Comparison of COVID-19 and Non-COVID-19 Pneumonia in Down Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
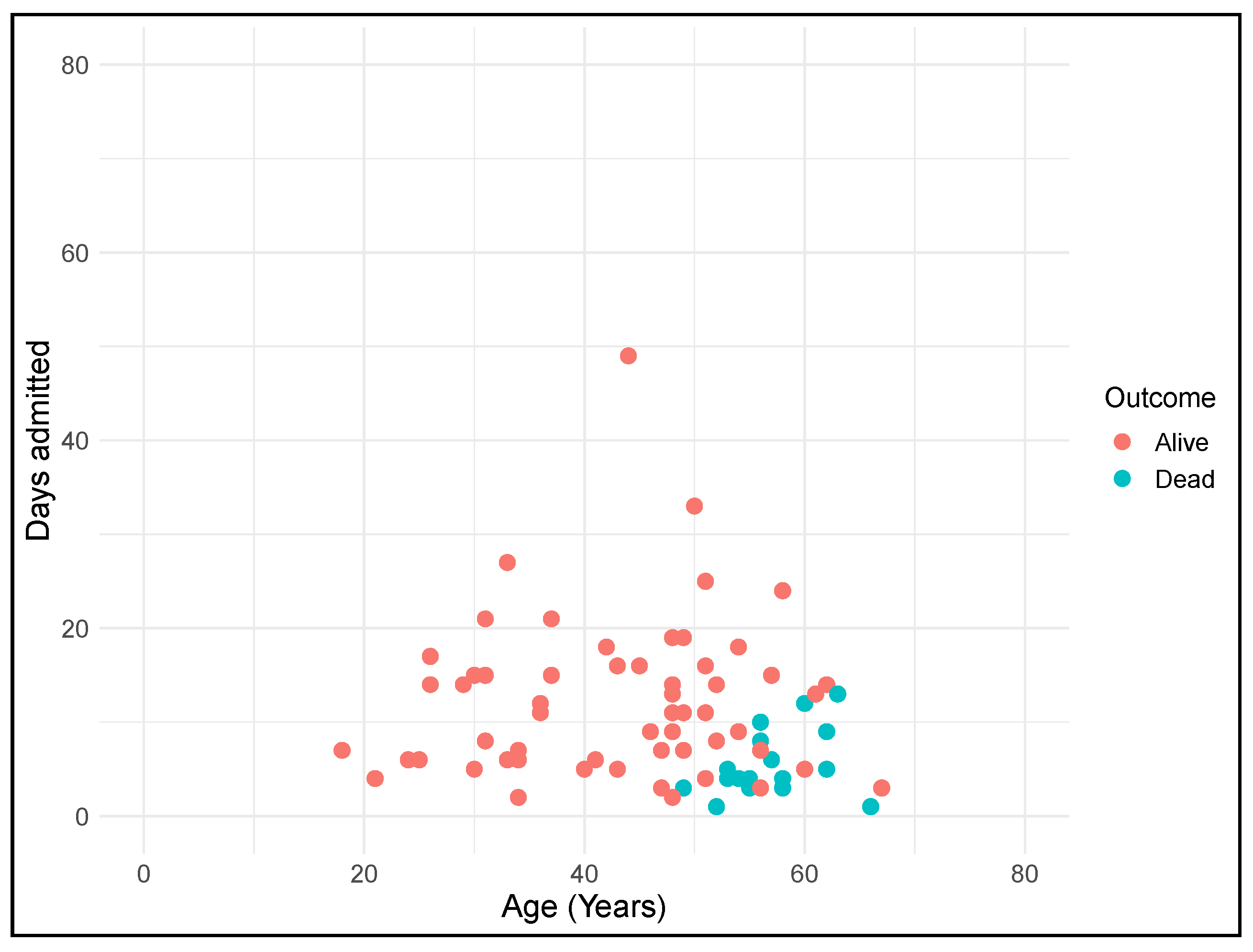
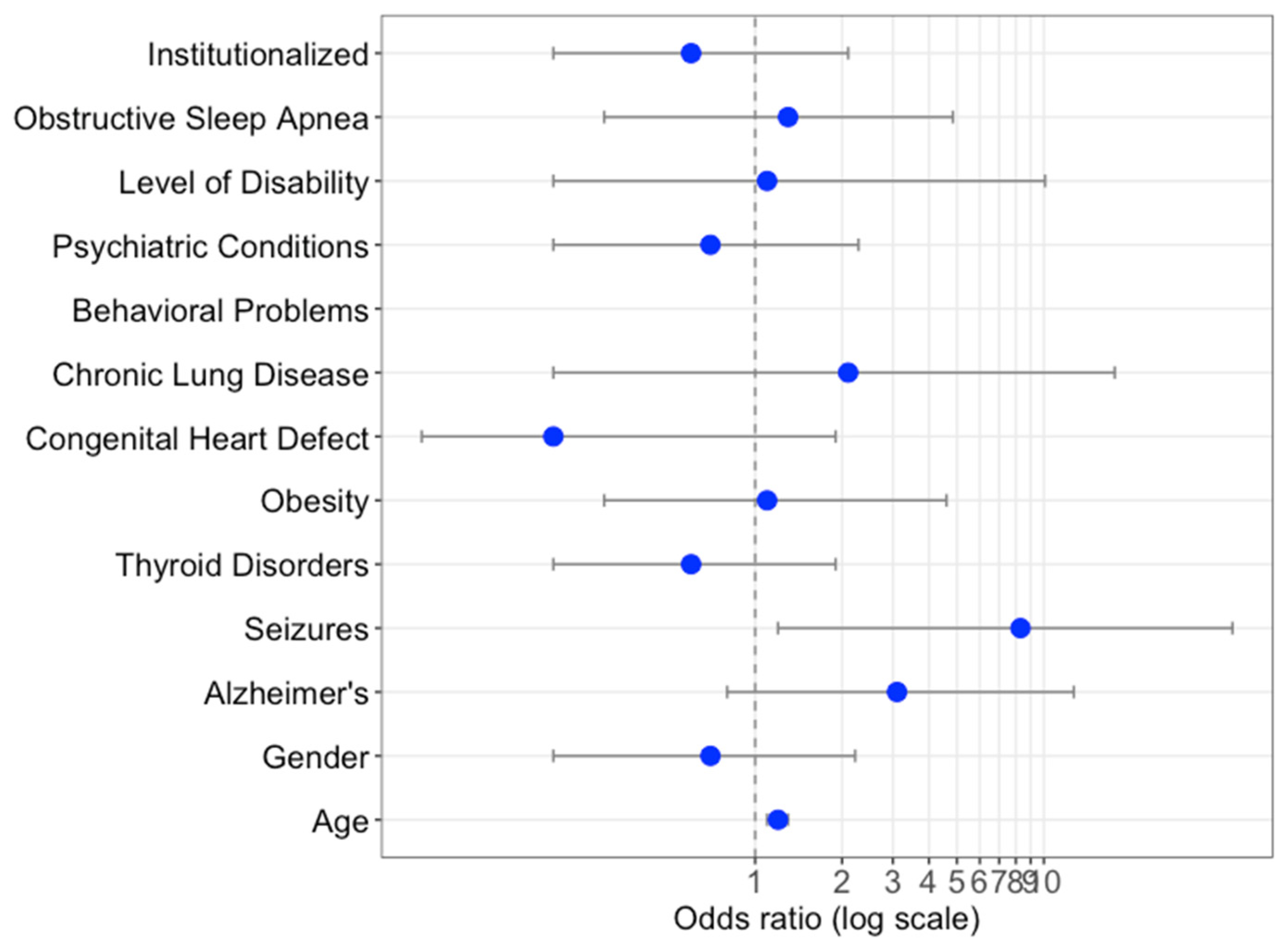
3.1. Socio-Demographic and Clinical Characteristics of the Spanish DS COVID-19 Patients
3.2. Comparison of COVID-19 and Other Pneumonia-Related Hospitalizations
3.3. Mortality Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wadman, M. People with Down syndrome face high risk from coronavirus. Science 2020, 370, 1384–1385. [Google Scholar] [CrossRef]
- Clift, M.A.K.; Coupland, C.A.; Keogh, R.H.; Hemingway, H.; Hippisley-Cox, J. COVID-19 Mortality Risk in Down Syndrome: Results From a Cohort Study of 8 Million Adults. Ann. Intern. Med. 2021, 174, 572–576. [Google Scholar] [CrossRef]
- De Cauwer, H.; Spaepen, A. Are patients with Down syndrome vulnerable to life-threatening COVID-19? Acta Neurol. Belg. 2021, 121, 685–687. [Google Scholar] [CrossRef]
- Perera, B.; Laugharne, R.; Henley, W.; Zabel, A.; Lamb, K.; Branford, D.; Courtanay, K.; Alexander, R.; Purandare, K.; Wijeratne, A.; et al. COVID-19 deaths in people with intellectual disability in the UK and Ireland: Descriptive study. BJPsych Open 2020, 6, E123. [Google Scholar] [CrossRef]
- De Toma, I.; Dierssen, M. Network analysis of Down syndrome and SARS-CoV-2 identifies risk and protective factors for COVID-19. Sci. Rep. 2021, 11, 1930. [Google Scholar] [CrossRef]
- De Cauwer, H. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: Implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies: Comment. Intern. Emerg. Med. 2020, 15, 1581–1582. [Google Scholar] [CrossRef]
- Villani, E.R.; Carfì, A.; Di Paola, A.; Palmieri, L.; Donfrancesco, C.; Noce, C.L.; Taruscio, D.; Meli, P.; Salerno, P.; Kodra, Y.; et al. Clinical characteristics of individuals with Down syndrome deceased with CoVID-19 in Italy—A case series. Am. J. Med Genet. Part A 2020, 182, 2964–2970. [Google Scholar] [CrossRef]
- Hüls, A.; Costa, A.C.; Dierssen, M.; Baksh, R.A.; Bargagna, S.; Baumer, N.T.; Brandão, A.C.; Carfi, A.; Carmona-Iragui, M.; Chicoine, B.A.; et al. Medical vulnerability of individuals with Down syndrome to severe COVID-19–data from the Trisomy 21 Research Society and the UK ISARIC4C survey. EClinicalMedicine 2021, 33, 100769. [Google Scholar] [CrossRef]
- Santoro, S.L.; Chicoine, B.; Jasien, J.M.; Kim, J.L.; Stephens, M.; Bulova, P.; Capone, G. Pneumonia and respiratory infections in Down syndrome: A scoping review of the literature. Am. J. Med. Genet. Part A 2021, 185, 286–299. [Google Scholar] [CrossRef]
- Pérez-Padilla, R.; Fernández, R.; García-Sancho, C.; Franco-Marina, F.; Aburto, O.; Lopez-Gatell, H.; Bojorquez, I. Pandemic (H1N1) 2009 Virus and Down Syndrome Patients. Emerg. Infect. Dis. 2010, 16, 1312–1314. [Google Scholar] [CrossRef]
- Bloemers, B.L.; Van Furth, A.M.; Weijerman, M.E.; Gemke, R.J.; Broers, C.J.; Ende, K.V.D.; Kimpen, J.L.; Strengers, J.L.; Bont, L.J. Down Syndrome: A Novel Risk Factor for Respiratory Syncytial Virus Bronchiolitis A Prospective Birth-Cohort Study. Pediatrice 2007, 120, e1076–e1081. [Google Scholar] [CrossRef] [PubMed]
- Löwensteyn, Y.N.; Phijffer, E.W.E.M.; Simons, J.V.L.; Scheltema, N.M.; Mazur, N.I.; Nair, H.; Bont, L.J.; on behalf of the RSV GOLD Study Group. Respiratory Syncytial Virus-related Death in Children with Down Syndrome. Pediatr. Infect. Dis. J. 2020, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Huls, A.; Costa, A.C.S.; Dierssen, M. An international survey on the impact of COVID-19 in individuals with Down syndrome. medRxiv 2020. [Google Scholar] [CrossRef]
- Connolly, A.; Gaehl, E.; Martin, H.; Morris, J.; Purandare, N. Underdiagnosis of dementia in primary care: Variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment. Health 2011, 15, 978–984. [Google Scholar] [CrossRef]
- Walker, I.F.; Lord, P.; Farragher, T.M. Variations in dementia diagnosis in England and association with general practice characteristics. Prim. Health Care Res. Dev. 2017, 18, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Malle, L.; Gao, C.; Hur, C.; Truong, H.Q.; Bouvier, N.M.; Percha, B.; Kong, X.-F.; Bogunovic, D. Individuals with Down syndrome hospitalized with COVID-19 have more severe disease. Genet. Med. 2021, 23, 576–580. [Google Scholar] [CrossRef]
- Aparicio, P.; Barba, R.; Moldenhauer, F.; Suárez, C.; De Asúa, D.R. Características de los adultos con síndrome de Down ingresados en los servicios de medicina interna españoles en el periodo 2005–2014. Rev. Clin. Esp. 2019, 220. [Google Scholar] [CrossRef]
- Beckhaus, A.A.; Castro-Rodriguez, J.A. Down Syndrome and the Risk of Severe RSV Infection: A Meta-analysis. Pediatrics 2018, 142, e20180225. [Google Scholar] [CrossRef] [Green Version]
- Piroth, L.; Cottenet, J.; Mariet, A.-S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2020, 17, 251–259. [Google Scholar] [CrossRef]
- Van Asten, L.; Harmsen, C.N.; Stoeldraijer, L.; Klinkenberg, D.; Teirlinck, A.C.; de Lange, M.M.; Meijer, A.; van de Kassteele, J.; van Gageldonk-Lafeber, A.B.; Hof, S.V.D.; et al. Excess Deaths during Influenza and Coronavirus Disease and Infection-Fatality Rate for Severe Acute Respiratory Syndrome Coronavirus 2, the Netherlands. Emerg. Infect. Dis. 2021, 27, 411–420. [Google Scholar] [CrossRef]
- Petersen, E. COVID-19 is not influenza. Lancet Respir. Med. 2021, 9, 219–220. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Maddukuri, G.; Al-Aly, Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: Cohort study. BMJ 2020, 371, m4677. [Google Scholar] [CrossRef]
- Illouz, T.; Biragyn, A.; Iulita, M.F.; Flores-Aguilar, L.; Dierssen, M.; De Toma, I.; Antonarakis, S.E.; Yu, E.; Herault, Y.; Potier, M.-C.; et al. Immune Dysregulation and the Increased Risk of Complications and Mortality Following Respiratory Tract Infections in Adults With Down Syndrome. Front. Immunol. 2021, 12, 2375. [Google Scholar] [CrossRef]
- Espinosa, J.M. Down Syndrome and COVID-19: A Perfect Storm? Cell Rep. Med. 2020, 1, 100019. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, C.G.; Chamorro-De-Vega, E.; Valerio, M.; Amor-Garcia, M.A.; Tejerina, F.; Sancho-Gonzalez, M.; Narrillos-Moraza, A.; Gimenez-Manzorro, A.; Manrique-Rodriguez, S.; Machado, M.; et al. COVID-19 in hospitalised patients in Spain: A cohort study in Madrid. Int. J. Antimicrob. Agents 2021, 57, 106249. [Google Scholar] [CrossRef]
- Borobia, A.; Carcas, A.; Arnalich, F.; Álvarez-Sala, R.; Monserrat-Villatoro, J.; Quintana, M.; Figueira, J.; Santos-Olmo, R.T.; García-Rodríguez, J.; Martín-Vega, A.; et al. A Cohort of Patients with COVID-19 in a Major Teaching Hospital in Europe. J. Clin. Med. 2020, 9, 1733. [Google Scholar] [CrossRef]
- Rosenthal, N.; Cao, Z.; Gundrum, J.; Sianis, J.; Safo, S. Risk Factors Associated With In-Hospital Mortality in a US National Sample of Patients With COVID-19. JAMA Netw. Open 2020, 3, e2029058. [Google Scholar] [CrossRef]
| COVID-19 Pneumonia | Non-COVID-19 Pneumonia | p | |
|---|---|---|---|
| n = 86 | n = 2832 | ||
| Age (years) | 46.4 (11.8) | 42.2 (13.9) | <0.01 * |
| (mean (SD)) | |||
| 16–40 years | 0.36 ** | ||
| Women | 24 (27.9%) | 1251 (44.2%) | |
| Men | 11 (45.8%) | 434 (34.7%) | |
| (n (%)) | 13 (56.2%) | 817 (65.3%) | |
| >40 years | 0.21 ** | ||
| Women | 61 (72.1%) | 1581 (55.8%) | |
| Men | 31 (50.8%) | 662 (41.9%) | |
| (n (%)) | 30 (49.2%) | 919 (58.1%) | |
| Length of stay | 10.3 (7.9) | 9.9 (9.4) | 0.79 ** |
| (mean (SD)) | |||
| Length of stay by discharge status (Days) (mean (SD)) | |||
| Alive | 12.1 (8.2) | 9.8 (8.6) | <0.001 * |
| Dead | 5.1 (3.3) | 11.9 (14.5) | 0.034 * |
| DS and COVID-19 n = 86 n (%) | Non-COVID Pneumoniae n = 2832 n (%) | χ2 (p) | |
|---|---|---|---|
| Obesity | 22 (25.5) | 246 (8.7) | <0.001 |
| Dementia | 22 (25.5) | 152 (5.4) | <0.001 |
| Epilepsy | 9 (10.4) | 389 (13.7) | 0.7 |
| COVID-19 Pneumonia n = 86 | Non-COVID Pneumoniae n = 2811 | p | |
|---|---|---|---|
| Overall in-hospital mortality rate (n (%)) | 23 (26.7) | 273 (9.7) | <0.001 * |
| Age of deceased patients (mean (SD)) | 55.5 (5) | 49.1 (11.7) | <0.001 ** |
| Proportion of deceased patients over 40 years (n (%)) | 23 (37) | 214 (13.7) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Real de Asua, D.; Mayer, M.A.; Ortega, M.d.C.; Borrel, J.M.; Bermejo, T.d.J.; González-Lamuño, D.; Manso, C.; Moldenhauer, F.; Carmona-Iragui, M.; Hüls, A.; et al. Comparison of COVID-19 and Non-COVID-19 Pneumonia in Down Syndrome. J. Clin. Med. 2021, 10, 3748. https://doi.org/10.3390/jcm10163748
Real de Asua D, Mayer MA, Ortega MdC, Borrel JM, Bermejo TdJ, González-Lamuño D, Manso C, Moldenhauer F, Carmona-Iragui M, Hüls A, et al. Comparison of COVID-19 and Non-COVID-19 Pneumonia in Down Syndrome. Journal of Clinical Medicine. 2021; 10(16):3748. https://doi.org/10.3390/jcm10163748
Chicago/Turabian StyleReal de Asua, Diego, Miguel A. Mayer, María del Carmen Ortega, Jose M. Borrel, Teresa de Jesús Bermejo, Domingo González-Lamuño, Coral Manso, Fernando Moldenhauer, María Carmona-Iragui, Anke Hüls, and et al. 2021. "Comparison of COVID-19 and Non-COVID-19 Pneumonia in Down Syndrome" Journal of Clinical Medicine 10, no. 16: 3748. https://doi.org/10.3390/jcm10163748
APA StyleReal de Asua, D., Mayer, M. A., Ortega, M. d. C., Borrel, J. M., Bermejo, T. d. J., González-Lamuño, D., Manso, C., Moldenhauer, F., Carmona-Iragui, M., Hüls, A., Sherman, S. L., Strydom, A., de la Torre, R., & Dierssen, M., on behalf of the Spanish Trisomy 21 Research Society COVID-19 Taskforce. (2021). Comparison of COVID-19 and Non-COVID-19 Pneumonia in Down Syndrome. Journal of Clinical Medicine, 10(16), 3748. https://doi.org/10.3390/jcm10163748







