Effect of Systemic Steroid Use for Immune-Related Adverse Events in Patients with Non-Small Cell Lung Cancer Receiving PD-1 Blockade Drugs
Abstract
:1. Introduction
2. Methods
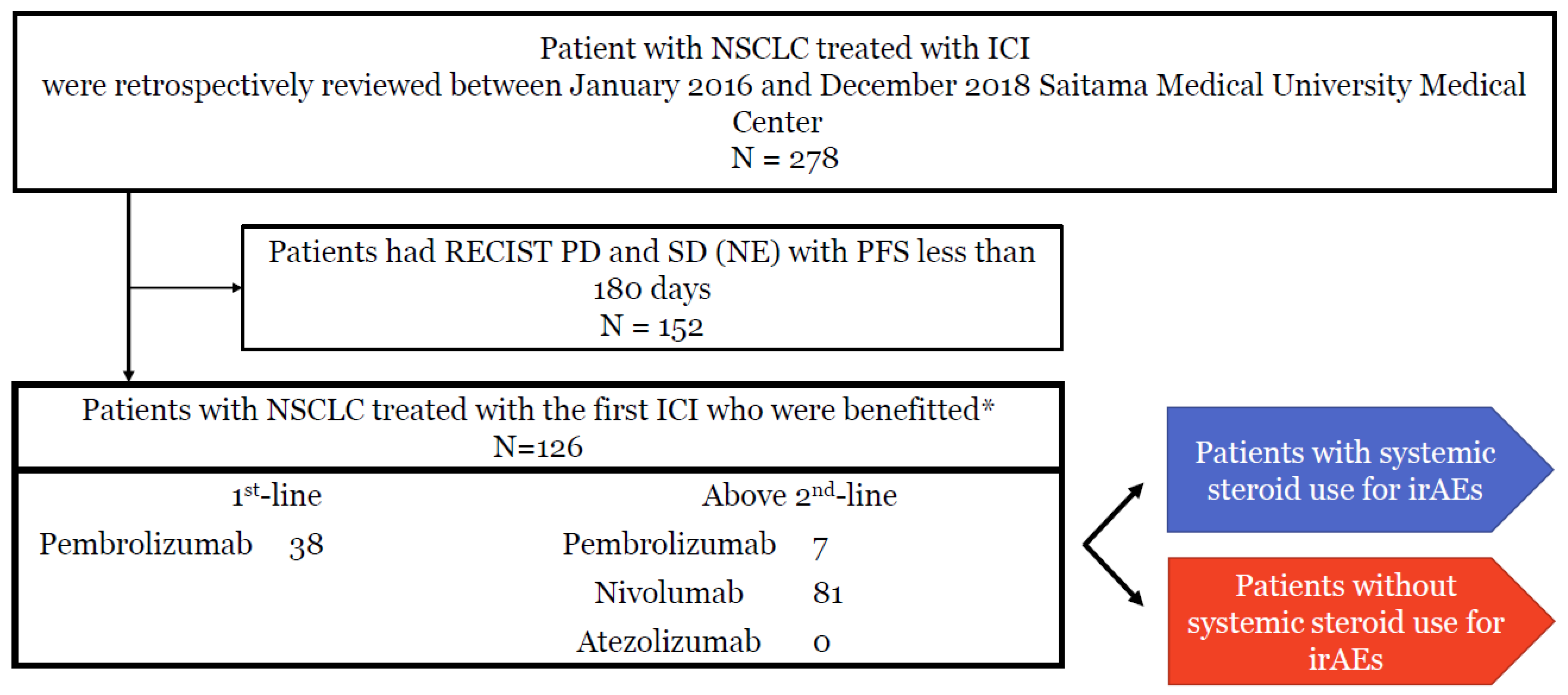
2.1. Study Design and Subjects
2.2. Treatment and Adverse Events
2.3. Assessment for Clinical Data
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
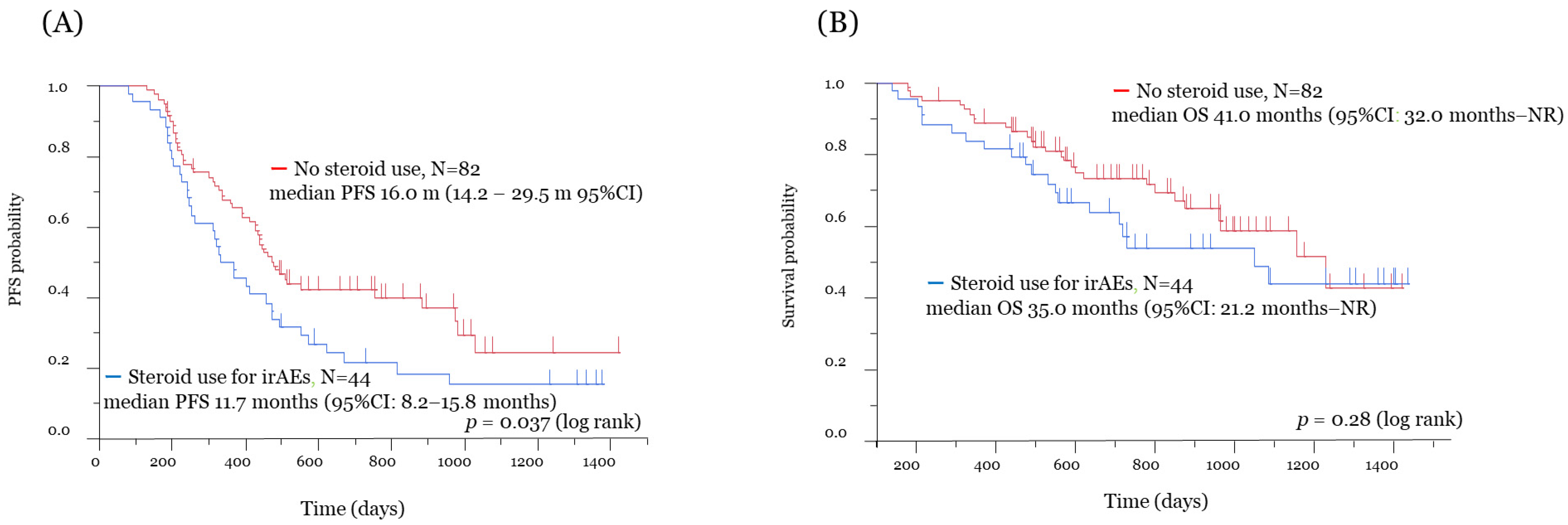
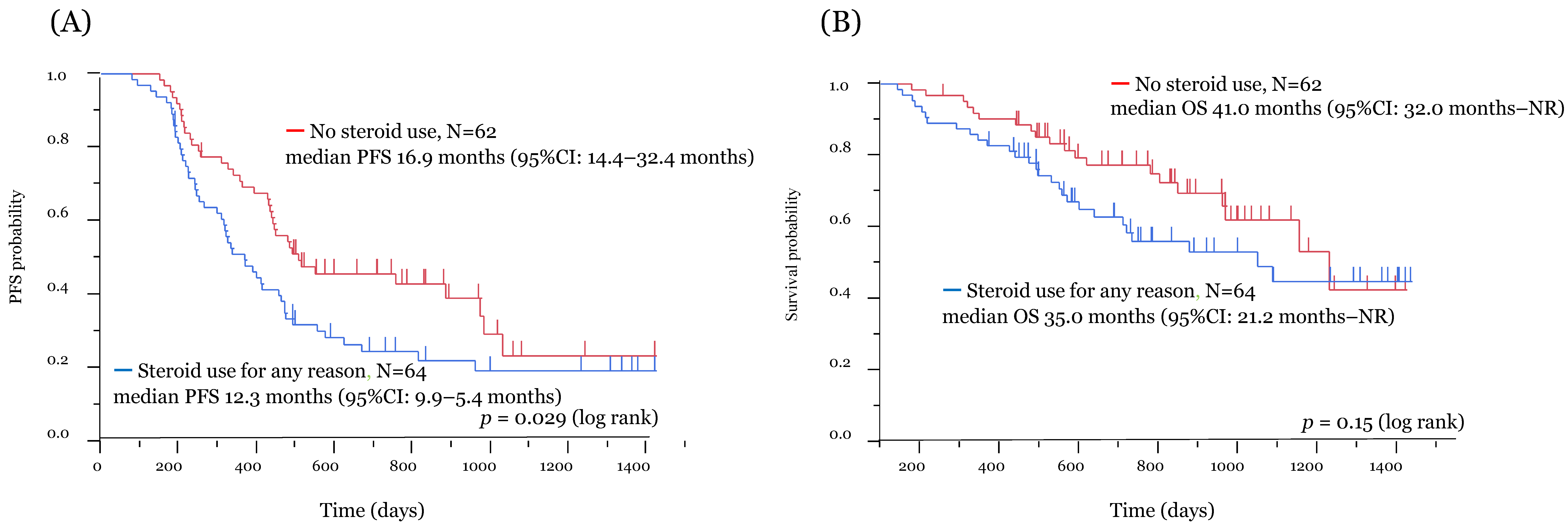
3.2. Efficacy and Survival Analysis
3.3. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events with Nivolumab Efficacy in Non–Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Caro, R.B.; Zurawski, B.; Kim, S.-W.; Costa, E.C.; Park, K.; Alexandru, A.; Lupinacci, L.; Jimenez, E.D.L.M.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Larkin, J.; Sileni, V.C.; Gonzalez, R.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Frontera, O.A.; Hammers, H.J.; Carducci, M.A.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1370–1385. [Google Scholar] [CrossRef]
- Meduri, G.U.; Annane, D.; Confalonieri, M.; Chrousos, G.P.; Rochwerg, B.; Busby, A.; Ruaro, B.; Meibohm, B. Pharmacological principles guiding prolonged glucocorticoid treatment in ARDS. Intensiv. Care Med. 2020, 46, 2284–2296. [Google Scholar] [CrossRef] [PubMed]
- Parrillo, J.E.; Fauci, A.S. Mechanisms of Glucocorticoid Action on Immune Processes. Annu. Rev. Pharmacol. Toxicol. 1979, 19, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients with Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Fucà, G.; Galli, G.; Poggi, M.; Russo, G.L.; Proto, C.; Imbimbo, M.; Ferrara, R.; Zilembo, N.; Ganzinelli, M.; Sica, A.; et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 2019, 4, e000457. [Google Scholar] [CrossRef] [Green Version]
- Shukuya, T.; Mori, K.; Amann, J.M.; Bertino, E.M.; Otterson, G.A.; Shields, P.G.; Morita, S.; Carbone, D.P. Relationship between Overall Survival and Response or Progression-Free Survival in Advanced Non–Small Cell Lung Cancer Patients Treated with Anti–PD-1/PD-L1 Antibodies. J. Thorac. Oncol. 2016, 11, 1927–1939. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Ricciuti, B.; Dahlberg, S.E.; Adeni, A.; Sholl, L.M.; Nishino, M.; Awad, M.M. Immune Checkpoint Inhibitor Outcomes for Patients with Non–Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J. Clin. Oncol. 2019, 37, 1927–1934. [Google Scholar] [CrossRef]
- Scott, S.C.; Pennell, N.A. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 1771–1775. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, Y.; Tamiya, A.; Isa, S.-I.; Nakahama, K.; Okishio, K.; Shiroyama, T.; Suzuki, H.; Inoue, T.; Tamiya, M.; Hirashima, T.; et al. Predictive Factors for Poor Progression-free Survival in Patients with Non-small Cell Lung Cancer Treated with Nivolumab. Anticancer Res. 2017, 37, 5857–5862. [Google Scholar] [CrossRef] [PubMed]
- Dumenil, C.; Massiani, M.-A.; Dumoulin, J.; Giraud, V.; Labrune, S.; Chinet, T.; Leprieur, E.G. Clinical factors associated with early progression and grade 3–4 toxicity in patients with advanced non-small-cell lung cancers treated with nivolumab. PLoS ONE 2018, 13, e0195945. [Google Scholar] [CrossRef] [Green Version]
- Drakaki, A.; Dhillon, P.K.; Wakelee, H.; Chui, S.Y.; Shim, J.; Kent, M.; Degaonkar, V.; Hoang, T.; McNally, V.; Luhn, P.; et al. Association of baseline systemic corticosteroid use with overall survival and time to next treatment in patients receiving immune checkpoint inhibitor therapy in real-world US oncology practice for advanced non-small cell lung cancer, melanoma, or urothelial carcinoma. OncoImmunology 2020, 9, 1824645. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, A.; Sugiyama, D.; Maeda, Y.; Warner, A.B.; Panageas, K.S.; Ito, S.; Togashi, Y.; Sakai, C.; Wolchok, J.D.; Nishikawa, H. Selective inhibition of low-affinity memory CD8+ T cells by corticosteroids. J. Exp. Med. 2019, 216, 2701–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, N.; Madi, A.; Zhang, H.; Klapholz, M.; Escobar, G.; Dulberg, S.; Christian, E.; Ferreira, M.; Dixon, K.O.; Fell, G.; et al. Endogenous Glucocorticoid Signaling Regulates CD8 + T Cell Differentiation and Development of Dysfunction in the Tumor Microenvironment. Immunity 2020, 53, 658–671. [Google Scholar] [CrossRef]
- Okoye, I.S.; Xu, L.; Walker, J.; Elahi, S. The glucocorticoids prednisone and dexamethasone differentially modulate T cell function in response to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade. Cancer Immunol. Immunother. 2020, 69, 1423–1436. [Google Scholar] [CrossRef]
- Haywood, A.; Duc, J.; Good, P.; Khan, S.; Rickett, K.; Vayne-Bossert, P.; Hardy, J.R. Systemic corticosteroids for the management of cancer-related breathlessness (dyspnoea) in adults. Cochrane Database Syst. Rev. 2019, 2, CD012704. [Google Scholar] [CrossRef] [Green Version]
- Grennan, D.; Wang, S. Steroid Side Effects. JAMA 2019, 322, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of Steroids Use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | Systemic Steroid Use for irAEs | No Steroid Use | p-Value |
|---|---|---|---|
| n = 44 | n = 82 | ||
| Age | |||
| Median (range) | 70 (43–86) | 70 (48–85) | |
| Gender | |||
| Male/Female | 38/6 | 69/13 | 0.80 |
| PS | |||
| 0 or 1/2 or 3 | 35/9 | 73/9 | 0.38 |
| Smoking history | |||
| Yes/No | 41/3 | 72/10 | 0.38 |
| Disease stage | |||
| III/IV/Postoperative recurrence | 8/28/8 | 15/43/24 | 0.40 |
| Histology | |||
| Ad/Sq/Others | 18/14/12 | 43/24/15 | 0.58 |
| Driver mutation/Translocation | |||
| EGFR/ALK/Wild type | 1/0/43 | 5/0/77 | 0.65 |
| PD-L1 (TPS) | |||
| <1%/1–49%/>50%/Unknown | 1/1/18/24 | 3/6/22/51 | 0.34 |
| Treatment line | |||
| 1st/2nd/3rd and higher | 17/20/7 | 21/46/15 | 0.33 |
| Efficacy by ICI treatment | |||
| CR and PR/SD/NE | 28/13/3 | 46/31/5 | 0.68 |
| Use of systemic steroid except for irAEs * | |||
| Yes/No | 5/39 | 15/67 | 0.11 |
| Bain metastasis | |||
| Yes/No | 11/33 | 20/62 | 0.99 |
| Re-administration ICI after irAEs | |||
| Yes/No | 27/17 | 46/36 | 0.58 |
| Antibiotics use until PD or within observation period | 27/17 | 28/54 | 0.004 |
| Yes/No |
| Factors | PFS | OS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age <70/≧70 | 0.75 | 0.48–1.14 | 0.18 | 0.72 | 0.47–1.11 | 0.14 | 0.48 | 0.26–0.88 | 0.02 | 0.46 | 0.25–0.83 | 0.01 |
| Gender: Male/Female | 0.75 | 0.33–1.36 | 0.33 | 1.24 | 0.57–3.28 | 0.60 | ||||||
| PS 0–1/2–3 | 0.65 | 0.38–1.20 | 0.16 | 0.61 | 0.35–1.13 | 0.11 | 0.48 | 0.25–1.02 | 0.06 | |||
| Smoking status: Never/Current or former | 0.99 | 0.46–1.89 | 0.98 | 0.83 | 0.25–2.04 | 0.71 | ||||||
| Disease stage: III or IV/Postoperative recurrence | 1.25 | 0.78–2.11 | 0.35 | 1.53 | 0.79–3.24 | 0.22 | ||||||
| Histology: Ad/Sq or Others | 0.82 | 0.53–1.24 | 0.34 | 0.66 | 0.36–1.17 | 0.16 | ||||||
| Driver mutation: EGFR/Wild | 2.30 | 0.80–5.19 | 0.11 | 2.78 | 0.96–6.38 | 0.06 | 1.14 | 0.28–3.12 | 0.83 | |||
| Treatment line: 1st/2nd and higher | 0.88 | 0.53–1.40 | 0.59 | 1.11 | 055–2.10 | 0.77 | ||||||
| Efficacy by ICI: CR and PR/SD or NE | 1.24 | 0.81–1.90 | 0.32 | 1.03 | 0.58–1.85 | 0.93 | ||||||
| Antibiotics: Yes/No | 0.99 | 0.65–1.52 | 0.98 | 1.43 | 0.75–2.96 | 0.29 | ||||||
| irAEs Grade≧3: Yes/No | 1.45 | 0.91–2.27 | 0.12 | 1.86 | 1.01–3.32 | 0.04 | 1.94 | 0.99–3.73 | 0.05 | |||
| Systemic steroid use to irAEs: Yes/No | 1.56 | 1.01–2.38 | 0.04 | 1.67 | 1.07–2.57 | 0.02 | 1.38 | 0.76–2.44 | 0.28 | 1.06 | 0.54–2.01 | 0.86 |
| Toxicities | All Grade | Grade ≥ 3 | ||||
|---|---|---|---|---|---|---|
| Steroid Use for irAEs n (%) | No Steroid Use n (%) | p-Value | Steroid Use for irAEs n (%) | No Steroid Use n (%) | p-Value | |
| Any irAE *1*4 | 44 (100) | 70 (85.4) | <0.01 | 28 (63.6) | 7 (8.5) | 0.99 |
| ILD | 21 (47.7) | 15 (18.3) | <0.01 | 7 (15.9) | 1 (1.2) | <0.01 |
| Thyroid dysfunction | 8 (18.2) | 7 (8.5) | 0.14 | 0 (0) | 0 (0) | 0.99 |
| Adrenal insufficiency | 9 (20.5) | 1 (1.2) | <0.01 | 3 (6.8) | 1 (1.2) | 0.12 |
| Liver dysfunction *2 | 13 (29.5) | 14 (17.1) | 0.11 | 5 (11.4) | 2 (2.4) | 0.05 |
| Renal dysfunction *3 | 5 (11.4) | 16 (19.5) | 0.32 | 1 (2.3) | 0 (0) | 0.35 |
| Rash | 15 (34.1) | 24 (29.3) | 0.69 | 3 (6.8) | 0 (0) | 0.04 |
| Fever | 6 (13.6) | 2 (2.4) | 0.02 | 1 (2.3) | 0 (0) | 0.35 |
| Diarrhea | 8 (18.2) | 8 (9.8) | 0.26 | 5 (11.4) | 0 (0) | <0.01 |
| Nervous disorder | 2 (4.5) | 2 (2.4) | 0.61 | 1 (2.3) | 0 (0) | 0.35 |
| Mucositis oral | 2 (4.5) | 3 (3.7) | 0.99 | 0 (0) | 1 (1.2) | 0.99 |
| CK | 2 (4.5) | 10 (12.2) | 0.21 | 0 (0) | 0 (0) | 0.99 |
| AMY | 6 (13.6) | 7 (8.5) | 0.54 | 0 (0) | 0 (0) | 0.99 |
| γGTP | 1 (2.3) | 4 (4.9) | 0.66 | 0 (0) | 0 (0) | 0.99 |
| Eosinophilia | 10 (22.7) | 19 (23.2) | 0.99 | 1 (2.3) | 0 (0) | 0.35 |
| First Author (Reference) | Eligible Subjects Who Received ICI | Number of Patients | ICI Regimens | Reason of Steroid Initiation | Timing of Steroid Initiation with PSL >10 mg | Survival after ICI Initiation | |||
|---|---|---|---|---|---|---|---|---|---|
| All Patients | Patients with Steroid Use | Patients without Steroid Use | PFS (Steroid Use vs. No Steroid Use) (p-Value) | OS (Steroid Use vs. No Steroid Use) (p-Value) | |||||
| Arbour *1 [11] | All patients (cohort 1) All patients (cohort 2) | 455 185 | 53 37 | 402 148 | Nivolumab Pembrolizumab Atezolizumab Durvalumab | Palliative or brain metastasis | ICI initiation | 1.7 m vs. 1.8 m (p < 0.001) 1.9 m vs. 2.6 m (p = 0.001) | 3.3 m vs. 9.4 m (p < 0.001) 5.4 m vs. 12.1 m (p < 0.001) |
| Fuca [12] | All patients | 151 | 35 | 116 | Anti-PD-(L)1 /Anti PD-L1 + anti-CTLA-4 | Palliative or brain metastasis | Within 28 days after ICI initiation | 1.98 m vs. 3.94 m (p = 0.003) | 4.83 m vs. 15.14 m (p < 0.01) |
| Ricciuti [15] | All patients | 650 | 93 | 557 | Anti-PD-1/PD-L1 Anti-CTLA-4 | Palliative or brain metastasis, etc. | Within 24 h after ICI initiation | 2.0 m vs. 3.4 m (p = 0.01) | 4.9 m vs. 11.2 m (p < 0.001) |
| Scott [16] | All patients | 210 | 66 | 144 | Nivolumab | Palliative or brain metastasis, COPD, irAEs | Within 30 days after ICI initiation | N/A | 4.3 m vs. 11.0 m (p = 0.006) |
| Present study | Patients with clinical benefit from ICI | 126 | 44 | 82 | Nivolumab Pembrolizumab | irAEs | All period of ICI treatment | 11.7 m vs. 16.0 m (p = 0.037) | 35.0 m vs. 41.0 m (p = 0.28) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouri, A.; Kaira, K.; Yamaguchi, O.; Hashimoto, K.; Miura, Y.; Shiono, A.; Shinomiya, S.; Imai, H.; Kobayashi, K.; Kagamu, H. Effect of Systemic Steroid Use for Immune-Related Adverse Events in Patients with Non-Small Cell Lung Cancer Receiving PD-1 Blockade Drugs. J. Clin. Med. 2021, 10, 3744. https://doi.org/10.3390/jcm10163744
Mouri A, Kaira K, Yamaguchi O, Hashimoto K, Miura Y, Shiono A, Shinomiya S, Imai H, Kobayashi K, Kagamu H. Effect of Systemic Steroid Use for Immune-Related Adverse Events in Patients with Non-Small Cell Lung Cancer Receiving PD-1 Blockade Drugs. Journal of Clinical Medicine. 2021; 10(16):3744. https://doi.org/10.3390/jcm10163744
Chicago/Turabian StyleMouri, Atsuto, Kyoichi Kaira, Ou Yamaguchi, Kousuke Hashimoto, Yu Miura, Ayako Shiono, Shun Shinomiya, Hisao Imai, Kunihiko Kobayashi, and Hiroshi Kagamu. 2021. "Effect of Systemic Steroid Use for Immune-Related Adverse Events in Patients with Non-Small Cell Lung Cancer Receiving PD-1 Blockade Drugs" Journal of Clinical Medicine 10, no. 16: 3744. https://doi.org/10.3390/jcm10163744
APA StyleMouri, A., Kaira, K., Yamaguchi, O., Hashimoto, K., Miura, Y., Shiono, A., Shinomiya, S., Imai, H., Kobayashi, K., & Kagamu, H. (2021). Effect of Systemic Steroid Use for Immune-Related Adverse Events in Patients with Non-Small Cell Lung Cancer Receiving PD-1 Blockade Drugs. Journal of Clinical Medicine, 10(16), 3744. https://doi.org/10.3390/jcm10163744






