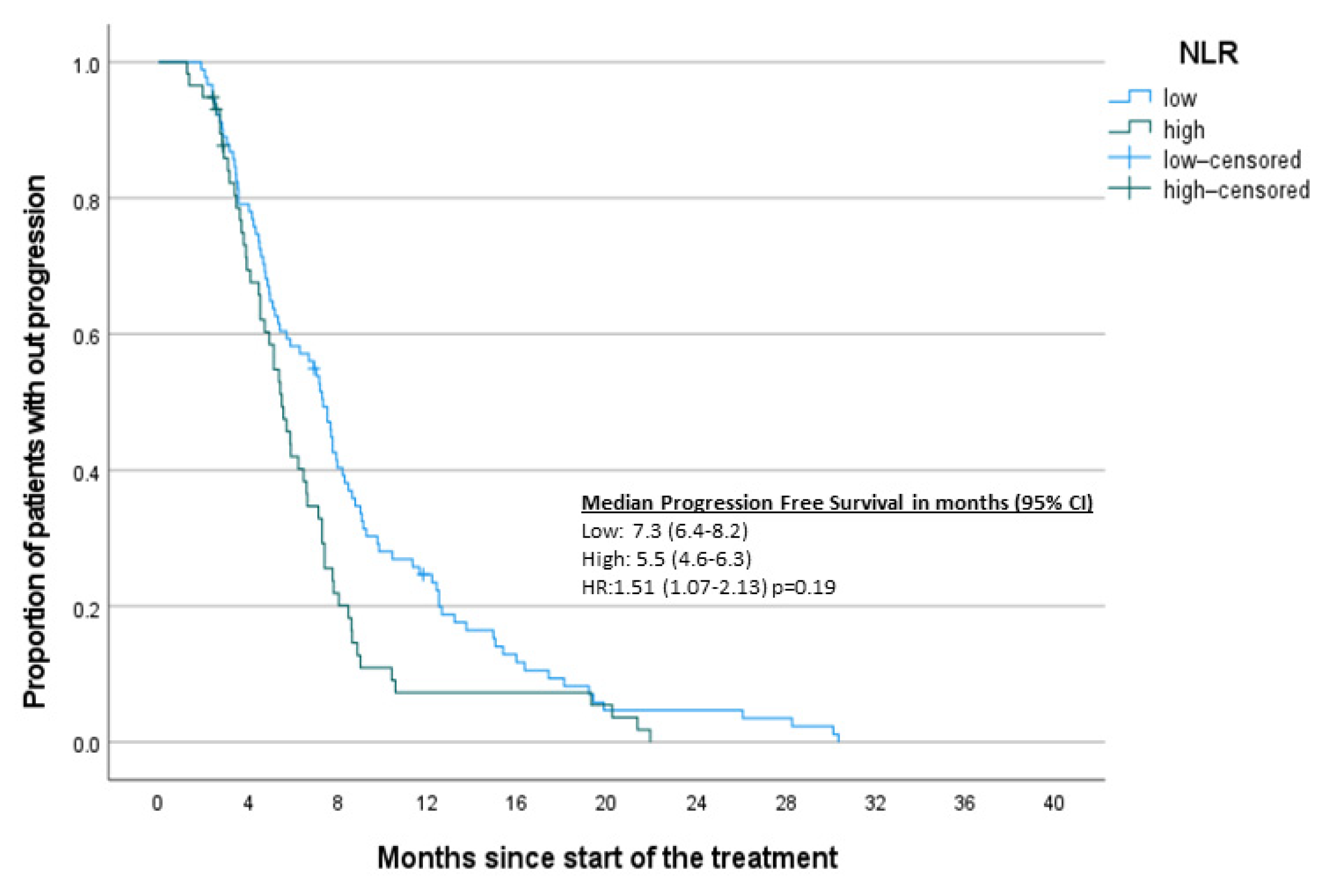
3. Results
3.1. Patient and Tumor Characteristics
3.2. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janunger, K.-G.; Hafström, L.; Glimelius, I.B. Chemotherapy in gastric cancer: A review and updated meta-analysis. Eur. J. Surg. 2002, 168, 597–608. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, I.D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef]
- Montagnani, F.; Turrisi, G.; Marinozzi, C.; Aliberti, C.; Fiorentini, I.G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2011, 1, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meerten, E.; Eskens, F.A.L.M.; Van Gameren, E.C.; Doorn, L.; van der Gaast, A. First-line treatment with oxaliplatin and capecitabine in patients with advanced or metastatic oesophageal cancer: A phase II study. Br. J. Cancer 2007, 96, 9. [Google Scholar] [CrossRef] [PubMed]
- Enzinger, P.C. CALGB 80403/ECOG 1206: A randomized phase II study of three standard chemotherapy regimens (ECF, IC, FOLFOX) plus cetuximab in metastatic esophageal and GE junction cancer. J. Clin. Oncol. 2010, 28, 4006. [Google Scholar] [CrossRef]
- Kim, G.M. A randomized phase II trial of S-1-oxaliplatin versus capecitabine–oxaliplatin in advanced gastric cancer. Eur. J. Cancer 2012, 48, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, B.; Huang, X.-T.; Li, Y.-S.; Wang, Y.; Liu, Z.-L. Doublet versus single agent as second-line treatment for advanced gastric cancer. Medicine 2016, 95, e2792. [Google Scholar] [CrossRef]
- Abbrederis, K. Weekly docetaxel monotherapy for advanced gastric or esophagogastric junction cancer: Results of a phase II study in elderly patients or patients with impaired performance status. Crit. Rev. Oncol. Hematol. 2008, 66, 84–90. [Google Scholar] [CrossRef]
- Ochenduszko, S. Comparison of efficacy and safety of first-line palliative chemotherapy with EOX and mDCF regimens in patients with locally advanced inoperable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma: A randomized phase 3 trial. Med. Oncol. Northwood Lond. Engl. 2015, 32, 242. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J. Clin. Oncol. 2006, 24, 31. [Google Scholar] [CrossRef]
- Guo, W. Phase III trial comparing XELOX regimen (oxaliplatin plus capecitabine) versus EOX regimen (epirubicin, oxaliplatin and capecitabine) as first-line treatment for advanced gastric cancer: EXELOX trial. J. Clin. Oncol. 2021, 39, 4014. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet Lond. Engl. 2010, 376, 9742. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Ajani, J.A. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet Lond. Engl. 2021, 398, 10294. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Diem, S. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Capone, M. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizunuma, M.; Yokoyama, Y.; Futagami, M.; Aoki, M.; Takai, Y.; Mizunuma, I.H. The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. Int. J. Clin. Oncol. 2015, 20, 989–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.R. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J. Surg. Oncol. 2011, 104, 504–510. [Google Scholar] [CrossRef]
- Lee, S. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 2013, 13, 350. [Google Scholar] [CrossRef] [Green Version]
- Xin-Ji, Z.; Yong-Gang, L.; Xiao-Jun, S.; Xiao-Wu, C.; Dong, Z.; Da-Jian, I.Z. The prognostic role of neutrophils to lymphocytes ratio and platelet count in gastric cancer: A. meta-analysis. Int. J. Surg. 2015, 21, 84–91. [Google Scholar] [CrossRef]
- Asher, V.; Lee, J.; Innamaa, A.; Bali, I.A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin. Transl. Oncol. 2011, 13, 499. [Google Scholar] [CrossRef]
- Hu, Z. Diagnostic value of hematological parameters platelet to lymphocyte ratio and hemoglobin to platelet ratio in patients with colon cancer. Clin. Chim. Acta 2020, 501, 48–52. [Google Scholar] [CrossRef]
- Cui, M. Platelet distribution width correlates with prognosis of non-small cell lung cancer. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Zhang, X.; Qin, Y.-Y.; Qin, J.-Q.; Lin, F.-Q. Mean platelet volume/platelet count ratio in colorectal cancer: A retrospective clinical study. BMC Cancer 2019, 19, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çetinkaya, E.; Şenol, K.; Saylam, B.; Tez, M. Red cell distribution width to platelet ratio: New and promising prognostic marker in acute pancreatitis. World J. Gastroenterol. WJG 2014, 20, 14450–14454. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Prev. Biomark. 2014, 23, 1204–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, G.; Turner, L.; Scully, M.; Kakkar, A. Platelets and cancer. Lancet Oncol. 2002, 3, 425–430. [Google Scholar] [CrossRef]
- Montagnana, M.; Danese, E. Red cell distribution width and cancer. Ann. Transl. Med. 2016, 4, 399. [Google Scholar] [CrossRef] [Green Version]
- Vano, Y.-A. Optimal cut-off for neutrophil-to-lymphocyte ratio: Fact or Fantasy? A prospective cohort study in metastatic cancer patients. PLoS ONE 2018, 13, e0195042. [Google Scholar] [CrossRef] [Green Version]
- Forget, P. The neutrophil-to-lymphocyte ratio (NLR) after surgery for hip fracture (HF). Arch. Gerontol. Geriatr. 2015, 60, 366–371. [Google Scholar] [CrossRef]
- Condado, J.F. Neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) can risk stratify patients in transcatheter aortic-valve replacement (TAVR). Int. J. Cardiol. 2016, 223, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Köse, N.; Yıldırım, T.; Akın, F.; Yıldırım, S.E.; Altun, İ. Prognostic role of NLR, PLR, and LMR in patients with pulmonary embolism. Bosn. J. Basic Med. Sci. 2020, 20, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.M. Neutrophil elastase–mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Webb, N.J.A.; Myers, C.R.; Watson, C.J.; Bottomley, M.J.; Brenchley, P.E.C. Activated human neutrophils express Vascular Endothelial Growth Factor (VEGF). Cytokine 1998, 10, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, F.; Cassatella, M.A.; Rossi, F.; Ceska, M.; Dewald, B.; Baggiolini, M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J. Exp. Med. 1991, 173, 771–774. [Google Scholar] [CrossRef]
- Fernández, C.A.; Yan, L.; Louis, G.; Yang, J.; Kutok, J.L.; Moses, M.A. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin. Cancer Res. 2005, 11, 5390–5395. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, A. Neutrophils suppress intraluminal NK cell–mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef] [Green Version]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Wang, K.; Xu, J.; Zhang, T.; Xue, D. Tumor-infiltrating lymphocytes in breast cancer predict the response to chemotherapy and survival outcome: A meta-analysis. Oncotarget 2016, 7, 44288–44298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Brodin, P.; Kalnicki, S.; Garg, M.; Guha, C.; Kabarriti, R. NLR as a prognostic factor in solid tumors. J. Clin. Oncol. 2018, 36, e24057. [Google Scholar] [CrossRef]
- Placke, T. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012, 72, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios-Acedo, A.L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Platelets, thrombo-inflammation, and cancer: Collaborating with the enemy. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cools-Lartigue, J.; Spicer, J.; Najmeh, S.; Ferri, L. Neutrophil extracellular traps in cancer progression. Cell. Mol. Life Sci. CMLS 2014, 71, 4179–4194. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-F.; Huang, Y.; Chen, Q.-X. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J. Surg. Oncol. 2014, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- Prabawa, I.P.Y. Pretreatment Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as a predictive value of hematological markers in cervical cancer. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Kaito, K. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br. J. Haematol. 2005, 128, 698–702. [Google Scholar] [CrossRef]
- Brækkan, S.K.; Mathiesen, E.B.; Njølstad, I.; Wilsgaard, T.; Størmer, J.; Hansen, J.B. Mean platelet volume is a risk factor for venous thromboembolism: The Tromsø study. J. Thromb. Haemost. 2010, 8, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Chen, W.; Tu, J.; Ni, C.; Meng, K. Prognostic value and clinicopathologic features of platelet distribution width in cancer: A meta-analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7130–7136. [Google Scholar] [CrossRef]
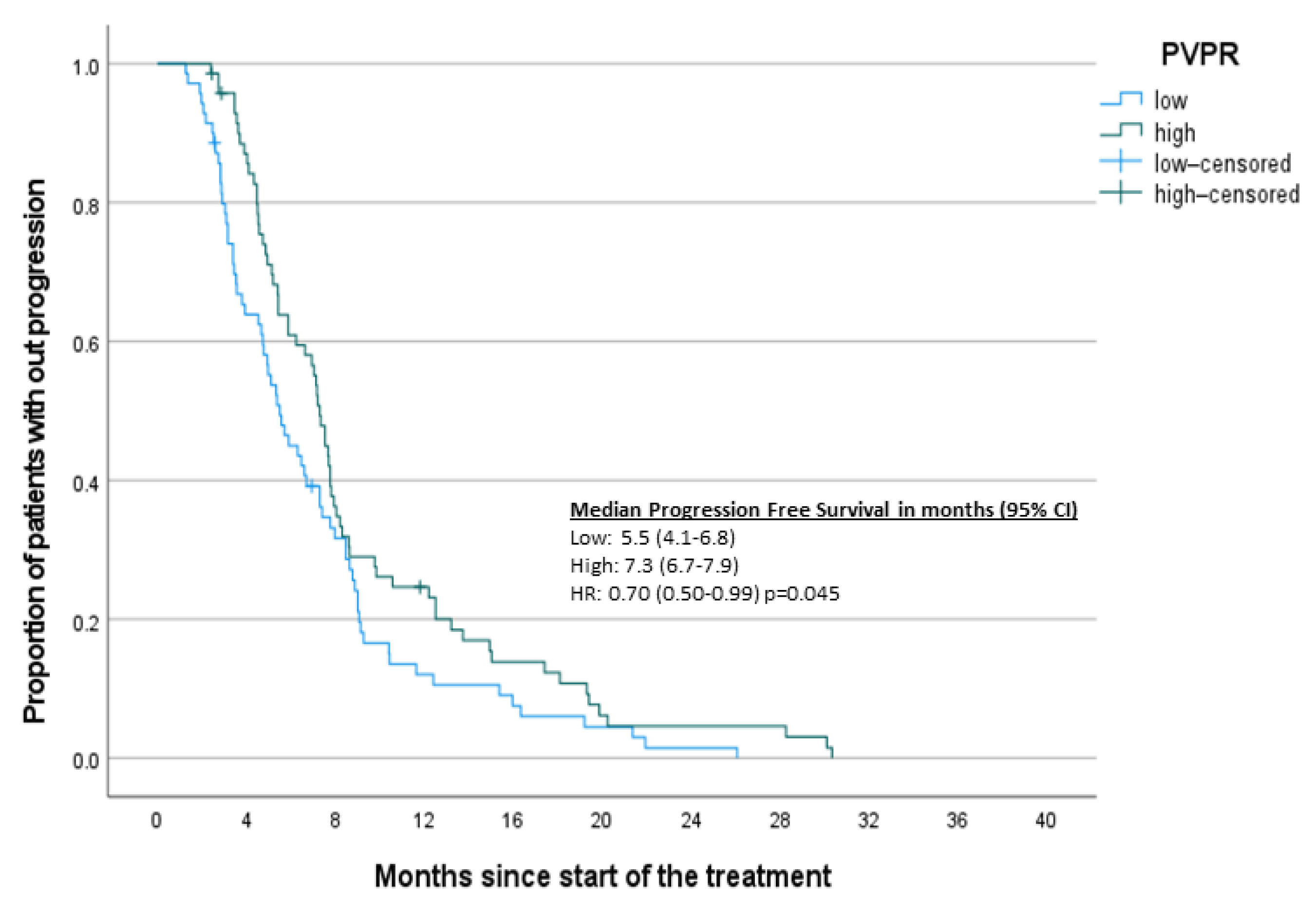
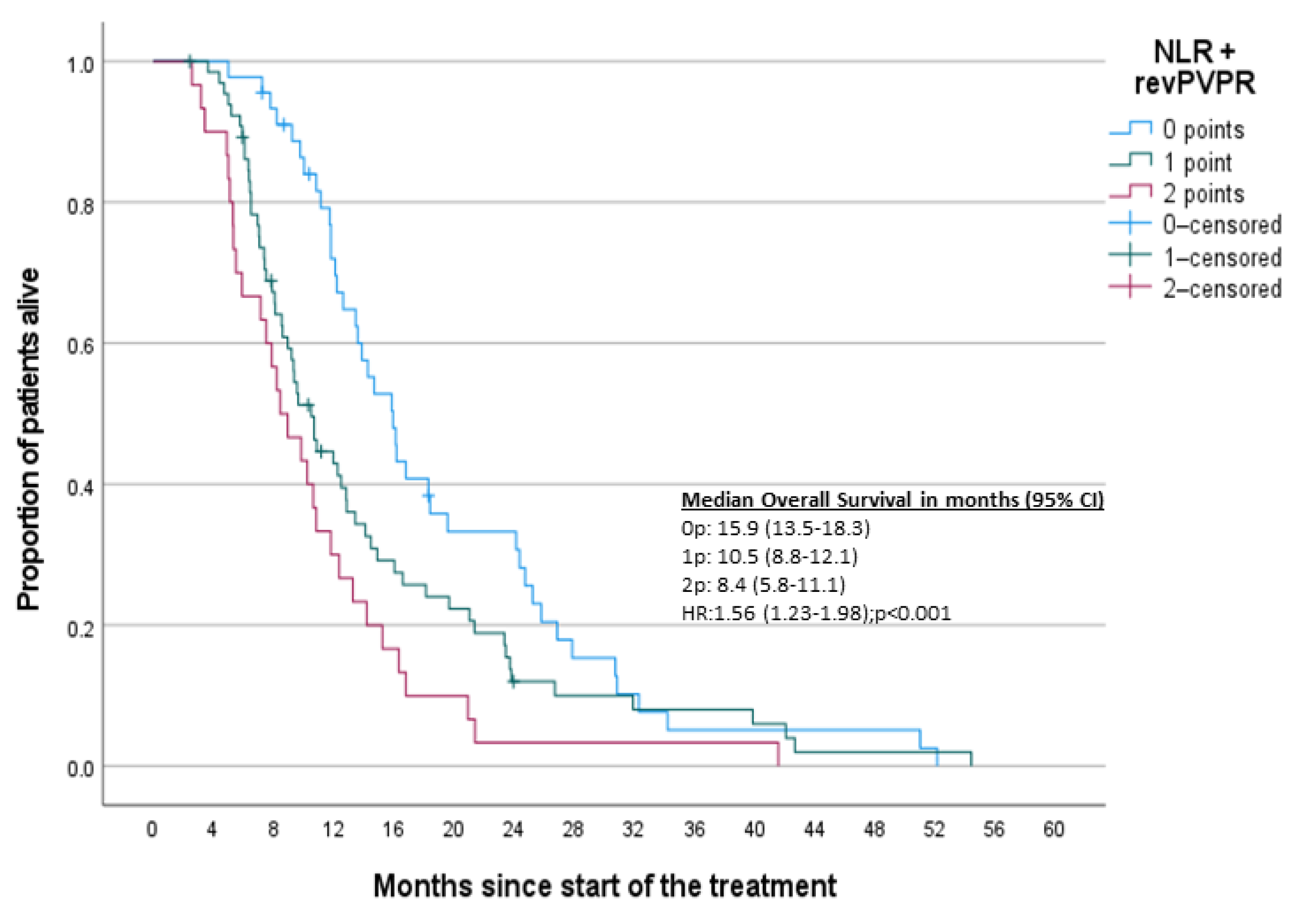
| Variable | n | q2 (q1–q3) | min–max |
| Age | 155 | 62 (56–70) | 32–82 |
| OS | 102 | 324 (191.25–474) | 65–1602 |
| PFS | 154 | 158 (84.75–239) | 8–880 |
| NLR | 151 | 3.26 (2.2–5.06) | 0.79–13 |
| PVPR | 143 | 0.34 (0.24–0.46) | 0.11–1.28 |
| PLR | 152 | 203.21 (139.11–279.42) | 52.09–938.24 |
| HPR | 154 | 0.37 (0.27–0.53) | 0.10–1.79 |
| PDW | 144 | 11.85 (10.67–13.53) | 8.4–20.9 |
| Variable | Categories | n | % |
| Gender | male/female | 109/46 | 70.3/29.7 |
| ECOG † | 0/1/2/NR | 24/92/31/8 | 15.5/59.4/20/5.2 |
| Grade | 1/2/3/4/NR | 3/30/78/1/43 | 1.9/19.4/50.3/0.6/27.7 |
| HER2 expression | 0-2/3/NR | 85/16/54 | 54.8/15.8/34/8 |
| Prior radical treatment ‡ | no/yes/NR | 41/114/3 | 26.5/73.5/1.9 |
| Prior gastrectomy | 0/1/NR | 87/63/5 | 56.1/40.6/3.2 |
| Bone metastases | no/yes/NR | 141/10/4 | 91/6.5/2.6 |
| Liver metastases | no/yes/NR | 98/52/5 | 63.2/33.5/3.2 |
| Number of metastatic sites | 0/1/2/3/NR | 21/91/27/10/6 | 13.5/58.7/17.4/6.5/3.9 |
| Type of chemotherapy | 1/2/3/4 | 19/59/62/15 | 12.3/38.1/40/9.7 |
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1. NLR | 1 | ||||
| 2. PVPR | −0.17 * | 1 | |||
| 3. PDW | −0.24 ** | 0.67 ** | 1 | ||
| 4. HPR | −0.14 | 0.93 ** | 0.54 ** | 1 | |
| 5. PLR | 0.40 ** | −0.28 ** | −0.17 | −0.34 ** | 1 |
| Model 1 *a | Model 2 *b | Model 3 *c | Model 4 *d | |
|---|---|---|---|---|
| OS | ||||
| n/Events ‡ | 131/122 | 133/124 | 126/117 | 93/85 |
| Harrell’s C-index (95% CI) ± | 0.65 (0.59; 0.71) | 0.67 (0.61; 0.73) | 0.64 (0.58; 0.7) | 0.65 (0.58; 0.73) |
| pPHA ¤ | 0.269 | 0.47 | 0.517 | 0.274 |
| PVPR | 0.66 (0.45–0.98) | 0.66 (0.44–0.98) | 0.63 (0.41–0.96) | 0.56 (0.33–0.93) |
| NLR | 1.74 (1.18–2.57) | 1.78 (1.2–2.64) | 1.85 (1.23–2.78) | 2.02 (1.2–3.37) |
| PFS | ||||
| n/Events ‡ | 130/125 | 132/127 | 125/120 | 92/87 |
| Harrell’s C-index (95% CI) ± | 0.62 (0.56; 0.68) | 0.65 (0.59; 0.71) | 0.65 (0.6; 0.71) | 0.64 (0.57; 0.72) |
| pPHA ¤ | 0.64 | 0.79 | 0.587 | 0.395 |
| PVPR | 0.73 (0.5–1.07) | 0.71 (0.48–1.05) | 0.71 (0.47–1.06) | 0.8 (0.49–1.33) |
| NLR | 1.5 (1.01–2.22) | 1.68 (1.13–2.49) | 1.98 (1.3–3.04) | 2.02 (1.19–3.45) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopka, K.; Micek, A.; Ochenduszko, S.; Streb, J.; Potocki, P.; Kwinta, Ł.; Wysocki, P.J. Combined Neutrophil-to-Lymphocyte and Platelet-Volume-to-Platelet Ratio (NLR and PVPR Score) Represents a Novel Prognostic Factor in Advanced Gastric Cancer Patients. J. Clin. Med. 2021, 10, 3902. https://doi.org/10.3390/jcm10173902
Konopka K, Micek A, Ochenduszko S, Streb J, Potocki P, Kwinta Ł, Wysocki PJ. Combined Neutrophil-to-Lymphocyte and Platelet-Volume-to-Platelet Ratio (NLR and PVPR Score) Represents a Novel Prognostic Factor in Advanced Gastric Cancer Patients. Journal of Clinical Medicine. 2021; 10(17):3902. https://doi.org/10.3390/jcm10173902
Chicago/Turabian StyleKonopka, Kamil, Agnieszka Micek, Sebastian Ochenduszko, Joanna Streb, Paweł Potocki, Łukasz Kwinta, and Piotr J. Wysocki. 2021. "Combined Neutrophil-to-Lymphocyte and Platelet-Volume-to-Platelet Ratio (NLR and PVPR Score) Represents a Novel Prognostic Factor in Advanced Gastric Cancer Patients" Journal of Clinical Medicine 10, no. 17: 3902. https://doi.org/10.3390/jcm10173902
APA StyleKonopka, K., Micek, A., Ochenduszko, S., Streb, J., Potocki, P., Kwinta, Ł., & Wysocki, P. J. (2021). Combined Neutrophil-to-Lymphocyte and Platelet-Volume-to-Platelet Ratio (NLR and PVPR Score) Represents a Novel Prognostic Factor in Advanced Gastric Cancer Patients. Journal of Clinical Medicine, 10(17), 3902. https://doi.org/10.3390/jcm10173902







