Secundum Type Atrial Septal Defect in Patients with Trisomy 21—Therapeutic Strategies, Outcome, and Survival: A Nationwide Study of the German National Registry for Congenital Heart Defects
Abstract
1. Introduction
2. Materials and Methods
Statistical Methods
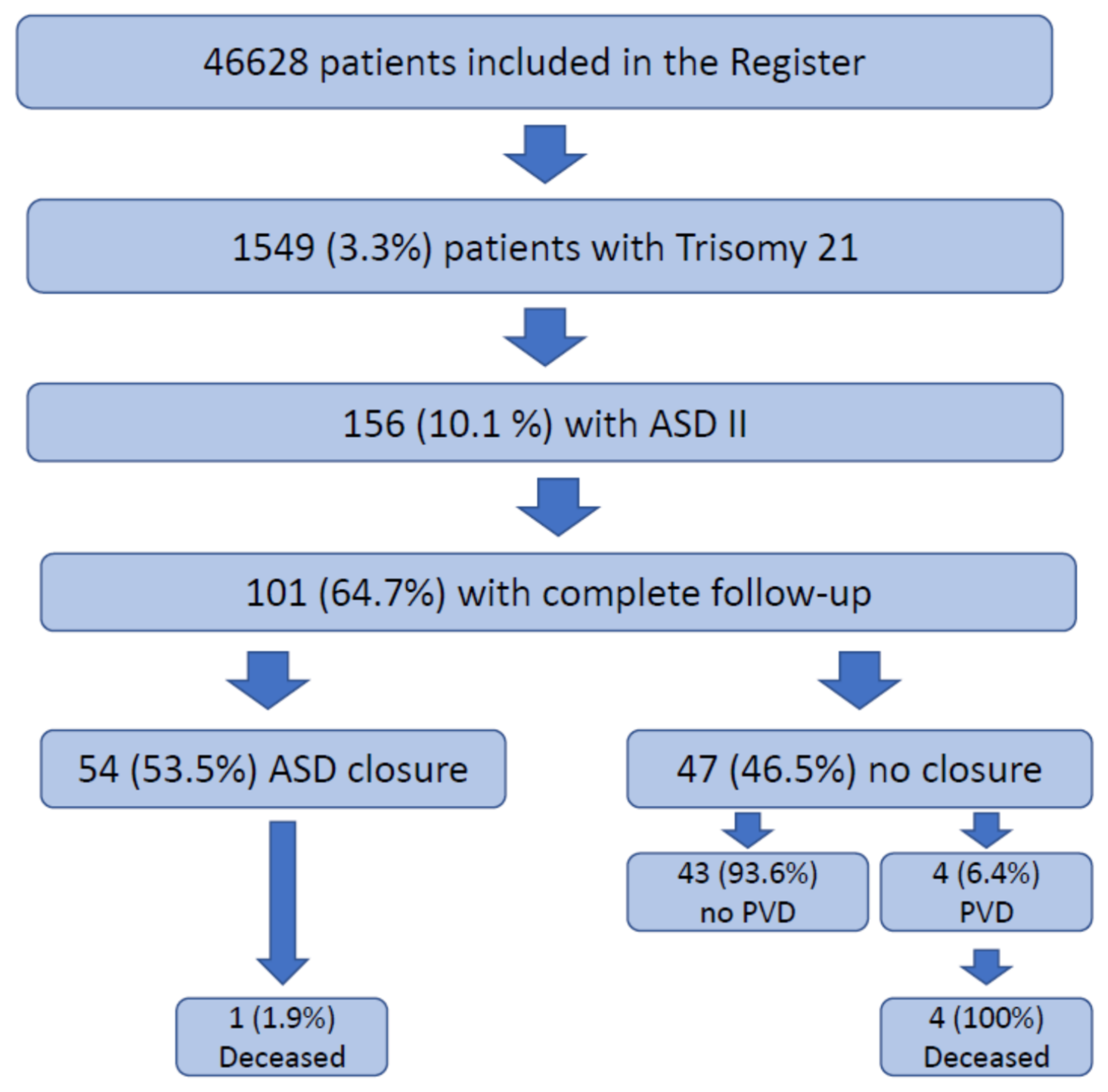
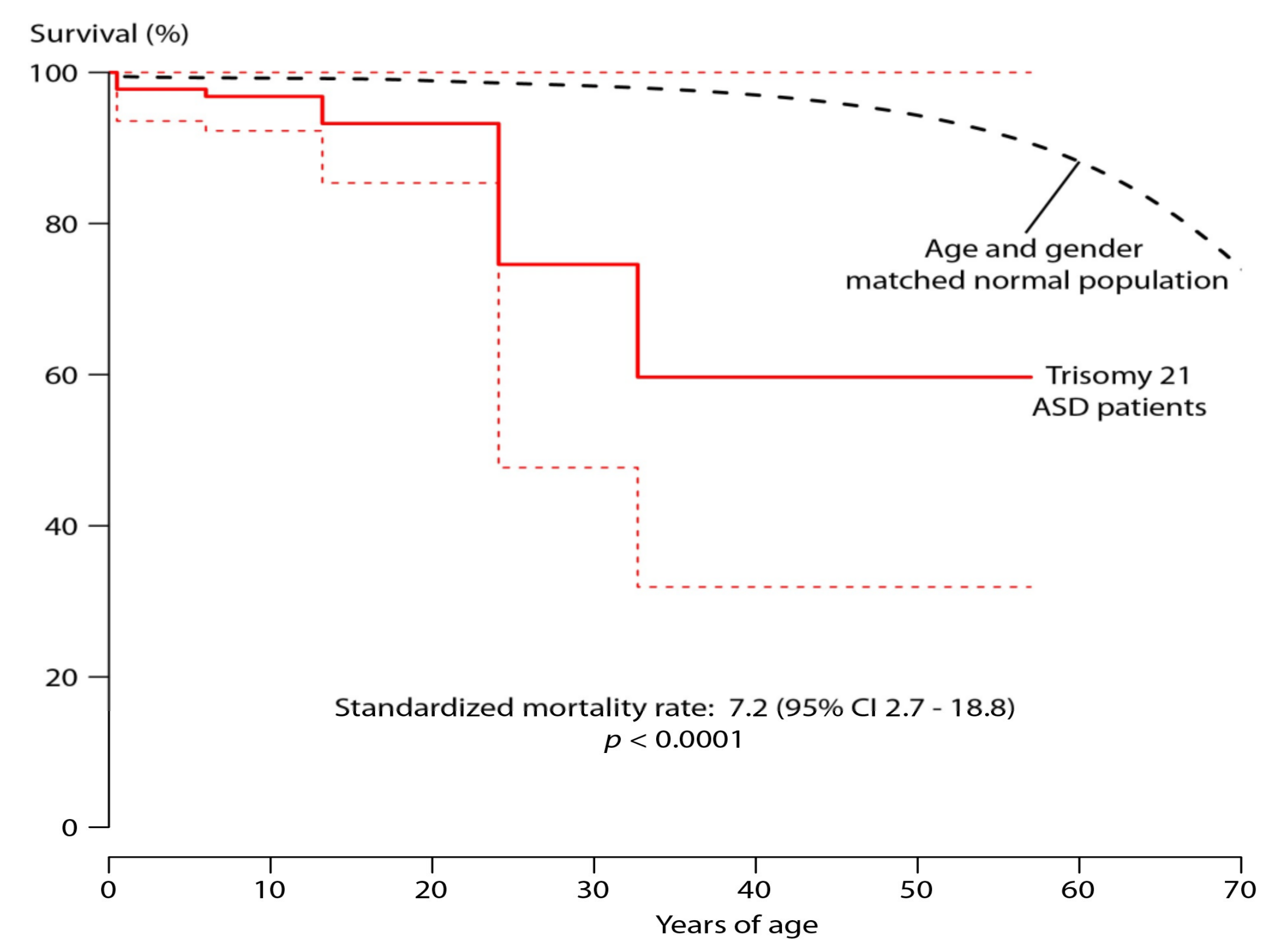
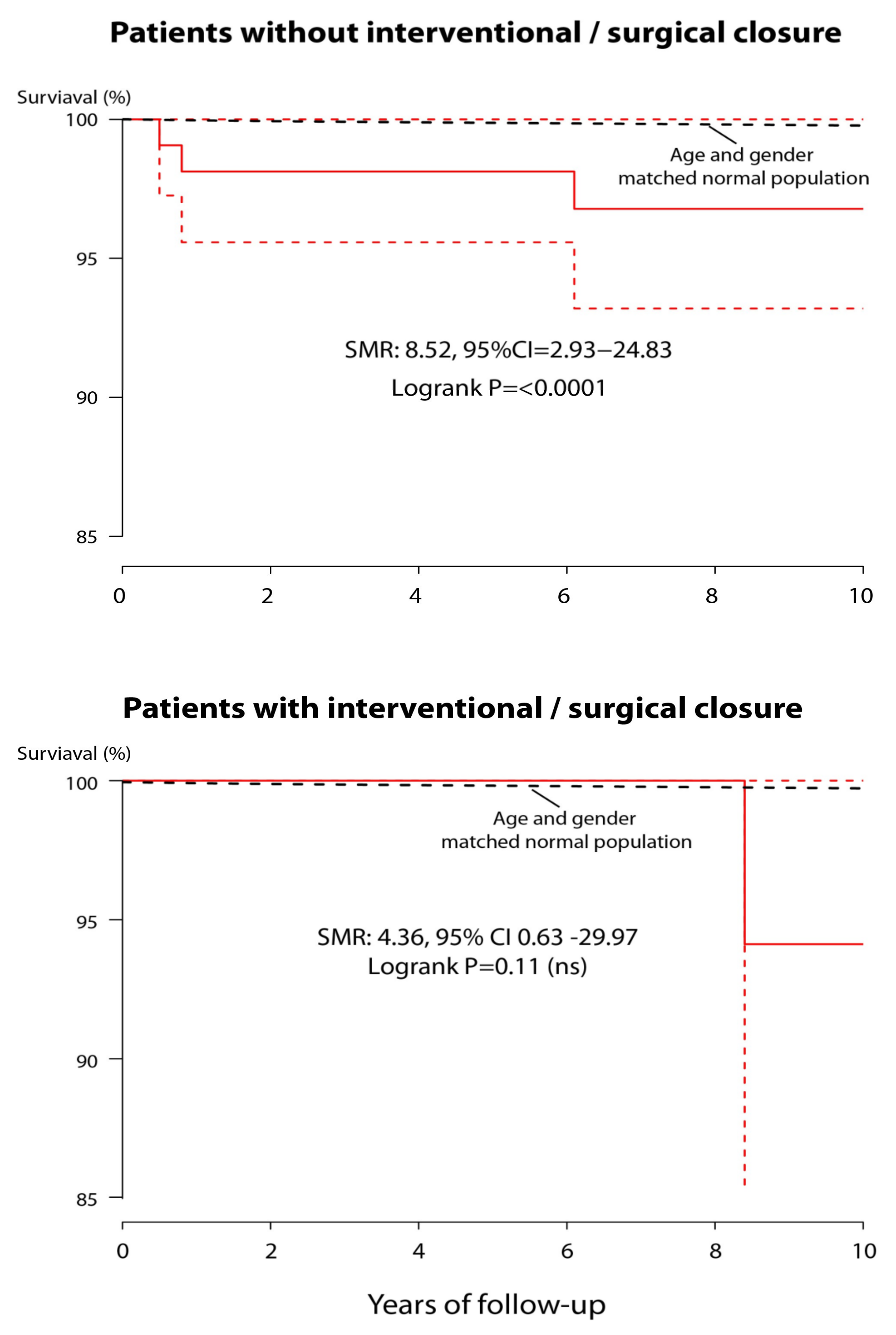
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfitzer, C.; Helm, P.C.; Rosenthal, L.M.; Berger, F.; Bauer, U.M.M.; Schmitt, K.R. Dynamics in prevalence of Down syndrome in children with congenital heart disease. Eur. J. Pediatr. 2018, 177, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Versacci, P.; Di Carlo, D.; Digilio, M.C.; Marino, B. Cardiovascular disease in Down syndrome. Curr. Opin. Pediatr. 2018, 30, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Driscoll, D.; Allen, H.D.; Atkins, D.L.; Brenner, J.; Dunnigan, A.; Franklin, W.; Gutgesell, H.P.; Herndon, P.; Shaddy, R.E.; Taubert, K.A. Guidelines for evaluation and management of common congenital cardiac problems in infants, children, and adolescents. A statement for healthcare professionals from the Committee on Congenital Cardiac Defects of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 1994, 90, 2180–2188. [Google Scholar]
- Spangler, J.G.; Feldt, R.H.; Danielson, G.K. Secundum atrial septal defect encountered in infancy. J. Thorac. Cardiovasc. Surg. 1976, 71, 398–401. [Google Scholar] [CrossRef]
- Campbell, M. Incidence of cardiac malformations at birth and later, and neonatal mortality. Br. Heart J. 1973, 35, 189. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.P.; Gatzoulis, M.A. Pulmonary vascular disease in adults with congenital heart disease. Circulation 2007, 115, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- D’Alto, M.; Mahadevan, V.S. Pulmonary arterial hypertension associated with congenital heart disease. Eur. Respir. Rev. 2012, 21, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Spicer, R.L. Cardiovascular disease in Down syndrome. Pediatr. Clin. N. Am. 1984, 31, 1331–1343. [Google Scholar] [CrossRef]
- Cooney, T.P.; Thurlbeck, W.M. Pulmonary hypoplasia in Down’s syndrome. New Engl. J. Med. 1982, 307, 1170–1173. [Google Scholar] [CrossRef]
- Béland, M.J.; Jacobs, J.P.; Tchervenkov, C.I.; Franklin, R.C. Report from the Executive of The International Working Group for Mapping and Coding of Nomenclatures for Paediatric and Congenital Heart Disease. Cardiol. Young 2002, 12, 425–430. [Google Scholar] [CrossRef]
- Finkelstein, D.M.; Muzikansky, A.; Schoenfeld, D.A. Comparing survival of a sample to that of a standard population. J. Natl. Cancer Inst. 2003, 95, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Hunt, C.E.; Lucas, R.V. Symptomatic atrial septal defect in infancy. Circulation 1973, 47, 1042–1048. [Google Scholar] [CrossRef]
- Phillips, S.J.; Okies, J.E.; Henken, D.; Sunderland, C.O.; Starr, A. Complex of secundum atrial septal defect and congestive heart failure in infants. J. Thorac. Cardiovasc. Surg. 1975, 70, 696–700. [Google Scholar] [CrossRef]
- Wyler, F.; Rutishauser, M. Symptomatic atrial septal defect in the neonate and infant. Helv. Paediatr. Acta 1976, 30, 399–408. [Google Scholar]
- Lammers, A.; Hager, A.; Eicken, A.; Lange, R.; Hauser, M.; Hess, J. Need for closure of secundum atrial septal defect in infancy. J. Thorac. Cardiovasc. Surg. 2005, 129, 1353–1357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Manes, A.; Palazzini, M.; Leci, E.; Bacchi Reggiani, M.L.; Branzi, A.; Galiè, N. Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: A comparison between clinical subgroups. Eur. Heart J. 2014, 35, 716–724. [Google Scholar] [CrossRef]
- Lammers, A.E.; Haworth, S.G.; Diller, G.P. Atrial septostomy in patients with pulmonary hypertension: Should it be recommended? Expert Rev. Respir. Med. 2011, 5, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Khalil, M.; Schmidt, D.; Bauer, J.; Esmaeili, A.; Apitz, C.; Voelkel, N.F.; Schranz, D. Creation of a restrictive atrial communication in pulmonary arterial hypertension (PAH): Effective palliation of syncope and end-stage heart failure. Pulm. Circ. 2018, 8, 2045894018776518. [Google Scholar] [CrossRef] [PubMed]
- Lammers, A.E.; Derrick, G.; Haworth, S.G.; Bonhoeffer, P.; Yates, R. Efficacy and long-term patency of fenestrated amplatzer devices in children. Catheter. Cardiovasc. Interv. 2007, 70, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Körten, M.A.; Helm, P.C.; Abdul-Khaliq, H.; Baumgartner, H.; Kececioglu, D.; Schlensak, C.; Bauer, U.M.; Diller, G.P. Eisenmenger syndrome and long-term survival in patients with Down syndrome and congenital heart disease. Heart 2016, 102, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Brida, M.; Diller, G.P.; Kempny, A.; Drakopoulou, M.; Shore, D.; Gatzoulis, M.; Uebing, A. Atrial septal defect closure in adulthood is associated with normal survival in the mid to longer term. Heart 2019, 105, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lammers, A.E.; Stegger, J.; Koerten, M.-A.; Helm, P.C.; Bauer, U.M.; Baumgartner, H.; Uebing, A.S. Secundum Type Atrial Septal Defect in Patients with Trisomy 21—Therapeutic Strategies, Outcome, and Survival: A Nationwide Study of the German National Registry for Congenital Heart Defects. J. Clin. Med. 2021, 10, 3807. https://doi.org/10.3390/jcm10173807
Lammers AE, Stegger J, Koerten M-A, Helm PC, Bauer UM, Baumgartner H, Uebing AS. Secundum Type Atrial Septal Defect in Patients with Trisomy 21—Therapeutic Strategies, Outcome, and Survival: A Nationwide Study of the German National Registry for Congenital Heart Defects. Journal of Clinical Medicine. 2021; 10(17):3807. https://doi.org/10.3390/jcm10173807
Chicago/Turabian StyleLammers, Astrid E., Julia Stegger, Marc-André Koerten, Paul C. Helm, Ulrike M. Bauer, Helmut Baumgartner, and Anselm S. Uebing. 2021. "Secundum Type Atrial Septal Defect in Patients with Trisomy 21—Therapeutic Strategies, Outcome, and Survival: A Nationwide Study of the German National Registry for Congenital Heart Defects" Journal of Clinical Medicine 10, no. 17: 3807. https://doi.org/10.3390/jcm10173807
APA StyleLammers, A. E., Stegger, J., Koerten, M.-A., Helm, P. C., Bauer, U. M., Baumgartner, H., & Uebing, A. S. (2021). Secundum Type Atrial Septal Defect in Patients with Trisomy 21—Therapeutic Strategies, Outcome, and Survival: A Nationwide Study of the German National Registry for Congenital Heart Defects. Journal of Clinical Medicine, 10(17), 3807. https://doi.org/10.3390/jcm10173807




