Competing Risks Model for Prediction of Small for Gestational Age Neonates and the Role of Second Trimester Soluble Fms-like Tyrosine Kinase-1
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Design
2.2. Outcome Measures
2.3. Statistical Analyses
3. Results
4. Discussion
4.1. Main Findings
4.2. Comparison with Previous Studies
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pilliod, R.A.; Cheng, Y.W.; Snowden, J.M.; Doss, A.E.; Caughey, A.B. The risk of intrauterine fetal death in the small-for-gestational-age fetus. Am. J. Obstet. Gynecol. 2012, 207, 318–416. [Google Scholar] [CrossRef] [Green Version]
- Trudell, A.S.; Cahill, A.G.; Tuuli, M.G.; Macones, G.A.; Odibo, A.O. Risk of stillbirth after 37 weeks in pregnancies complicated by small-for-gestational-age fetuses. Am. J. Obstet. Gynecol. 2013, 208, e1–e7. [Google Scholar]
- Bukowski, R.; Burgett, A.D.; Gei, A.; Saade, G.R.; Hankins, G.D. Impairment of fetal growth potential and neonatal encephalopathy. Am. J. Obstet. Gynecol. 2003, 188, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 2006, 49, 270–283. [Google Scholar] [CrossRef]
- McCowan, L.M.; Figueras, F.; Anderson, N.H. Evidence-based national guidelines for the management of suspected fetal growth restriction: Comparison, consensus, and controversy. Am. J. Obstet. Gynecol. 2018, 218, S855–S868. [Google Scholar] [CrossRef] [Green Version]
- Lindqvist, P.G.; Molin, J. Does antenatal identification of small-for-gestational-age fetuses significantly improve their outcome? Ultrasound Obstet. Gynecol. 2005, 25, 258–264. [Google Scholar] [CrossRef]
- Papastefanou, I.; Wright, D.; Nicolaides, K.H. Competing risks model for prediction of small for gestational age neonates from maternal characteristics and medical history. Ultrasound Obstet. Gynecol. 2020, 56, 196–205. [Google Scholar] [CrossRef]
- Papastefanou, I.; Wright, D.; Syngelaki, A.; Lolos, M.; Anampousi, K.; Nicolaides, K.H. Competing-risks model for prediction of small-for-gestational-age neonates from maternal characteristics and serum pregnancy-associated plasma protein-A at 11–13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2020, 56, 541–548. [Google Scholar] [CrossRef]
- Papastefanou, I.; Wright, D.; Lolos, M.; Anampousi, K.; Mamalis, M.; Nicolaides, K.H. Competing-risks model for prediction of small-for-gestational-age neonates from maternal characteristics, serum PAPP-A and PlGF at 11–13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2021, 57, 392–400. [Google Scholar] [CrossRef]
- Papastefanou, I.; Wright, D.; Syngelaki, A.; Souretis, K.; Chrysanthopoulou, E.; Nicolaides, K.H. Competing-risks model for prediction of small-for-gestational-age neonates from biophysical and biochemical markers at 11–13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2021, 57, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, I.; Nowacka, U.; Syngelaki, A.; Dragoi, V.; Karamanis, G.; Wright, D.; Nicolaides, K.H. Competing risks model for prediction of small for gestational age neonates from estimated fetal weight at 19–24 weeks’ gestation. Ultrasound Obstet. Gynecol. 2021, 57, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, I.; Nowacka, U.; Syngelaki, A.; Mansukhani, T.; Karamanis, G.; Wright, D.; Nicolaides, K.H. Competing risks model for prediction of small for gestational age neonates from biophysical markers at 19–24 weeks’ gestation. Am. J. Obstet. Gynecol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, I.; Nowacka, U.; Buerger, O.; Akolekar, R.; Wright, D.; Nicolaides, K.H. Evaluation of the RCOG guideline in the prediction of small for gestational age neonates and comparison with the competing risks model. BJOG 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, U.; Papastefanou, I.; Bouariou, A.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Second trimester contingent screening for small for gestational age neonates. Ultrasound Obstet. Gynecol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.M.; Wright, D.; Casanova, C.; Campanero, M.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 19–24 weeks’ gestation. Am. J. Obstet. Gynecol. 2016, 214, 619.e1–619.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, D.; Krajewska, K.; Bogdanova, A.; Wright, A.; Nicolaides, K.H. Maternal serum soluble fms-like tyrosine kinase-1 at 22 and 32 weeks in the prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 2016, 47, 755–761. [Google Scholar] [CrossRef]
- Lesmes, C.; Gallo, D.M.; Gonzalez, R.; Poon, L.C.; Nicolaides, K.H. Prediction of small-for-gestational-age neonates: Screening by maternal serum biochemical markers at 19–24 weeks. Ultrasound Obstet. Gynecol. 2015, 46, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Papageorghiou, A.T.; Yu, C.K.H.; Bindra, R.; Pandis, G.; Nicolaides, K.N. Multicentre screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet. Gynecol. 2001, 18, 441–449. [Google Scholar] [CrossRef]
- Albaiges, G.; Missfelder-Lobos, H.; Lees, C.; Parra, M.; Nicolaides, K.H. One-stage screening for pregnancy complications by color Doppler assessment of the uterine arteries at 23 weeks’ gestation. Obstet. Gynecol. 2000, 96, 559–564. [Google Scholar] [CrossRef]
- Poon, L.C.; Zymeri, N.A.; Zamprakou, A.; Syngelaki, A.; Nicolaides, K.H. Protocol for measurement of mean arterial pressure at 11–13 weeks’ gestation. Fetal Diagn. Ther. 2012, 31, 42–48. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Hammami, A.; Mazer Zumaeta, A.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Ultrasonographic estimation of fetal weight: Development of new model and assessment of performance of previous models. Ultrasound Obstet. Gynecol. 2018, 52, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, H.P.; Fleming, J.E. A critical evaluation of sonar crown rump length measurements. Br. J. Obstet Gynaecol. 1975, 82, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Snijders, R.J.; Nicolaides, K.H. Fetal biometry at 14–40 weeks’ gestation. Ultrasound Obstet. Gynecol. 1994, 4, 34–48. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 202. Obstet. Gynecol. 2019, 133, e1–e25. [Google Scholar]
- Nicolaides, K.H.; Wright, D.; Syngelaki, A.; Wright, A.; Akolekar, R. Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound Obstet. Gynecol. 2018, 52, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Gilks, W.R.; Thomas, A.; Spiegelhalter, D.J. A language and program for complex Bayesian modelling. Statistician 1994, 43, 169–177. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 1 June 2021).
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 2021, 152 (Suppl. 1), 3–57. [Google Scholar] [CrossRef]

| Variables | Total (n = 40241) | Non-SGA (n = 35468) | SGA (n = 4773) | p-Value |
|---|---|---|---|---|
| Maternal age (years) | 31.9 (27.9–35.5) | 32.0 (28.0–35.5) | 31.4 (27.0–35.3) | <0.0001 |
| Maternal weight (kg) | 67.2 (59.9–78.1) | 68.0 (60.0–79.0) | 63.8 (56.4–73.8) | <0.0001 |
| Maternal height (cm) | 165 (161–170) | 165 (161–170) | 163 (158–167) | <0.0001 |
| Body mass index (kg/m2) | 24.6 (22.0–28.5) | 24.7 (22.1–28.6) | 24.0 (21.4–27.6) | <0.0001 |
| Gestational age at assessment (w) | 21.6 (21.1–22.0) | 21.6 (21.1–22.0) | 21.6 (21.1–22.0) | 0.241 |
| Racial origin | ||||
| White | 31195 (77.5) | 28036 (79.1) | 3159 (62.2) | <0.0001 |
| Black | 5226 (13.0) | 4334 (12.2) | 892 (18.7) | <0.0001 |
| South Asian | 1923 (4.8) | 1487 (4.2) | 436 (9.1) | <0.0001 |
| East Asian | 784 (2.0) | 669 (1.9) | 115 (2.4) | 0.016 |
| Mixed | 1113 (2.8) | 942 (2.7) | 171 (3.6) | 0.0003 |
| Conception | ||||
| Natural | 38433 (95.5) | 33897 (95.6) | 4536 (95.0) | 0.101 |
| Ovulation induction | 295 (0.7) | 255 (0.7) | 40 (0.8) | 0.415 |
| In vitro fertilization | 1513 (3.8) | 1316 (3.7) | 197 (4.1) | 0.167 |
| Medical history | ||||
| Chronic hypertension | 425 (1.1) | 323 (0.9) | 102 (2.1) | <0.0001 |
| Diabetes mellitus | 354 (0.9) | 315 (0.9) | 39 (0.8) | 0.681 |
| SLE/APS | 85 (0.2) | 68 (0.2) | 17 (0.4) | 0.031 |
| Cigarette smokers | 3016 (7.5) | 2324 (6.6) | 692 (14.5) | <0.0001 |
| Family history of preeclampsia | 1451 (3.6) | 1246 (3.5) | 205 (4.3) | 0.007 |
| Parity | ||||
| Nulliparous | 18954 (47.1) | 16241 (45.8) | 2713 (56.8) | <0.0001 |
| Parous with previous SGA | 2818 (7.0) | 2033 (5.7) | 785 (16.5) | <0.0001 |
| Parous with previouspreeclampsia and (or) SGA | 3563 (8.9) | 2701 (7.6) | 862 (18.1) | <0.0001 |
| Inter-pregnancy interval (years) | 2.7 (1.7–4.7) | 2.7 (1.7–4.6) | 3.2 (1.8–5.8) | <0.0001 |
| Preeclampsia | 1197 (3.0) | 846 (2.4) | 351 (7.4) | <0.0001 |
| Gestational hypertension | 1095 (2.7) | 859 (2.4) | 236 (4.9) | <0.0001 |
| Term | Estimate (Upper and Lower 95 Credibility Limits) | SD |
|---|---|---|
| log10 MoM sFlt-1 | ||
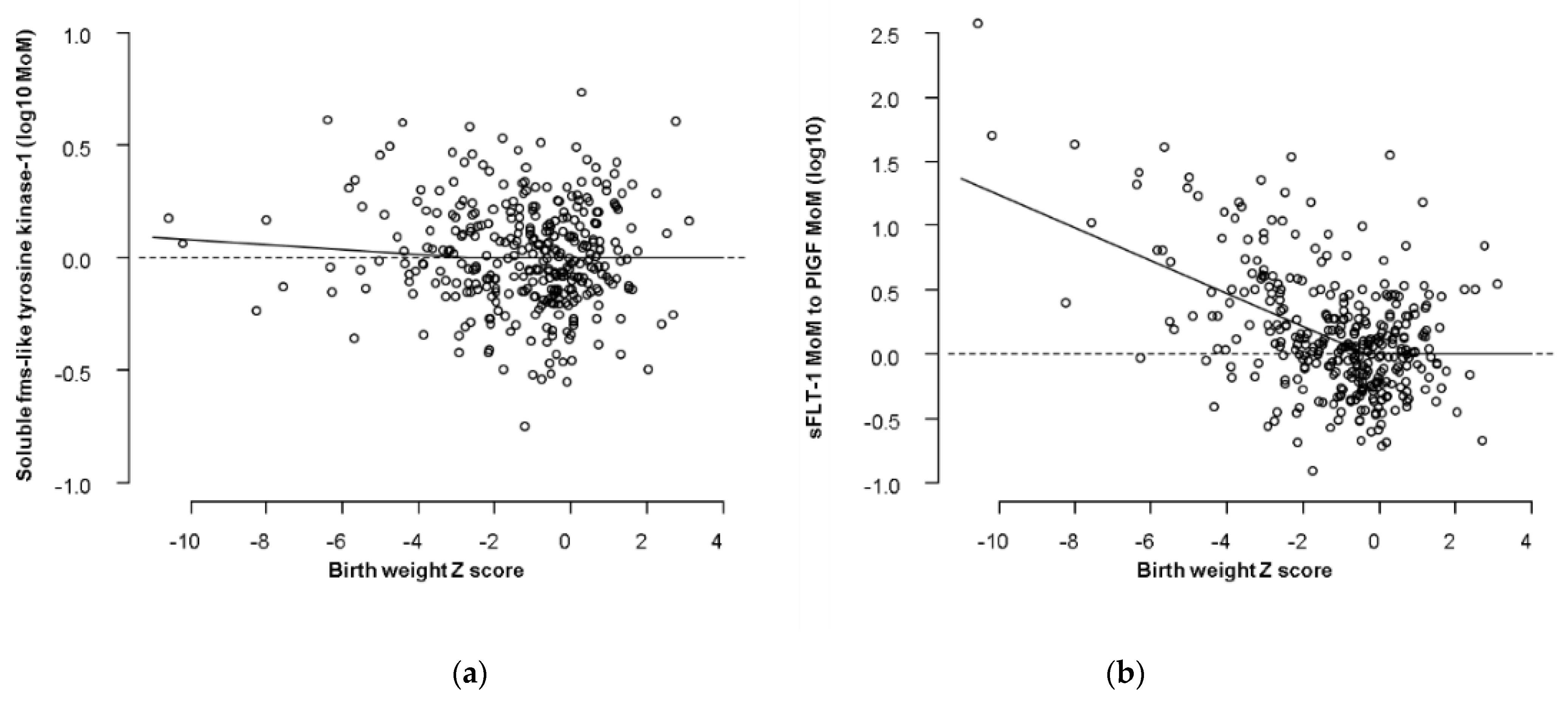
| Intercept | −0.028181411 (−0.101200000 to 0.044080000) | 0.034100921 |
| Birth weight Z score | −0.011182582 (−0.032760000 to 0.013000250) | 0.011519052 |
| (GA–33)−1 | 0.001131449 (−0.001348025 to 0.004564025) | 0.001472069 |
| SD for log10 MoM sFlt-1 | 0.233381804 (0.216500000 to 0.252000000) | 0.009013250 |
| log10 (MoM sFlt-1/MoM PlGF) | ||
| Intercept | −0.25555636 (−0.3528000 to −0.1639000) | 0.04816489 |
| Birth weight Z score | −0.12802946 (−0.1524000 to −0.1030000) | 0.01262894 |
| GA-40 | −0.01624357 (−0.0224600 to −0.0102000) | 0.00319910 |
| SD for log10 (MoM sFlt-1 / MoM PlGF) | 0.31564903 (0.3135000 to 0.3178000) | 0.00111485 |
| Method of Screening | N | Comparison of Detection by the Two Methods of Screening n (%) vs. n (%) | Difference in Detection between the Two Methods of Screening n (%; 95% CI) | p-Value |
|---|---|---|---|---|
| <32 weeks | ||||
| All SGA <10th percentile | ||||
| MF vs MF+ sFlt-1 | 131 | 50 (38.2) vs. 51 (38.9) | 1 (0.7; −0.7 to 2.1) | 0.318 |
| MF vs MF+ sFlt-1/PlGF | 131 | 50 (38.2) vs. 71 (54.2) | 21 (16.0; 9.7 to 22.3) | 0.0006 |
| MF+PlGF vs MF+ sFlt-1/PlGF | 131 | 81 (61.8) vs. 71 (54.2) | −10 (−7.6; −12.1 to −3.1) | 0.016 |
| SGA <10th percentile with PE | ||||
| MF vs MF+ sFlt-1 | 43 | 16 (37.2) vs. 17 (39.5) | 1 (2.3; −2.2 to 6.8) | 0.347 |
| MF vs MF+ sFlt-1/PlGF | 43 | 16 (37.2) vs. 29 (67.3) | 13 (30.1; 16.4 to 43.8) | 0.020 |
| MF+PlGF vs MF+ sFlt-1/PlGF | 43 | 30 (69.8) vs. 29 (67.3) | −1 (−2.5; −7.2 to 2.2) | 0.057 |
| SGA <10th percentile no PE | ||||
| MF vs MF+ sFlt-1 | 88 | 34 (38.6) vs. 33 (37.5) | −1 (1.2; −3.5 to 1.1) | 0.322 |
| MF vs MF+ sFlt-1/PlGF | 88 | 34 (38.6) vs. 48 (54.1) | 14 (15.5; 7.9 to 23.1) | 0.006 |
| MF+PlGF vs MF+ sFlt-1/PlGF | 88 | 53 (60.2) vs. 48 (54.1) | −5 (−6.1; −11.1 to −1.1) | 0.083 |
| <32 weeks | ||||
| All SGA <3rd percentile | ||||
| MF vs MF+ sFlt-1 | 105 | 41 (39.1) vs. 40 (38.1) | −1 (−1; −2.9 to 0.9) | 0.482 |
| MF vs MF+ sFlt-1/PlGF | 105 | 41 (39.1) vs. 60 (57.1) | 19 (18; 10.7 to 25.4) | 0.0009 |
| MF+PlGF vs MF+ sFlt-1/PlGF | 105 | 71 (67.6) vs. 60 (57.1) | −11 (−10.5; −16.4 to −4.6) | 0.021 |
| SGA <3rd percentile with PE | ||||
| MF vs MF+ sFlt-1 | 41 | 16 (39.1) vs. 41 (39.1) | 0 (0; −0.2 to 0.2) | 1 |
| MF vs MF+ sFlt-1/PlGF | 41 | 16 (39.1) vs. 23 (56.1) | 7 (17; 5.5 to 28.5) | 0.034 |
| MF+PlGF vs MF+ sFlt-1/PlGF | 41 | 29 (70.7) vs. 23 (56.1) | −6 (−14.6; −25.4 to −3.8) | 0.057 |
| SGA <3rd percentile no PE | ||||
| MF vs MF+ sFlt-1 | 64 | 25 (39.1) vs. 24 (37.5) | −1 (−1.6; −4.7 to 1.5) | 0.317 |
| MF vs MF+ sFlt-1/PlGF | 64 | 25 (39.1) vs. 37 (57.8) | 12 (18.7; 9.2 to 28.3) | 0.011 |
| MF+PlGF vs MF+ sFlt-1/PlGF | 64 | 44 (68.8) vs. 37 (57.8) | −7 (−11; −18.7 to −3.3) | 0.070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, U.; Papastefanou, I.; Bouariu, A.; Syngelaki, A.; Nicolaides, K.H. Competing Risks Model for Prediction of Small for Gestational Age Neonates and the Role of Second Trimester Soluble Fms-like Tyrosine Kinase-1. J. Clin. Med. 2021, 10, 3786. https://doi.org/10.3390/jcm10173786
Nowacka U, Papastefanou I, Bouariu A, Syngelaki A, Nicolaides KH. Competing Risks Model for Prediction of Small for Gestational Age Neonates and the Role of Second Trimester Soluble Fms-like Tyrosine Kinase-1. Journal of Clinical Medicine. 2021; 10(17):3786. https://doi.org/10.3390/jcm10173786
Chicago/Turabian StyleNowacka, Urszula, Ioannis Papastefanou, Alexandra Bouariu, Argyro Syngelaki, and Kypros H. Nicolaides. 2021. "Competing Risks Model for Prediction of Small for Gestational Age Neonates and the Role of Second Trimester Soluble Fms-like Tyrosine Kinase-1" Journal of Clinical Medicine 10, no. 17: 3786. https://doi.org/10.3390/jcm10173786
APA StyleNowacka, U., Papastefanou, I., Bouariu, A., Syngelaki, A., & Nicolaides, K. H. (2021). Competing Risks Model for Prediction of Small for Gestational Age Neonates and the Role of Second Trimester Soluble Fms-like Tyrosine Kinase-1. Journal of Clinical Medicine, 10(17), 3786. https://doi.org/10.3390/jcm10173786







