Pelvic Organ Prolapse Is Associated with Osteoporosis in Korean Women: Analysis of the Health Insurance Review and Assessment Service National Patient Sample
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
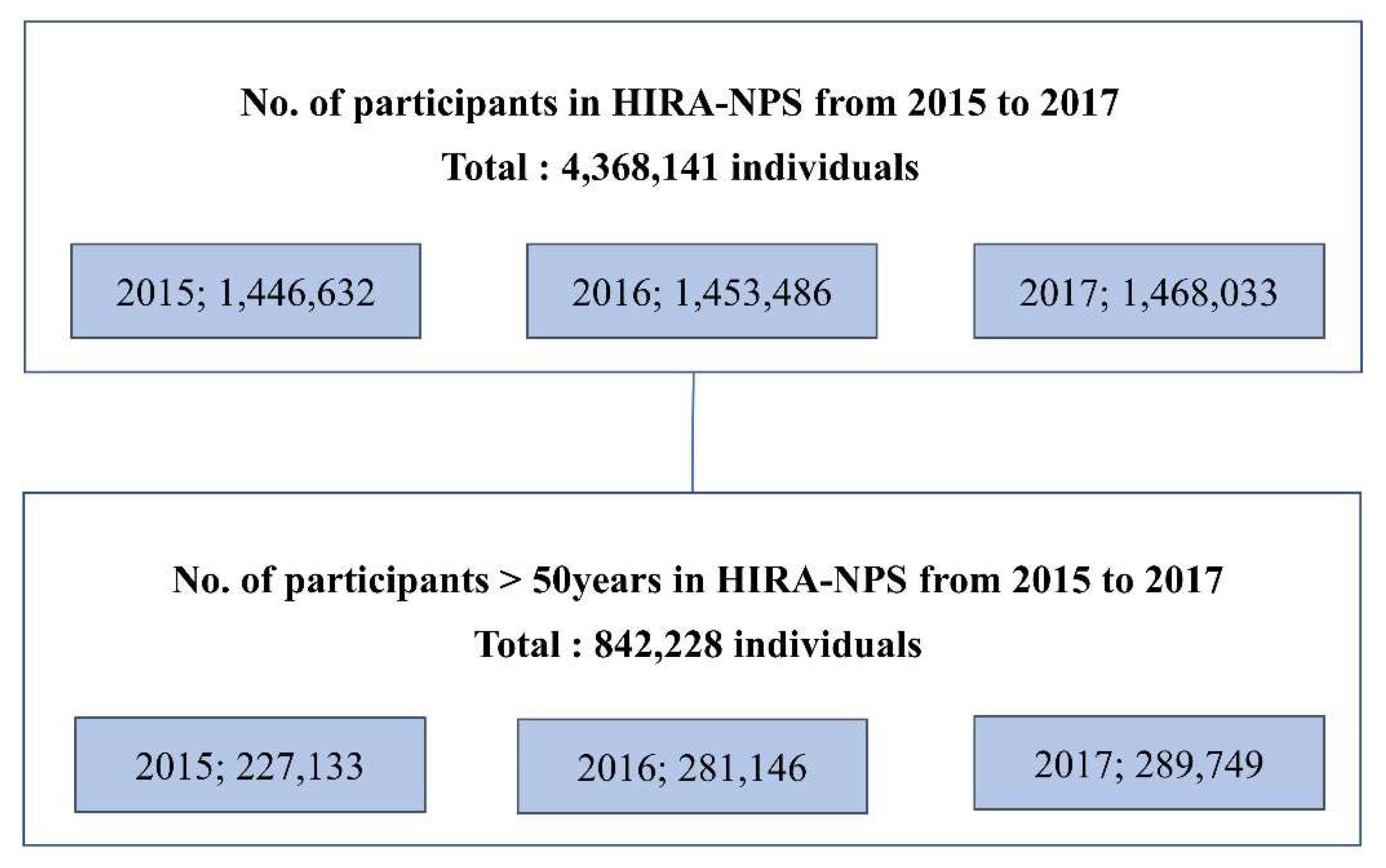
2.2. Study Design
2.3. Statistical Analysis
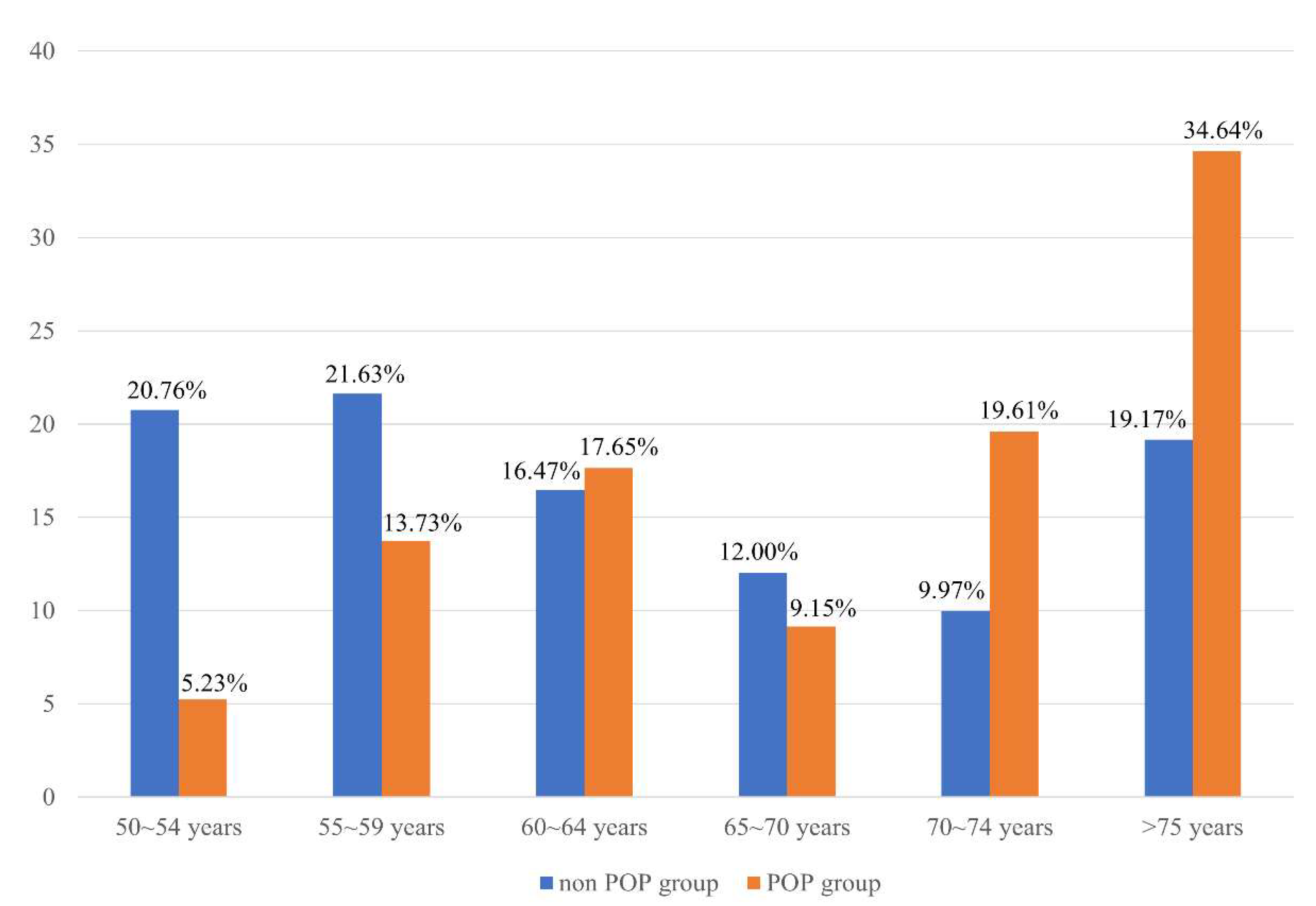
3. Results
Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barber, M.D. Pelvic organ prolapse. BMJ 2016, 354, i3853. [Google Scholar] [CrossRef]
- Glaser, D.L.; Kaplan, F.S. Osteoporosis: Definition and clinical presentation. Spine 1997, 22 (Suppl. 24), 12S–16S. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, S.L.; Clark, A.; Nygaard, I.; Aragaki, A.; Barnabei, V.; McTiernan, A. Pelvic organ prolapse in the Women’s Health Initiative: Gravity and gravidity. Am. J. Obstet. Gynecol. 2002, 186, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.-Y.; Burlet, N. Osteoporosis: A still increasing prevalence. Bone 2006, 38, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.T.; Kim, J.M. Pelvic Organ Support and Prevalence by Pelvic Organ Prolapse-Quantification (POP-Q) in Korean Women. J. Urol. 2006, 175, 1769–1772. [Google Scholar] [CrossRef]
- Korea Society for Bone and Mineral Research, National Health Insurance Service. Osteoporosis and Osteoporotic Fracture Fact Sheet 2019; Korea Society for Bone and Mineral Research, National Health Insurance Service: Seoul, Korea, 2019. [Google Scholar]
- Yoon, H.K.; Lee, Y.-K.; Ha, Y.-C. Characteristics of Patients Diagnosed with Osteoporosis in South Korea: Results from the National Claim Registry. J. Bone Metab. 2017, 24, 59–63. [Google Scholar] [CrossRef] [Green Version]
- World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/data/gho/publications/world-health-statistics (accessed on 20 December 2020).
- Kim, S.; Harvey, M.-A.; Johnston, S. A Review of the Epidemiology and Pathophysiology of Pelvic Floor Dysfunction: Do Racial Differences Matter? J. Obstet. Gynaecol. Can. 2005, 27, 251–259. [Google Scholar] [CrossRef]
- DeLancey, J.O. What’s new in the functional anatomy of pelvic organ prolapse? Curr. Opin. Obstet. Gynecol. 2016, 28, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Sferra, R.; Pompili, S.; D’Alfonso, A.; Sabetta, G.; Gaudio, E.; Carta, G.; Festuccia, C.; Colapietro, A.; Vetuschi, A. Neurovascular alterations of muscularis propria in the human anterior vaginal wall in pelvic organ prolapse. J. Anat. 2017, 235, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.R.; Avery, N.C.; Tarlton, J.F.; Eckford, S.D.; Abrams, P.; Bailey, A.J. Changes in metabolism of collagen in genitourinary prolapse. Lancet 1996, 347, 1658–1661. [Google Scholar] [CrossRef]
- Lee, S.W.; Cho, H.H.; Kim, M.-R.; You, Y.O.; Kim, S.Y.; Hwang, Y.B.; Kim, J.H. Association between pelvic organ prolapse and bone mineral density in postmenopausal women. J. Obstet. Gynaecol. 2015, 35, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.Y.; Glinter, H.; Marcus-Braun, N. Narrative review of the epidemiology, diagnosis and pathophysiology of pelvic organ prolapse. Int. Braz. J. Urol. 2020, 46, 5–14. [Google Scholar] [CrossRef]
- Appelman-Dijkstra, N.M.; Papapoulos, S.E. Modulating Bone Resorption and Bone Formation in Opposite Directions in the Treatment of Postmenopausal Osteoporosis. Drugs 2015, 75, 1049–1058. [Google Scholar] [CrossRef] [Green Version]
- Young, M.F. Bone matrix proteins: Their function, regulation, and relationship to osteoporosis. Osteoporos. Int. 2003, 14, 35–42. [Google Scholar] [CrossRef]
- Pal, L. Pelvic Organ Prolapse and Relationship with Skeletal Integrity. Womens Health 2009, 5, 325–333. [Google Scholar] [CrossRef]
- Pal, L.; Hailpern, S.M.; Santoro, N.F.; Freeman, R.; Barad, D.; Kipersztok, S.; Barnabei, V.M.; Wassertheil-Smoller, S. Association of pelvic organ prolapse and fractures in postmenopausal women: Analysis of baseline data from the Women’s Health Initiative Estrogen Plus Progestin trial. Menopause 2008, 15, 59–66. [Google Scholar] [CrossRef]
- Pal, L.; Hailpern, S.M.; Santoro, N.F.; Freeman, R.; Barad, D.; Kipersztok, S.; Barnabei, V.M.; Wassertheil-Smoller, S. Increased incident hip fractures in postmenopausal women with moderate to severe pelvic organ prolapse. Menopause 2011, 18, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Yoldemir, T.; Erenus, M. Should we consider assessment of bone mineral density earlier in postmenopausal women with pelvic organ prolapse? Climacteric 2011, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Kim, J.-A.; Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef]
- Yuk, J.S.; Lee, J.H.; Hur, J.Y.; Shin, J.H. The prevalence and treatment pattern of clinically diagnosed pelvic organ prolapse: A Korean National Health Insurance Database-based cross-sectional study 2009–2015. Sci. Rep. 2018, 8, 1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leblanc, D.; Schneider, M.; Angele, P.; Vollmer, G.; Docheva, D. The effect of estrogen on tendon and ligament metabolism and function. J. Steroid Biochem. Mol. Biol. 2017, 172, 106–116. [Google Scholar] [CrossRef]
- Gong, R.; Xia, Z. Collagen changes in pelvic support tissues in women with pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 185–189. [Google Scholar] [CrossRef]
- Martin, R.; Ishida, J. The relative effects of collagen fiber orientation, porosity, density, and mineralization on bone strength. J. Biomech. 1989, 22, 419–426. [Google Scholar] [CrossRef]
- Väänänen, H.K.; Härkönen, P.L. Estrogen and bone metabolism. Maturitas 1996, 23, S65–S69. [Google Scholar] [CrossRef]
- Meyer, I.; Morgan, S.L.; Markland, A.D.; Szychowski, J.M.; Richter, H.E. Pelvic floor disorder symptoms and bone strength in postmenopausal women. Int. Urogynecol. J. 2020, 31, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Bodner-Adler, B.; Bodner, K.; Stinglmeier, A.; Kimberger, O.; Halpern, K.; Koelbl, H.; Umek, W. Prolapse surgery versus vaginal pessary in women with symptomatic pelvic organ prolapse: Which factors influence the choice of treatment? Arch. Gynecol. Obstet. 2019, 299, 773–777. [Google Scholar] [CrossRef] [Green Version]
| N81 | Female genital prolapse |
| N81.1 | Female cystocele |
| N81.2 | Incomplete uterovaginal prolapse |
| N81.3 | Complete uterovaginal prolapse |
| N81.4 | Uterovaginal prolapse, unspecified |
| N81.6 | Female rectocele |
| N81.8 | Other female genital prolapse |
| N81.9 | Female genital prolapse, unspecified |
| N99.3 | Prolapse of the vaginal vault after a hysterectomy |
| R4113 | Insertion of a pessary |
| R3620 | Repair of cystocele |
| R0408 | Anterior colporrhaphy |
| R0410 | Posterior colporrhaphy |
| R0412 | Anterior and posterior colporrhaphy |
| R04202 | Vaginal hysterectomy |
| R04203 | Vaginal hysterectomy with anterior and posterior colporrhaphy |
| R04204 | Manchester surgery |
| Q3020 | Correction of rectocele |
| M80 | Osteoporosis with pathological fracture |
| M80.0 | Postmenopausal osteoporosis with pathological fracture |
| M80.00 | Postmenopausal osteoporosis with pathological fracture, multiple sites |
| M80.01 | Postmenopausal osteoporosis with pathological fracture, shoulder region |
| M80.02 | Postmenopausal osteoporosis with pathological fracture, upper arm |
| M80.03 | Postmenopausal osteoporosis with pathological fracture, forearm |
| M80.04 | Postmenopausal osteoporosis with pathological fracture, hand |
| M80.05 | Postmenopausal osteoporosis with pathological fracture, pelvic region and thigh |
| M80.06 | Postmenopausal osteoporosis with pathological fracture, lower leg |
| M80.07 | Postmenopausal osteoporosis with pathological fracture, ankle and foot |
| M80.08 | Postmenopausal osteoporosis with pathological fracture, other |
| M80.09 | Postmenopausal osteoporosis with pathological fracture, site unspecified |
| M80.1 | Postoophorectomy osteoporosis with pathological fracture |
| M80.10 | Postoophorectomy osteoporosis with pathological fracture, multiple sites |
| M80.11 | Postoophorectomy osteoporosis with pathological fracture, shoulder region |
| M80.12 | Postoophorectomy osteoporosis with pathological fracture, upper arm |
| M80.13 | Postoophorectomy osteoporosis with pathological fracture, forearm |
| M80.14 | Postoophorectomy osteoporosis with pathological fracture, hand |
| M80.15 | Postoophorectomy osteoporosis with pathological fracture, pelvic region and thigh |
| M80.16 | Postoophorectomy osteoporosis with pathological fracture, lower leg |
| M81.0 | Postmenopausal osteoporosis |
| M82.0 | Osteoporosis in diseases classified elsewhere |
| Year, 2015 | Without Osteoporosis | With Osteoporosis | Total |
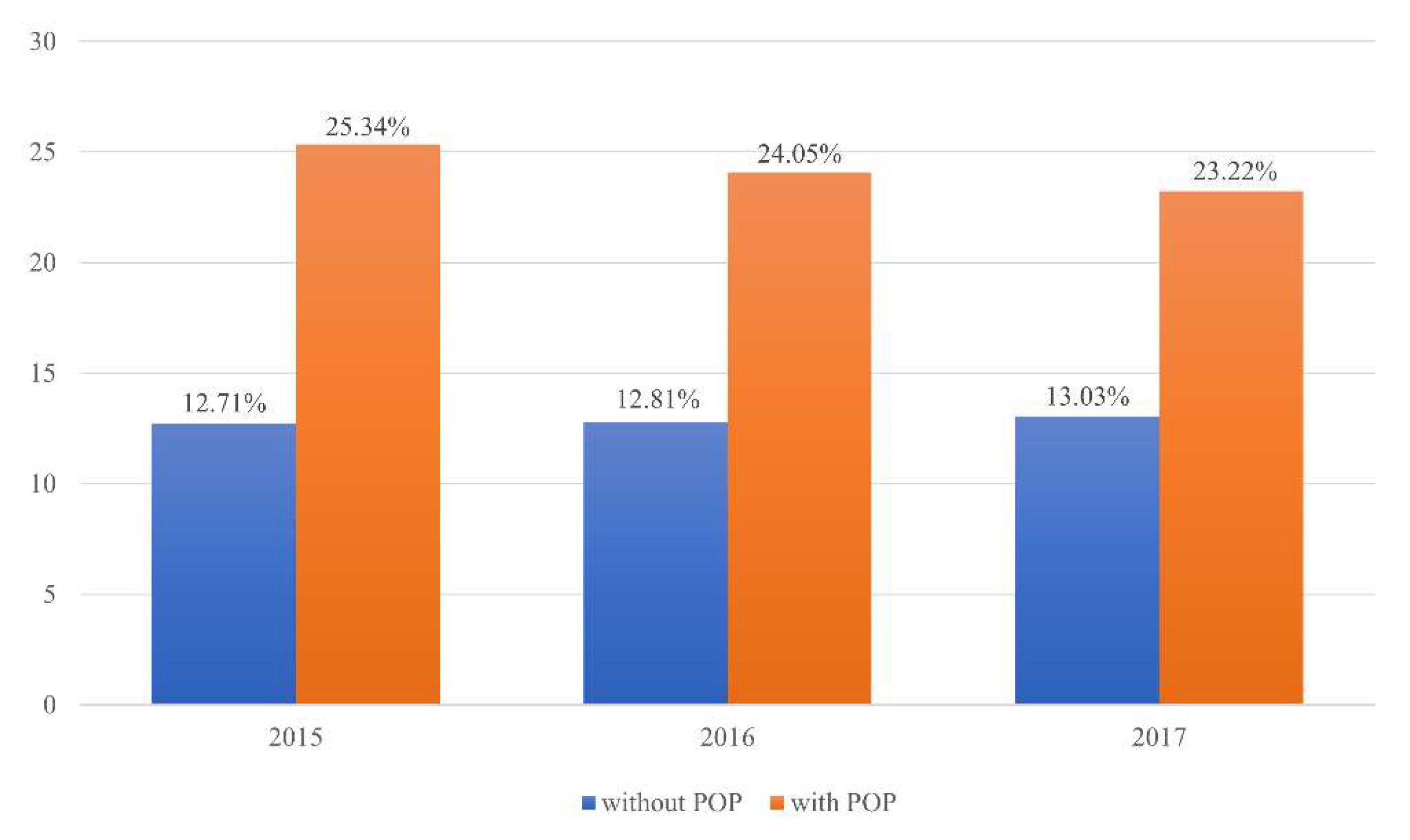
| Without POP, n (%) | 236,665 (87.29%) | 34,447 (12.71%) | 271,112 |
| With POP, n (%) | 165 (74.66%) | 56 (25.34%) | 221 |
| χ2 test < 0.0001 | 236,830 | 34,503 | 271,333 |
| Year, 2016 | Without Osteoporosis | With Osteoporosis | Total |
| Without POP, n (%) | 244,919 (87.19%) | 35,990 (12.81%) | 271,112 |
| With POP, n (%) | 180 (75.95%) | 57 (24.05%) | 221 |
| χ2 test < 0.0001 | 245,099 | 36,047 | 281,146 |
| Year, 2017 | Without Osteoporosis | With Osteoporosis | Total |
| Without POP, n (%) | 251,797 (86.97%) | 37,741 (13.03%) | 289,538 |
| With POP, n (%) | 162 (76.78%) | 49 (23.22%) | 221 |
| χ2 test < 0.0001 | 251,959 | 37,790 | 289,749 |
| Year, 2015 | Without Osteoporosis | With Osteoporosis | Total |
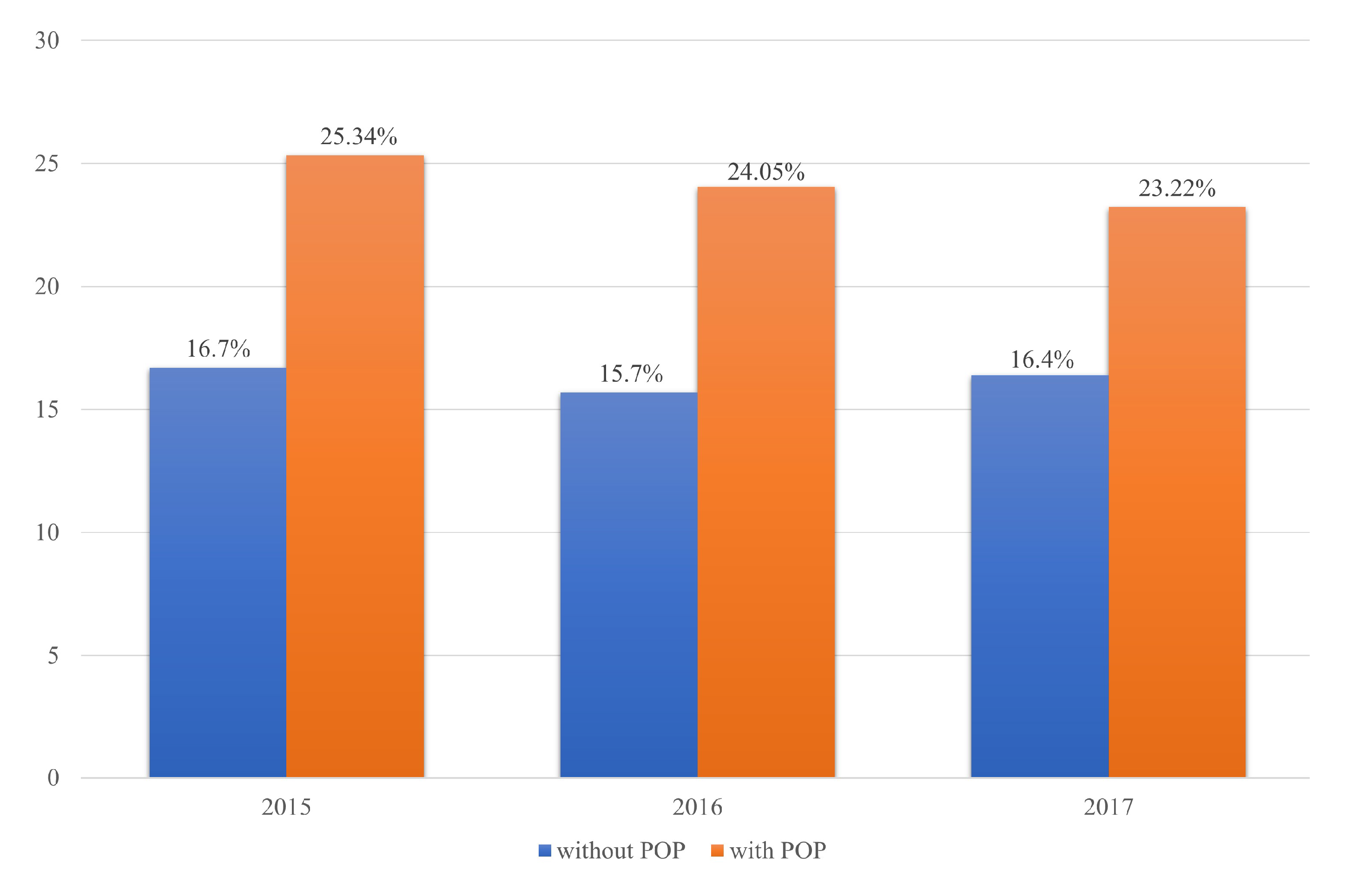
| Without POP, n (%) | 1841 (83.3%) | 369 (16.7%) | 2210 |
| With POP, n (%) | 165 (74.66%) | 56 (25.34%) | 221 |
| χ2 test = 0.0013 | 2006 | 425 | 2431 |
| Year, 2016 | Without Osteoporosis | With Osteoporosis | Total |
| Without POP, n (%) | 1998 (84.3%) | 372 (15.7%) | 2370 |
| With POP, n (%) | 180 (75.95%) | 57 (24.05%) | 237 |
| χ2 test = 0.0009 | 2178 | 429 | 2607 |
| Year, 2017 | Without Osteoporosis | With Osteoporosis | Total |
| Without POP, n (%) | 1764 (83.6%) | 346 (16.4%) | 2110 |
| With POP, n (%) | 162 (76.78%) | 49 (23.22%) | 221 |
| χ2 test = 0.0119 | 1926 | 395 | 2321 |
| Year, 2015–2017 | Without Osteoporosis | With Osteoporosis | Total |
|---|---|---|---|
| Without POP, n (%) | 733,381 (87.1%) | 108,178 (12.9%) | 841,559 |
| With POP, n (%) | 507 (75.8%) | 162 (24.2%) | 669 |
| χ2 test = 0.0119 | 733,888 | 108,340 | 842,228 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, Y.-R.; Lee, S.-R.; Kim, S.-H.; Chae, H.-D. Pelvic Organ Prolapse Is Associated with Osteoporosis in Korean Women: Analysis of the Health Insurance Review and Assessment Service National Patient Sample. J. Clin. Med. 2021, 10, 3751. https://doi.org/10.3390/jcm10163751
Ko Y-R, Lee S-R, Kim S-H, Chae H-D. Pelvic Organ Prolapse Is Associated with Osteoporosis in Korean Women: Analysis of the Health Insurance Review and Assessment Service National Patient Sample. Journal of Clinical Medicine. 2021; 10(16):3751. https://doi.org/10.3390/jcm10163751
Chicago/Turabian StyleKo, Yoo-Ra, Sa-Ra Lee, Sung-Hoon Kim, and Hee-Dong Chae. 2021. "Pelvic Organ Prolapse Is Associated with Osteoporosis in Korean Women: Analysis of the Health Insurance Review and Assessment Service National Patient Sample" Journal of Clinical Medicine 10, no. 16: 3751. https://doi.org/10.3390/jcm10163751
APA StyleKo, Y.-R., Lee, S.-R., Kim, S.-H., & Chae, H.-D. (2021). Pelvic Organ Prolapse Is Associated with Osteoporosis in Korean Women: Analysis of the Health Insurance Review and Assessment Service National Patient Sample. Journal of Clinical Medicine, 10(16), 3751. https://doi.org/10.3390/jcm10163751






