Hyperthyroidism in Pregnancy: The Delicate Balance between Too Much or Too Little Antithyroid Drug
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Data Collection
2.2.1. Evaluation of the Thyroid Function and Treatment
2.2.2. Evaluation of the Pregnancy Outcome
2.3. Statistics
3. Results
3.1. Demographics
3.2. Etiology of Hyperthyroidism
3.3. Treatment with ATD
3.4. Control of Thyroid Function during Pregnancy
3.5. Postpartum Recurrence/Relapse/Aggravation of Hyperthyroidism
3.6. Pregnancy Outcome
4. Discussion
4.1. Difficulties in the Diagnosis of Hyperthyroidism during Pregnancy
4.2. Treatment with ATD and Thyroid Function Control in Pregnant Women with Hyperthyroidism
4.3. Fetal and Neonatal Outcomes
4.4. Fetal and Neonatal Hyperthyroidism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seo, G.H.; Kim, T.H.; Chung, J.H. Antithyroid Drugs and Congenital Malformations: A Nationwide Korean Cohort Study. Ann. Intern. Med. 2018, 168, 405–413. [Google Scholar] [CrossRef]
- Quintino-Moro, A.; Zantut-Wittmann, D.E.; Tambascia, M.; Machado, H.d.C.; Fernandes, A. High Prevalence of Infertility among Women with Graves’ Disease and Hashimoto’s Thyroiditis. Int. J. Endocrinol. 2014, 2014, 982705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, D.S.; Laurberg, P. Hyperthyroidism in Pregnancy. Lancet Diabetes Endocrinol. 2013, 1, 238–249. [Google Scholar] [CrossRef] [Green Version]
- Dong, A.C.; Stagnaro-Green, A. Differences in Diagnostic Criteria Mask the True Prevalence of Thyroid Disease in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid Off. J. Am. Thyroid Assoc. 2019, 29, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.M.; Mestman, J.H. Transient Non-Autoimmune Hyperthyroidism of Early Pregnancy. J. Thyroid Res. 2011, 2011, 142413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glinoer, D. The Regulation of Thyroid Function in Pregnancy: Pathways of Endocrine Adaptation from Physiology to Pathology. Endocr. Rev. 1997, 18, 404–433. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L.; Knøsgaard, L. Management of Thyrotoxicosis during Pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101414. [Google Scholar] [CrossRef]
- Andersen, S.L.; Olsen, J.; Wu, C.S.; Laurberg, P. Spontaneous Abortion, Stillbirth and Hyperthyroidism: A Danish Population-Based Study. Eur. Thyroid J. 2014, 3, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Korevaar, T.I.M.; Muetzel, R.; Medici, M.; Chaker, L.; Jaddoe, V.W.V.; de Rijke, Y.B.; Steegers, E.A.P.; Visser, T.J.; White, T.; Tiemeier, H.; et al. Association of Maternal Thyroid Function during Early Pregnancy with Offspring IQ and Brain Morphology in Childhood: A Population-Based Prospective Cohort Study. Lancet Diabetes Endocrinol. 2016, 4, 35–43. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 315–389. [Google Scholar] [CrossRef] [Green Version]
- Kahaly, G.J.; Bartalena, L.; Hegedüs, L.; Leenhardt, L.; Poppe, K.; Pearce, S.H. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur. Thyroid J. 2018, 7, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N. Management of thyrotoxicosis: Preconception, pregnancy, and the postpartum period. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2019, 25, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tonacchera, M.; Chiovato, L.; Bartalena, L.; Cavaliere, A.F.; Vitti, P. Treatment of Graves’ Hyperthyroidism with Thionamides: A Position Paper on Indications and Safety in Pregnancy. J. Endocrinol. Investig. 2020, 43, 257–265. [Google Scholar] [CrossRef]
- Andersen, S.L.; Olsen, J.; Laurberg, P. Antithyroid Drug Side Effects in the Population and in Pregnancy. J. Clin. Endocrinol. Metab. 2016, 101, 1606–1614. [Google Scholar] [CrossRef] [Green Version]
- Bliddal, S.; Rasmussen, A.K.; Sundberg, K.; Brocks, V.; Feldt-Rasmussen, U. Antithyroid Drug-Induced Fetal Goitrous Hypothyroidism. Nat. Rev. Endocrinol. 2011, 7, 396–406. [Google Scholar] [CrossRef]
- Amino, N.; Tanizawa, O.; Mori, H.; Iwatani, Y.; Yamada, T.; Kurachi, K.; Kumahara, Y.; Miyai, K. Aggravation of Thyrotoxicosis in Early Pregnancy and after Delivery in Graves’ Disease. J. Clin. Endocrinol. Metab. 1982, 55, 108–112. [Google Scholar] [CrossRef]
- Laurberg, P. Remission of Graves’ Disease during Anti-Thyroid Drug Therapy. Time to Reconsider the Mechanism? Eur. J. Endocrinol. 2006, 155, 783–786. [Google Scholar] [CrossRef] [Green Version]
- Kiserud, T.; Piaggio, G.; Carroli, G.; Widmer, M.; Carvalho, J.; Neerup Jensen, L.; Giordano, D.; Cecatti, J.G.; Abdel Aleem, H.; Talegawkar, S.A.; et al. The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS Med. 2017, 14, e1002220. [Google Scholar] [CrossRef]
- Smith, T.J.; Hegedüs, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef] [Green Version]
- Glinoer, D.; Spencer, C.A. Serum TSH Determinations in Pregnancy: How, When and Why? Nat. Rev. Endocrinol. 2010, 6, 526–529. [Google Scholar] [CrossRef]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid Function and Human Reproductive Health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stricker, R.; Echenard, M.; Eberhart, R.; Chevailler, M.-C.; Perez, V.; Quinn, F.A.; Stricker, R. Evaluation of Maternal Thyroid Function during Pregnancy: The Importance of Using Gestational Age-Specific Reference Intervals. Eur. J. Endocrinol. 2007, 157, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weeke, J.; Dybkjaer, L.; Granlie, K.; Eskjaer Jensen, S.; Kjaerulff, E.; Laurberg, P.; Magnusson, B. A Longitudinal Study of Serum TSH, and Total and Free Iodothyronines during Normal Pregnancy. Acta Endocrinol. 1982, 101, 531–537. [Google Scholar] [CrossRef]
- Lee, R.H.; Spencer, C.A.; Mestman, J.H.; Miller, E.A.; Petrovic, I.; Braverman, L.E.; Goodwin, T.M. Free T4 Immunoassays Are Flawed during Pregnancy. Am. J. Obstet. Gynecol. 2009, 200, 260.e1–260.e6. [Google Scholar] [CrossRef] [PubMed]
- Rodien, P.; Brémont, C.; Sanson, M.L.; Parma, J.; Van Sande, J.; Costagliola, S.; Luton, J.P.; Vassart, G.; Duprez, L. Familial Gestational Hyperthyroidism Caused by a Mutant Thyrotropin Receptor Hypersensitive to Human Chorionic Gonadotropin. N. Engl. J. Med. 1998, 339, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Delange, F.; Lecomte, P. Iodine Supplementation: Benefits Outweigh Risks. Drug Saf. 2000, 22, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Varlas, V. Fetal Thyroid Status in Normal Pregnancy and Premature Birth Euthyroid Women Without Goitre from Areas With or Without Iodine Deficiency. Acta Endocrinol. Buchar. 2006, 2, 403–418. [Google Scholar] [CrossRef]
- Ursu, H. Iodine Status after a Decade of Universal Salt Iodization in Romania: A Bicentric Study in Urban Areas. Acta Endocrinol. Buchar. 2014, 10, 9–20. [Google Scholar] [CrossRef]
- Ursu, H.I.; Toader, O.D.; Podia-Igna, C.; Delia, C.E.; Firta, A.R.; Tupea, C.C.; Tudor, L.M.; Gheorghiu, M.L.; Suciu, N. Iodine Status in Pregnant Women after a Decade of Universal Salt Iodization in Romania. Acta Endocrinol. Buchar. Rom. 2005 2016, 12, 161–167. [Google Scholar] [CrossRef]
- Andersen, S.L.; Olsen, J.; Wu, C.S.; Laurberg, P. Birth Defects after Early Pregnancy Use of Antithyroid Drugs: A Danish Nationwide Study. J. Clin. Endocrinol. Metab. 2013, 98, 4373–4381. [Google Scholar] [CrossRef]
- Morales, D.R.; Fonkwen, L.; Nordeng, H.M.E. Antithyroid Drug Use during Pregnancy and the Risk of Birth Defects in Offspring: Systematic Review and Meta-Analysis of Observational Studies with Methodological Considerations. Br. J. Clin. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.M.; Leveno, K.J. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number. Obstet. Gynecol. 2020, 135, e261–e274. [Google Scholar] [CrossRef]
- Mandel, S.J.; Cooper, D.S. The Use of Antithyroid Drugs in Pregnancy and Lactation. J. Clin. Endocrinol. Metab. 2001, 86, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Millar, L.K.; Wing, D.A.; Leung, A.S.; Koonings, P.P.; Montoro, M.N.; Mestman, J.H. Low Birth Weight and Preeclampsia in Pregnancies Complicated by Hyperthyroidism. Obstet. Gynecol. 1994, 84, 946–949. [Google Scholar]
- Yoshihara, A.; Noh, J.; Yamaguchi, T.; Ohye, H.; Sato, S.; Sekiya, K.; Kosuga, Y.; Suzuki, M.; Matsumoto, M.; Kunii, Y.; et al. Treatment of Graves’ Disease with Antithyroid Drugs in the First Trimester of Pregnancy and the Prevalence of Congenital Malformation. J. Clin. Endocrinol. Metab. 2012, 97, 2396–2403. [Google Scholar] [CrossRef] [Green Version]
- Laurberg, P.; Andersen, S.L. Antithyroid Drug Use in Pregnancy and Birth Defects: Why Some Studies Find Clear Associations, and Some Studies Report None. Thyroid Off. J. Am. Thyroid Assoc. 2015, 25, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Laurberg, P.; Andersen, S.L. Therapy of Endocrine Disease: Antithyroid Drug Use in Early Pregnancy and Birth Defects: Time Windows of Relative Safety and High Risk? Eur. J. Endocrinol. 2014, 171, R13–R20. [Google Scholar] [CrossRef] [Green Version]
- Rovet, J.F. The Role of Thyroid Hormones for Brain Development and Cognitive Function. Endocr. Dev. 2014, 26, 26–43. [Google Scholar] [CrossRef]
- Momotani, N.; Noh, J.Y.; Ishikawa, N.; Ito, K. Effects of Propylthiouracil and Methimazole on Fetal Thyroid Status in Mothers with Graves’ Hyperthyroidism. J. Clin. Endocrinol. Metab. 1997, 82, 3633–3636. [Google Scholar] [CrossRef]
- Andersen, S.L.; Andersen, S.; Vestergaard, P.; Olsen, J. Maternal Thyroid Function in Early Pregnancy and Child Neurodevelopmental Disorders: A Danish Nationwide Case-Cohort Study. Thyroid Off. J. Am. Thyroid Assoc. 2018, 28, 537–546. [Google Scholar] [CrossRef]
- Nanu, M.; Ardeleanu, I.S.; Brezan, F.; Nanu, I.; Apostol, A.; Moldovanu, F.; Lazarescu, H.; Gheorghiu, M.L.; Kozma, A. Neonatal Screening for Congenital Hypothyroidism in Romania: Data from Medilog Medical Information Registry. Acta Endocrinol. Buchar. Rom. 2005 2019, 15, 209–214. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.M.; Smits, I.H.; Fliers, E.; Bisschop, P.H. Maternal Thyrotropin Receptor Antibody Concentration and the Risk of Fetal and Neonatal Thyrotoxicosis: A Systematic Review. Thyroid Off. J. Am. Thyroid Assoc. 2018, 28, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Varlas, V.N.; Duică, F.; Antoniadis, P.; Danila, C.A.; Cretoiu, D.; Suciu, N.; Crețoiu, S.M.; Voinea, S.C. Pregnancy-Related Extracellular Vesicles Revisited. Int. J. Mol. Sci. 2021, 22, 3904. [Google Scholar] [CrossRef]
- Huel, C.; Guibourdenche, J.; Vuillard, E.; Ouahba, J.; Piketty, M.; Oury, J.F.; Luton, D. Use of Ultrasound to Distinguish between Fetal Hyperthyroidism and Hypothyroidism on Discovery of a Goiter. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2009, 33, 412–420. [Google Scholar] [CrossRef]
- Varlas, V.; Bostan, G.; Gheorghiu, M.L. Fetal Thyroid: Ultrasonographic and Hormonal Evaluation in Normal Pregnancy, Premature Birth and Preeclamptic IUGR. Proc. 5th Rom. Congr. Ultrasound Obstet. Gynecol. Mures Rom. 2017, 634–639. [Google Scholar]
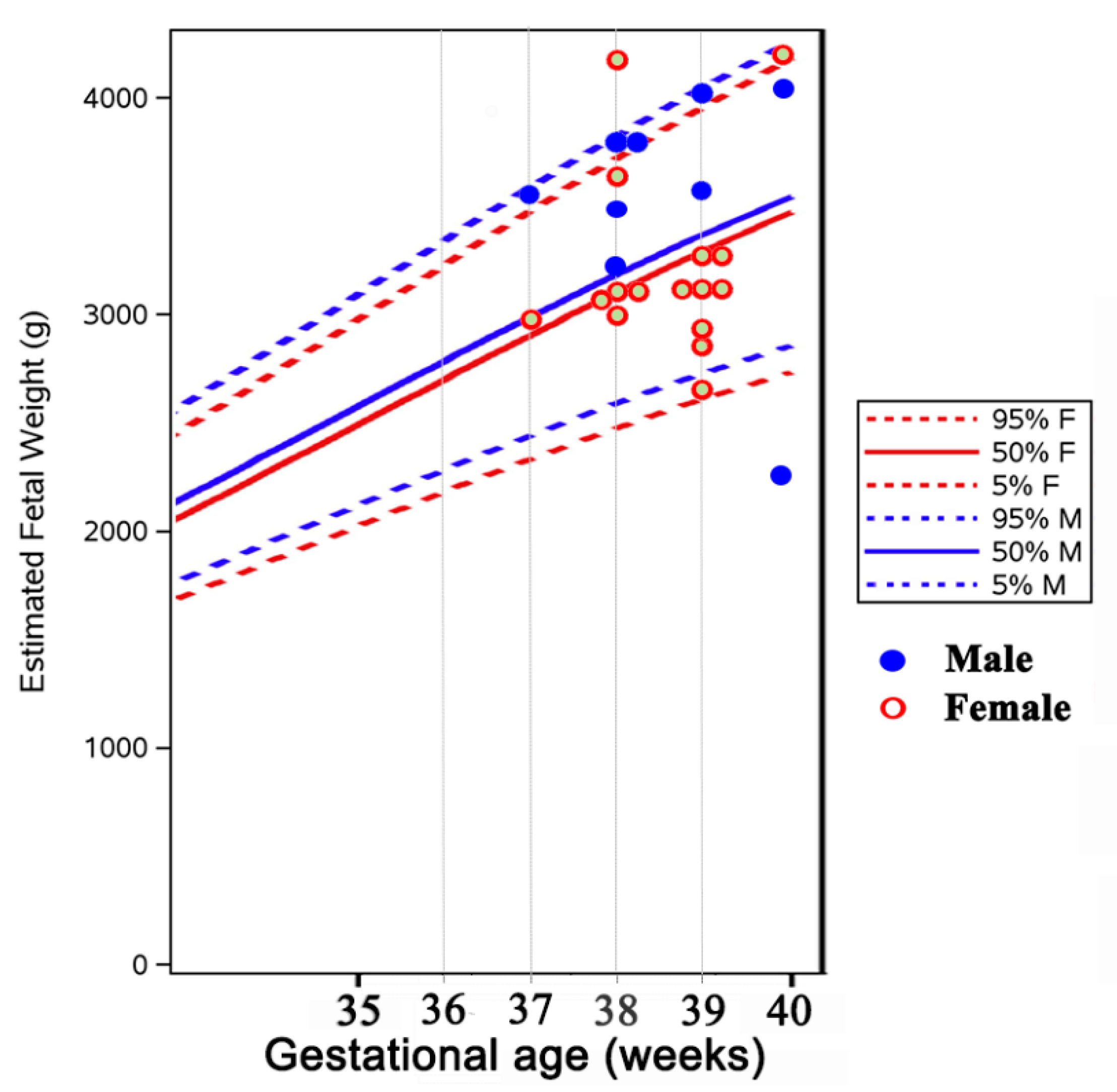
| Patients | n = 29 Women and 36 Single Fetus Pregnancies |
| Age at pregnancy, years, mean ± SD | 30.3 ± 4.7 (range 22–41) |
| Etiology of hyperthyroidism | Graves’ disease (GD): n = 26 (33 pregnancies) Hyperfunctioning autonomous nodule: n = 1 Transient gestational thyrotoxicosis: n = 2 |
| Graves ophthalmopathy | n = 7 (6 mild, 1 moderate) (7/22 evaluable GD women, 31.8%) |
| Treatment with antithyroid drugs (ATD) during pregnancy | Treatment: 28 pregnancies Methimazole (M): 22 pregnancies (78.5% of treated women) Propylthiouracil (P): 2 pregnancies (7.1%) Switch from M to P: 4 pregnancies (14.2%) No treatment: 8 pregnancies (2 with GTT, 1 subclinical GD, 5 euthyroid in previous GD remission before conception) |
| Treatment withdrawal during pregnancy | 16 out of 28 treated pregnancies (57.1%), withdrawal maintained until delivery in 14 out of 28 pregnancies (50%)
|
| Recurrence or aggravation of thyrotoxicosis after delivery | 20 out of 24 pregnancies in ATD treated women (83.3%, all with GD); 3 out of 5 women with GD remission and no treatment be fore & after conception (60%) Median interval ± SD until recurrence/aggravation (range): 3 ± 2.6 months (range 1–10 months) |
| No./Age (Years) | Before Pregnancy | First Trimester | Second Trimester | Third Trimester | Postpartum H. Recurrence/Aggravation (Months) | ||
|---|---|---|---|---|---|---|---|
| Dose/Thyroid Status | TRAb (IU/mL) | Dose/Thyroid Status | Dose/Thyroid Status | Dose/Thyroid Status | Pregnancy Outcome/Delivery | ||
| I. Hyperthyroid women treated with ATD more than 6 months until pregnancy | |||||||
| 1.1 (27) | M 2.5/2d/N | 8.5→<0.3 | -/N | -/N | -/N | girl 3300 g, A8, C-section 39w | 4 |
| 2 * (36) | M 2.5/2d/N | n.a | M 2.5/2d/N | M 2.5/2d/N | -/N | girl, 2950 g, C-section 37w | 2 |
| 1.2 (30) | M 2.5/N | 5.15 | M 2.5/2d/N/SH | M 2.5/SH/hT/N | -/N | girl, 3300 g, A9, C-section 39w | 3 |
| 3 (30) | M 2.5/N | n.a | M 2.5/N | M 2.5/N | M 2.5/N | girl, 2950 g, A7, C-section 38 | No on M 10 |
| 4 (37) | M 5/N | 1.66 | -/N | -/N | -/N | boy, 3500 g, C-section 36w | 4 |
| 5.1 (25) | M 5/N | n.a | -/N | -/N | -/N | Intrauterine death &, C-section 40w | 7 |
| 5.2 (26) | M 5/N | n.a | -/N | -/N | -/N | girl, 2800 g, A9, C-section 38w | 7–8 |
| 5.3 (29) | M 5/N | 6.72 | -/N | -/N | -/N | boy, 3750 g, A10, C-section 39w | 7 |
| 6 (31) | M 5/N | 1.37 | -/N | -/N | -/hT/N | girl, 3150 g, A9/10, Vaginal 39w | n.a |
| 7.2 (31) | M 5/N | n.a | M 2.5–5/N | -/N | -/N | boy 3500 g, A10, C-section 39w | 1 |
| 8.1 (27) | M 5/N | n.a | P 50/SH/N | P 25–37.5/N | P 12.5/2d/N | boy, Vaginal at term | 3 |
| 9 (26) | M 10/N | 2.07 | -/N | -/n.a | -/N | girl, 2750 g, A10, C-section 39w | 3 (SH) |
| 10 (34) | M 10/N | 0.31 | M 10/N/H | M 7.5–10/SH/N | M 2.5–5/N | girl, 3000 g, A10, Vaginal 28w | No on M 5 |
| 11 (31) | M 10/H | n.a | M 10/N | M 10/N | M 10/n.a | boy, 3700 g, A9-10, C- section | n.a |
| 12 (29) | M 10/N | n.a | M 10/N | M 6.25–7.5 hT/N | M 5–6.25/hT/N | boy, 2250 g, A9, C-section 40w | No on M 5 |
| 13 (23) | M 10/n.a | n.a | P 150–250/SH | P 150/SH | P 150/SH | girl, 2700 g, A10, C-section 39w | 3 |
| 14 (31) | M 10/n.a | 3.69 ^ | M 10/n.a | P 50–100/SH | P 50/SH | girl, 4250 g, A9-10, C-section | n.a |
| 15.1 (36) | M 10/N | n.a | P 100–150/H | P 100–150/N | P 100/N | boy, 3700 g, A10, C-section 38w | 3 |
| 15.2 (38) | P 200/SH | n.a | P 50–150/SH | P 50/SH | -/SH | girl, 3600 g, A10, C-section 38w | 1 |
| 16 (22) | P 300/SH | 13.73 | P 300/n.a | P 150/SH | P 150/SH | girl, 3140 g, A9, C-section 39w | 3 |
| II. Overt hyperthyroid women diagnosed shortly before or during pregnancy | |||||||
| IIa. Women treated with ATD less than 6 weeks until pregnancy | |||||||
| 17 (26) | M 20 1 month/H | 4.19 | M 30/H | M 10/SH/N | -/N | girl, 4250 g, A9, Vaginal 40w | 1.5 |
| 18.1 (25) | M 40–20 1 month/H | n.a | M 15/SH | Spontaneous abortion at 5w | 10 | ||
| IIb. Women who started ATD during pregnancy | |||||||
| 19 (26) | M 6 months, stop 3.5 years | n.a | - | M 45/H | M 30/H | boy; neonate death; Vaginal PD 32w | n.a |
| 20 (29) | - | 24.5 ^ | - | M 2.5–30/H/N | M2.5/2d/-/hT | girl, 3100 g, C-section | 10 (not tested before) |
| 7.1 (29) | - | 5.44 ^ | - | M 5–10/H | M 5/SH | girl, 3100 g, A9, C-section 39w | 3 |
| 21 (30) | - | 1.89; 1.57 postP | M 10–7.5/H | M 2.5–5/H-SH | -/SH | girl, 2490 g, Vaginal 39w | 2 (borderline SH), then N |
| 18.2 (26) | M 7 months, stop 5 months | 1.56 ^ | -/H | M 5/H | M 5/n.a | boy, 4000 g, A9, Vaginal 40w | 4 |
| 22 (40) | - | 36 | - | - | M 15–20/H | girl, 3170, A9, C-section 38w | Stable mild H at 3 weeks |
| III. Women with hyperthyroidism not treated with ATD during pregnancy | |||||||
| IIIa. Transient gestational thyrotoxicosis | |||||||
| 23 (33) | -/N | 0.41 | -/SH | -/N | -/n.a | girl, Vaginal 39w | No |
| 24 (30) | -/N | 0.28 ^ | -H/SH | -/N | -/n.a | n.a | n.a |
| IIIb. GD with subclinical hyperthyroidism | |||||||
| 25 (33) | M 4 months, stop 2.5 years/SH | 5.55 | -/SH | -/N | -/N | girl **, n.a | n.a |
| IV. Women with hyperthyroidism in remission after previous ATD treatment (euthyroid, not treated during pregnancy) | |||||||
| 26 (35) | M 9 months, stop 4 years/N | 13.2 postP | -/N | -/N | -/N | girl 3150 g, A9/9, Vaginal | 3 |
| 27 (32) | M 4 years, stop 3 years/N | 10.3 postP | -/N | -/N | -/N | boy, 3150 g, A10, C-section 39w | 4 |
| 28 (26) | M 9 months, stop 3 months/N | 2.41 | -/N | -/N | -/N | boy, 3260 g, A8, C-section at term | No |
| 8.2 (31) | M 4 years, stop 1 year/N | 1.5 | -/N | LT4-12.5-37.5/Sh/N | LT4-50/Sh | Child, Vaginal at term | No |
| 29 (41) | M 1 year, stop 1.3 years/N | 3.88→0.3 1.94 postP | -/n.a | LT4-25-37.5/N-hT | LT4 50-62.5/hT | Child, C-section at term | 6.5 |
| Serum TSH (mIU/L) | Serum FT4 (ng/dL) | |||||
|---|---|---|---|---|---|---|
| Women treated more than 6 months before pregnancy (n = 20 pregnancies, 16 women) | ||||||
| T1 | T2 | T3 | T1 | T2 | T3 | |
| Mean value (range) | 0.40 (nd–1.06) | 0.61 (nd–2.12) | 0.57 (nd–1.9) | 1.09 (0.82–1.12) | 1.03 (0.71–2.04) | 0.92 (0.63–1.44) |
| At least once below normal | 7/19 (37%) | 6/17 (35%) | 4/18 (22%) | 0/17 (0%) | 2/17 (11.7%) | 2/17 (11.7%) |
| At least once above normal | 0/19 (0%) | 0/17 (0%) | 0/19 (0%) | 1/17(5.8%) | 0/17 (0%) | 0/17 (0%) |
| Methimazole mg/day (median, range) | 5 (2.5 at 2 days–10) | 4.37 (2.5 at 2 days–10) | 3.75 (2.5–10) | |||
| n= 18 | n= 6 | n= 4 | - | - | - | |
| Propylthiouracil mg/day (median, range) | 150 (50–300) | 75 (25–150) | 75 (12.5–150) | |||
| n= 5 | n= 6 | n= 5 | - | - | - | |
| No treatment by the end of trimester | 7/20 (35%) | 12/20 (60%) | 11/20 (55%) | - | - | - |
| Women who started treatment shortly before (<6 weeks) or during pregnancy (n = 8 pregnancies, 7 women) | ||||||
| Mean value (range) | nd | nd | 0.24 (nd–1.2) | 1.96 (1.3–2.63) | 2.43 (1.18–5) | 1.07 (0.59–1.73) |
| At least once below normal | 3/3 (100%) | 6/6 (100%) | 4/6 (80%) | 0/3 | 0/6 | 1/5 (20%) |
| At least once above normal | 0/3 (0%) p = 0.032 | 0/6 (0%) p = 0.013 | 0/6 (0%) p = 0.12 | 2/3 (66%) p = 0.045 | 5/6 (83%) p < 0.001 | 1/5 (20%) p = 0.22 |
| Methimazole mg/day (median, range) | 20 (10–30) n = 3 | 10 (5–45) n = 6 | 5 (2.5/2 days–30) n = 5 | - | - | - |
| Propylthiouracil | - | - | - | - | - | - |
| No treatment by the end of trimester | 0/3 (0%) p = 0.52 | 0/6 (0%) p = 0.017 | 3/7 * (42.8%) p = 1 | - | - | - |
| KERRYPNX | Women with Hyperthyroidism (Previous or Current) n = 29 Women, 36 Single Fetus Pregnancies | Control Normal Women n = 39 Women, 39 Single Fetus Pregnancies | p Value |
| Age at pregnancy, years, mean ± SD | 30.3 ± 4.7 29.6 ± 4.5 in ATD treated women | 27.0 ± 4.1 | <0.01 |
| Pregnancy outcome | 31 live children (91.1% of 34 evaluated; 2 unknown *) Women treated for more than 6 months before pregnancy (20 pregnancies): 1 fetal death at 40 weeks due to a true umbilical cord knot Women who started ATD shortly before or during pregnancy (8 pregnancies): 1 spontaneous abortion at 5 weeks 1 premature delivery at 32 weeks, with neonate death within 24 h (respiratory distress) Women in GD remission before pregnancy (5 pregnancies): 5 live children (1 unknown) Untreated hyperthyroidism (3 pregnancies): 1 live child (2 unknown *) | 39 live children | |
| Delivery type | Vaginal: 10 (28.5%) C-section: 23 (65.7%); 85% in women treated >6 months; 57% in women diagnosed shortly before or during pregnancy, p = 0.049 Unknown: 2 (5.7%) | Vaginal: 17 (43.5%) C-section: 22 (56.5%) | 0.32 |
| Mean gestational age (weeks) | 38.4 ± 1.7 38.5 in women treated > 6 months, 38.0 in women treated shortly before or during pregnancy (p = NS) | 39.05 ± 1.2 | 0.11 |
| Children Sex F:M Congenital anomalies Follow-up >2 years Fetal/neonatal hyperthyroidism Fetal/neonatal goiter or congenital hypothyroidism | n = 35 * | n = 39 | |
| 20 F:11 M (4 unknown) | 15 F:24 M | 0.053 | |
| 1 small atrial sept defect—ostium secundum (in 25 live children of ATD treated women, 4%) | None | ||
| 13 of 25 children of ATD treated women (52%) | N/A | ||
| 1 child (in 30 live children from GD women, 3.3%) | 0 cases | ||
| 0 cases | 0 cases | ||
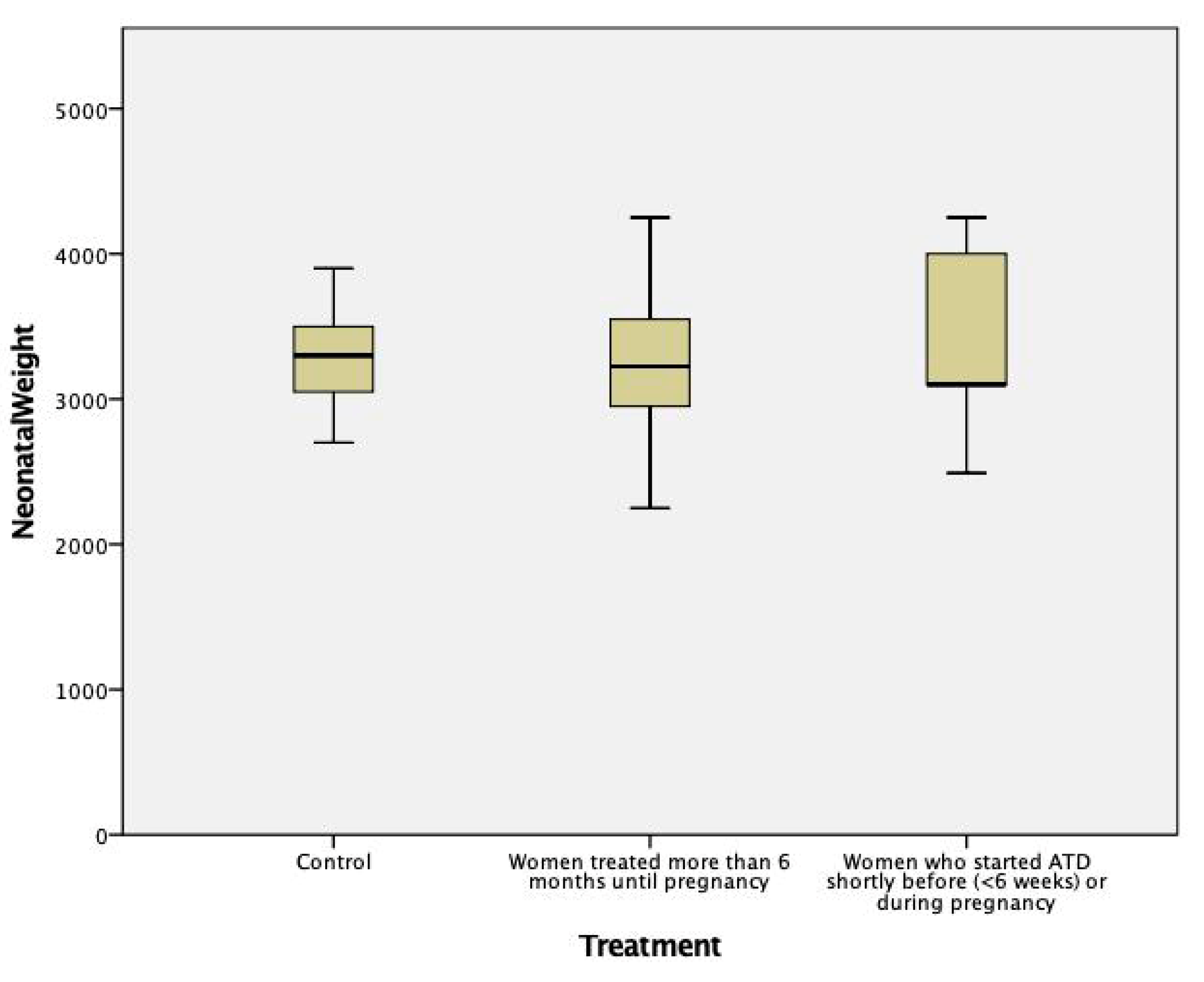
| Children’s birth weight, mean ± SD, grams | (a) All women treated during pregnancy (n = 24 children): 3267 ± 515 | 3342 ± 469 | 0.56 |
| (b) Women treated more than 6 months before, and during pregnancy (n = 18 children): 3238 ± 480 | 0.45 | ||
| (c) Women who stopped ATD before week 10 (n = 6 children): 3208 ± 391 | 0.47 | ||
| (d) Women diagnosed and treated shortly before (n = 1) or only during pregnancy (n = 5): 3352 ± 652 p = NS between groups (b), (c), (d) | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghiu, M.L.; Bors, R.G.; Gheorghisan-Galateanu, A.-A.; Pop, A.L.; Cretoiu, D.; Varlas, V.N. Hyperthyroidism in Pregnancy: The Delicate Balance between Too Much or Too Little Antithyroid Drug. J. Clin. Med. 2021, 10, 3742. https://doi.org/10.3390/jcm10163742
Gheorghiu ML, Bors RG, Gheorghisan-Galateanu A-A, Pop AL, Cretoiu D, Varlas VN. Hyperthyroidism in Pregnancy: The Delicate Balance between Too Much or Too Little Antithyroid Drug. Journal of Clinical Medicine. 2021; 10(16):3742. https://doi.org/10.3390/jcm10163742
Chicago/Turabian StyleGheorghiu, Monica Livia, Roxana Georgiana Bors, Ancuta-Augustina Gheorghisan-Galateanu, Anca Lucia Pop, Dragos Cretoiu, and Valentin Nicolae Varlas. 2021. "Hyperthyroidism in Pregnancy: The Delicate Balance between Too Much or Too Little Antithyroid Drug" Journal of Clinical Medicine 10, no. 16: 3742. https://doi.org/10.3390/jcm10163742
APA StyleGheorghiu, M. L., Bors, R. G., Gheorghisan-Galateanu, A.-A., Pop, A. L., Cretoiu, D., & Varlas, V. N. (2021). Hyperthyroidism in Pregnancy: The Delicate Balance between Too Much or Too Little Antithyroid Drug. Journal of Clinical Medicine, 10(16), 3742. https://doi.org/10.3390/jcm10163742









