High-Resolution 18F-FDG PET/CT for Assessing Three-Dimensional Intraoperative Margins Status in Malignancies of the Head and Neck, a Proof-of-Concept
Abstract
:1. Introduction
2. Materials and Methods
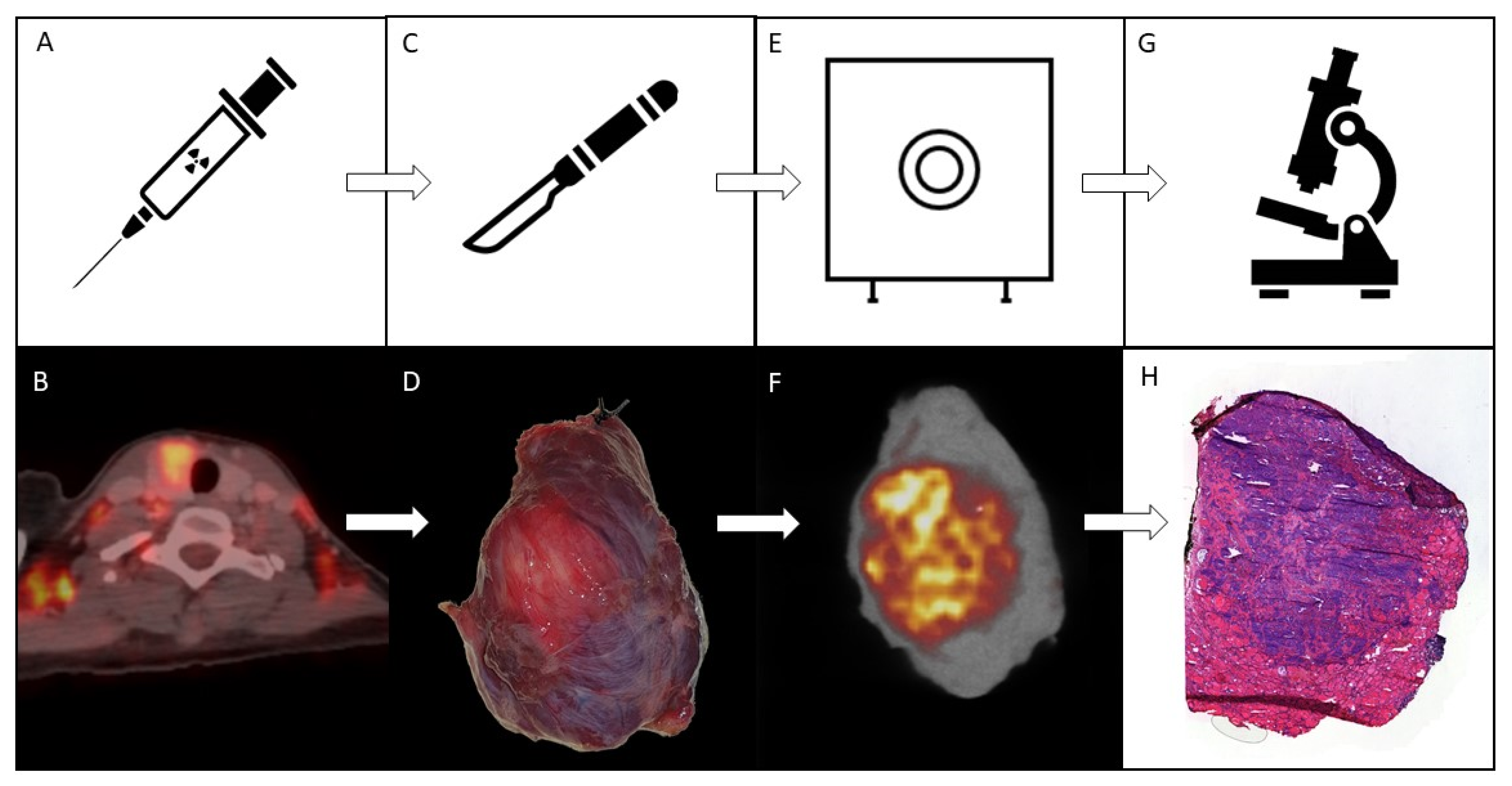
2.1. Study Flow
2.2. Scanning
2.3. Pathology
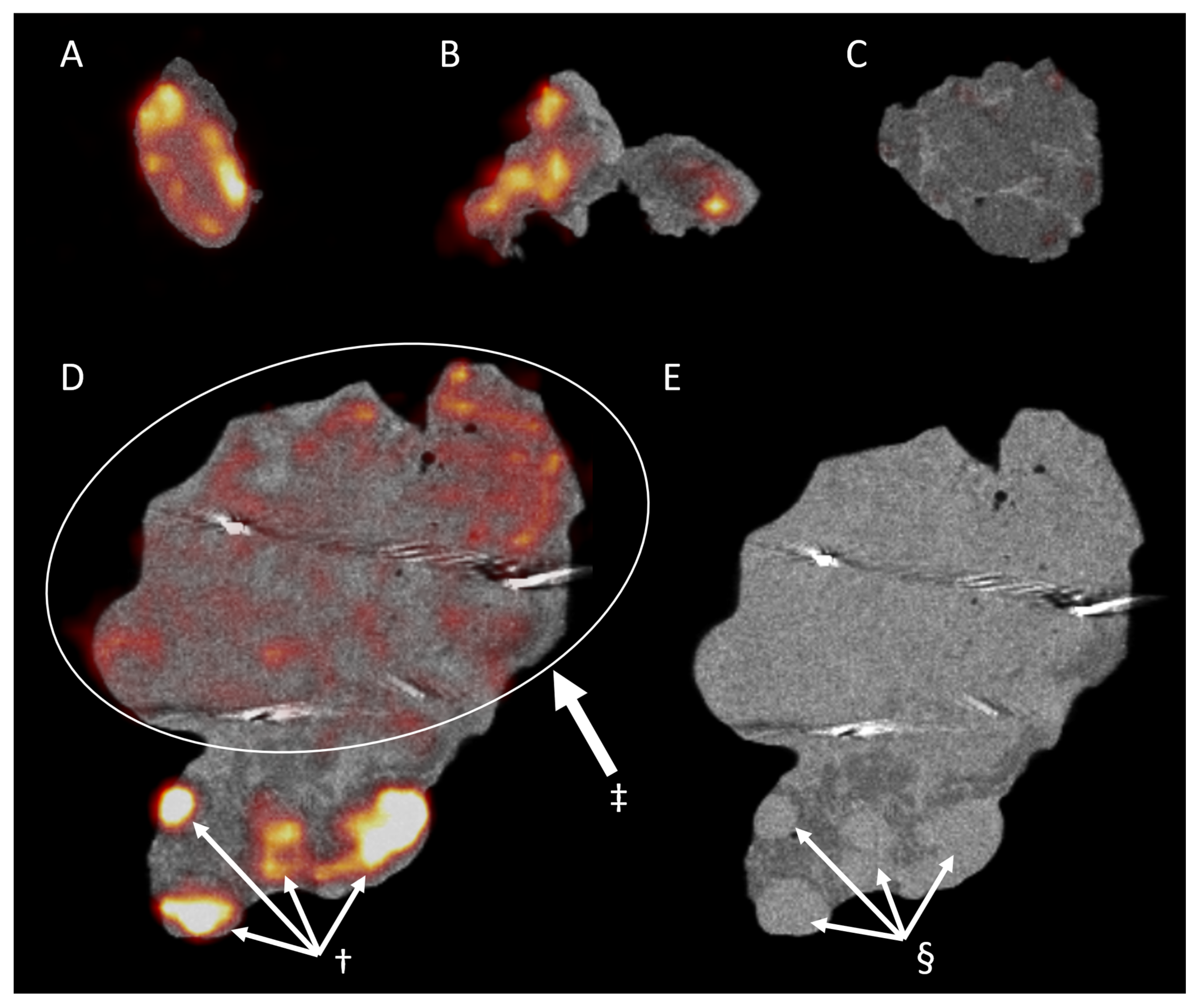
3. Results
3.1. (Pre)Surgical Flow
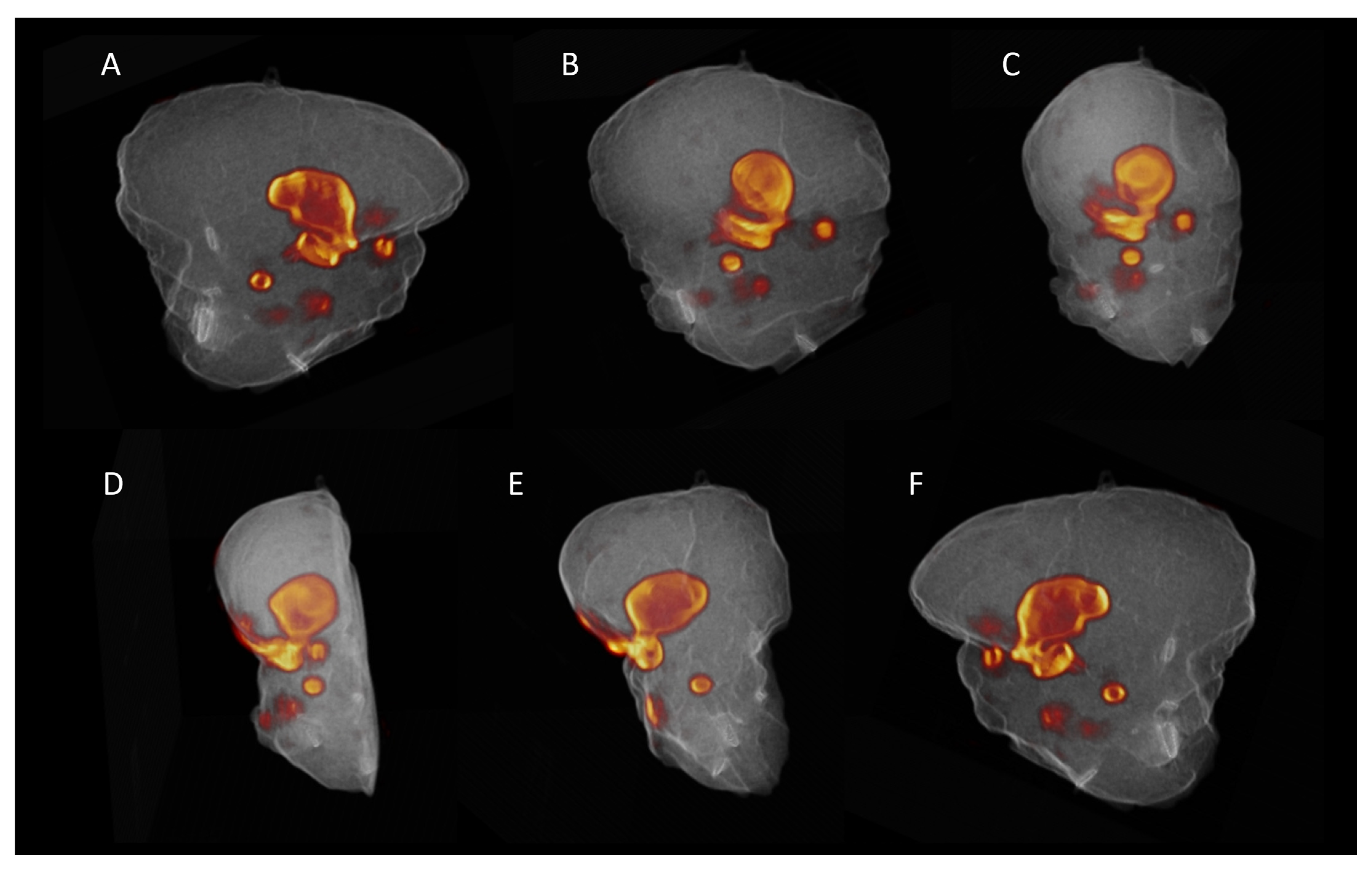
3.2. Ex Vivo Specimen PET/CT
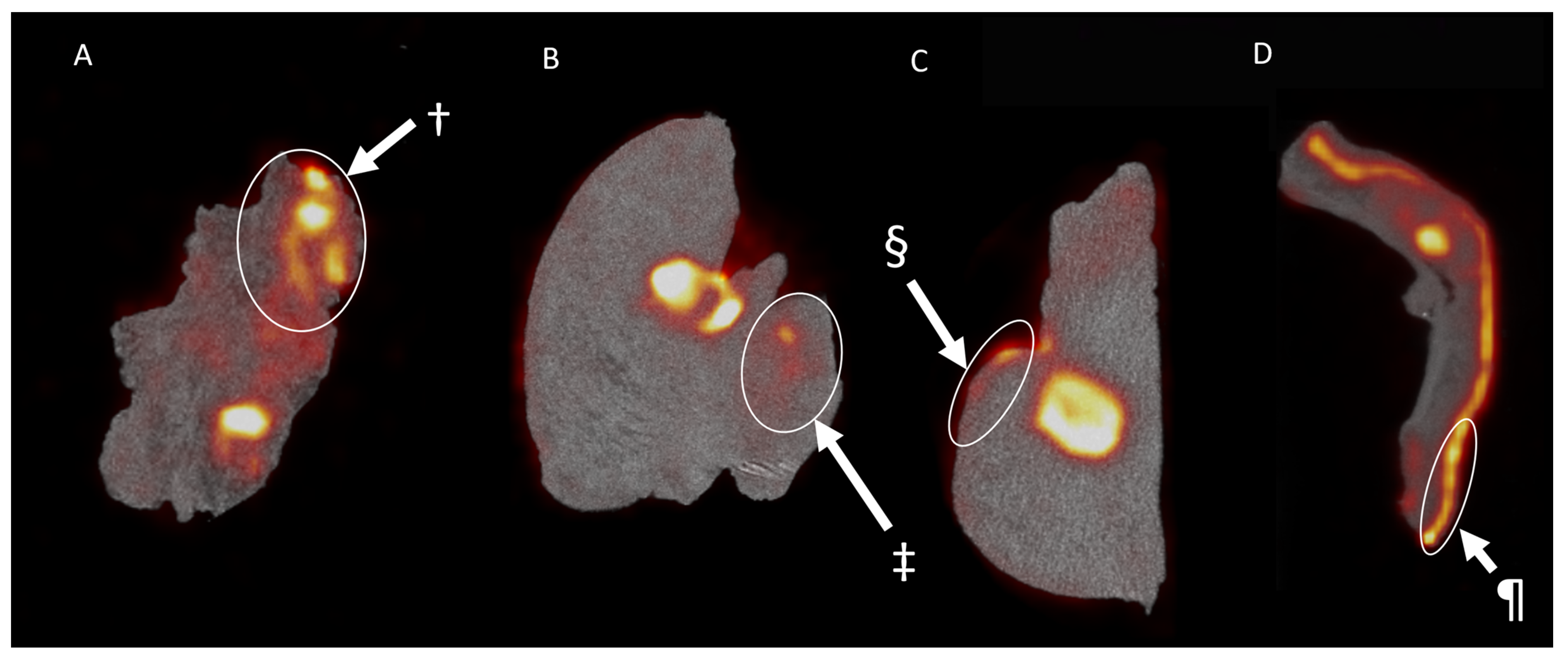
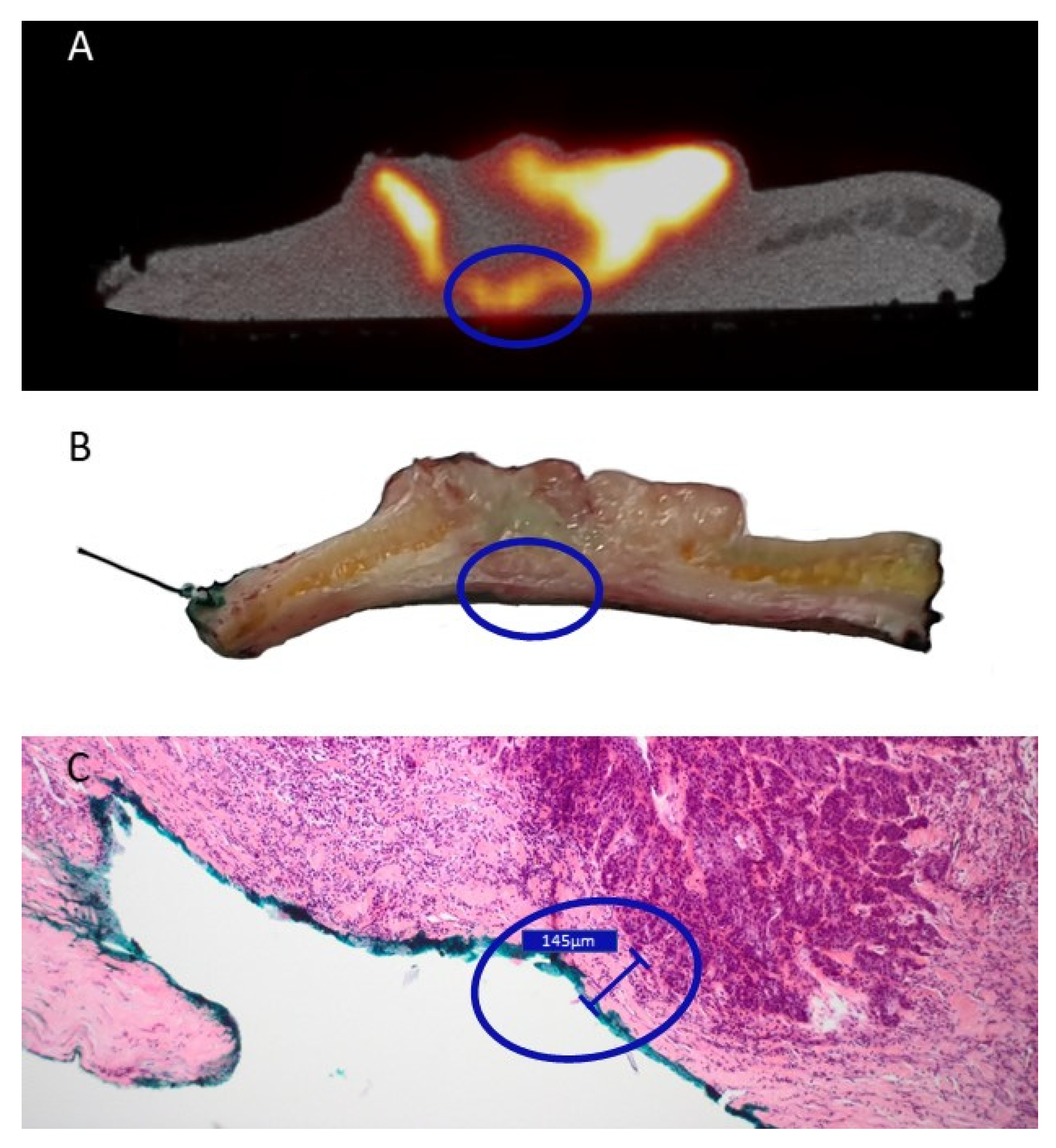
3.3. Pathology and Correlation with the Imaging Results
3.4. Lymph Nodes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and neck cancers, version 2.2020. JNCCN J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Puram, S.V.; Silverman, D.A.; Old, M.O.; Rocco, J.W.; Kang, S.Y. Margin Analysis in Head and Neck Cancer: State of the Art and Future Directions. Ann. Surg. Oncol. 2019, 26, 4070–4080. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.W. Perspective review of what is needed for molecular-specific fluorescence-guided surgery. J. Biomed. Opt. 2018, 23, 100601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso, E.M.; Aaboubout, Y.; van der Sar, L.C.; Mast, H.; Sewnaik, A.; Hardillo, J.A.; ten Hove, I.; Nunes Soares, M.R.; Ottevanger, L.; Bakker Schut, T.C.; et al. Performance of Intraoperative Assessment of Resection Margins in Oral Cancer Surgery: A Review of Literature. Front. Oncol. 2021, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Ajmani, G.S.; Nocon, C.C.; Wang, C.H.; Bhayani, M.K. Assessment of adjuvant therapy in resected head and neck cancer with high-risk features. Oral Oncol. 2017, 74, 15–20. [Google Scholar] [CrossRef]
- Rathod, S.; Livergant, J.; Klein, J.; Witterick, I.; Ringash, J. A systematic review of quality of life in head and neck cancer treated with surgery with or without adjuvant treatment. Oral Oncol. 2015, 51, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Sisson, K.; Moncrieff, M. A meta-analysis of margin size and local recurrence in oral squamous cell carcinoma. Oral Oncol. 2015, 51, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Brouwer de Koning, S.G.; Schaeffers, A.W.M.A.; Schats, W.; van den Brekel, M.W.M.; Ruers, T.J.M.; Karakullukcu, M.B. Assessment of the deep resection margin during oral cancer surgery: A systematic review. Eur. J. Surg. Oncol. 2021. [Google Scholar] [CrossRef]
- Kho, E.; De Boer, L.L.; Van De Vijver, K.K.; Van Duijnhoven, F.; Peeters, M.J.T.F.D.V.; Sterenborg, H.J.C.M.; Ruers, T.J.M. Hyperspectral imaging for resection margin assessment during cancer surgery. Clin. Cancer Res. 2019, 25, 3572–3580. [Google Scholar] [CrossRef] [Green Version]
- Nicolson, F.; Kircher, M.F.; Stone, N.; Matousek, P. Spatially offset Raman spectroscopy for biomedical applications. Chem. Soc. Rev. 2021, 50, 556–568. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Singh, B.; Stack, B.C.; Thacker, S.; Gaysinskiy, V.; Bartel, T.; Lowe, V.; Cool, S.; Entine, G.; Nagarkar, V. A hand-held beta imaging probe for FDG. Ann. Nucl. Med. 2013, 27, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepan, K.O.; Li, M.M.; Kang, S.Y.; Puram, S.V. Molecular margins in head and neck cancer: Current techniques and future directions. Oral Oncol. 2020, 110, 104893. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, S.J.; Tjalma, J.J.J.; Koller, M.; Linssen, M.D.; Vonk, J.; Dobosz, M.; Jorritsma-Smit, A.; Kleibeuker, J.H.; Hospers, G.A.P.; Havenga, K.; et al. Back-Table Fluorescence-Guided Imaging for Circumferential Resection Margin Evaluation Using Bevacizumab-800CW in Patients with Locally Advanced Rectal Cancer. J. Nucl. Med. 2020, 61, 655–661. [Google Scholar] [CrossRef] [Green Version]
- Woolgar, J.A.; Triantafyllou, A. A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol. 2005, 41, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Hinni, M.L.; Ferlito, A.; Brandwein-Gensler, M.S.; Takes, R.P.; Silver, C.E.; Westra, W.H.; Seethala, R.R.; Rodrigo, J.P.; Corry, J.; Bradford, C.R.; et al. Surgical margins in head and neck cancer: A contemporary review. Head Neck 2013, 35, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.; Subramaniam, R.M. Role of Non-FDG-PET/CT in Head and Neck Cancer. Semin. Nucl. Med. 2021, 51, 68–78. [Google Scholar] [CrossRef]
- Göker, M.; Marcinkowski, R.; Van Bockstal, M.; Keereman, V.; Van Holen, R.; Van Dorpe, J.; Vandenberghe, S.; Brans, B.; Depypere, H.; Van den Broecke, R. 18F-FDG micro-PET/CT for intra-operative margin assessment during breast-conserving surgery. Acta Chir. Belg. 2020, 120, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.A.; Berg, E.; Schmall, J.P.; Omidvari, N.; Leung, E.K.; Abdelhafez, Y.G.; Tang, S.; Deng, Z.; Dong, Y.; Lv, Y.; et al. Performance evaluation of the uEXPLORER Total-body PET/CT scanner based on NEMA NU 2-2018 with additional tests to characterize long axial field-of-view PET scanners. J. Nucl. Med. 2020, 62, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowski, R.; Mollet, P.; Van Holen, R.; Vandenberghe, S. Sub-millimetre DOI detector based on monolithic LYSO and digital SiPM for a dedicated small-animal PET system. Phys. Med. Biol. 2016, 61, 2196–2212. [Google Scholar] [CrossRef]
- Loening, A.M.; Gambhir, S.S. AMIDE: A Free Software Tool for Multimodality Medical Image Analysis. Mol. Imaging 2003, 2, 153535002003031. [Google Scholar] [CrossRef] [Green Version]
- Szyszko, T.A.; Cook, G.J.R. PET/CT and PET/MRI in head and neck malignancy. Clin. Radiol. 2018, 73, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Blankemeyer, E.; Mollet, P.; Surti, S.; Van Holen, R.; Karp, J.S. Performance evaluation of the MOLECUBES β-CUBE—A high spatial resolution and high sensitivity small animal PET scanner utilizing monolithic LYSO scintillation detectors. Phys. Med. Biol. 2018, 63, 155013. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.R.; Koontz, N.A. Normal Patterns and Pitfalls of FDG Uptake in the Head and Neck. Semin. Ultrasound CT MRI 2019, 40, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Boeckmann, J.; Bartel, T.; Siegel, E.; Bodenner, D.; Stack, B.C. Can the pathology of a thyroid nodule be determined by positron emission tomography uptake? Otolaryngol.—Head Neck Surg. 2012, 146, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, F.; Wu, Y.; Fang, Q.; Zhang, X.; Du, W.; Zhang, X.; Chen, D.; Luo, R. Predictive Value of Occult Metastasis and Survival Significance of Metabolic Tumor Volume Determined by PET-CT in cT1-2N0 Squamous Cell Carcinoma of the Tongue. Front. Oncol. 2020, 10, 2583. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Saitoh, M.; Notani, K.I.; Tei, K.; Totsuka, Y.; Takinami, S.I.; Kanegae, K.; Inubushi, M.; Tamaki, N.; Kitagawa, Y. Assessment of cervical lymph node metastases using FDG-PET in patients with head and neck cancer. Ann. Nucl. Med. 2008, 22, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lowe, V.J.; Duan, F.; Subramaniam, R.M.; Sicks, J.R.D.; Romanoff, J.; Bartel, T.; Jian, Q.; Nussenbaum, B.; Richmon, J.; Arnold, C.D.; et al. Multicenter trial of [18F]fluorodeoxyglucose positron emission tomography/computed tomography staging of head and neck cancer and negative predictive value and surgical impact in the n0 neck: Results from acrin 6685. J. Clin. Oncol. 2019, 37, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Heidkamp, J.; Scholte, M.; Rosman, C.; Manohar, S.; Fütterer, J.J.; Rovers, M.M. Novel imaging techniques for intraoperative margin assessment in surgical oncology: A systematic review. Int. J. Cancer 2021, ijc.33570. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Dorrius, M.D.; de Jongh, S.J.; Jansen, L.; de Vries, J.; Schröder, C.P.; Zhang, G.J.; de Vries, E.G.E.; van der Vegt, B.; van Dam, G.M. Micro-computed tomography (micro-CT) for intraoperative surgical margin assessment of breast cancer: A feasibility study in breast conserving surgery. Eur. J. Surg. Oncol. 2018, 44, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, Specificity, and Predictors of Positive 68Ga–Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.A.; Freudlsperger, C.; Plinkert, P.K.; Debus, J.; et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845. [Google Scholar] [CrossRef]
- Meershoek, P.; van Den, N.S.; Brouwer, O.R.; Teertstra, H.J.; Lange, C.A.H.; Valdés-Olmos, R.A.; van der Hiel, B.; Balm, A.J.M.; Klop, W.M.; van Leeuwen, F.W.B. Three-dimensional tumor margin demarcation using the hybrid tracer indocyanine green-99mTc-nanocolloid: A proof-of-concept study in tongue cancer patients scheduled for sentinel node biopsy. J. Nucl. Med. 2019, 60, 764–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell’Oglio, P.; de Vries, H.M.; Mazzone, E.; KleinJan, G.H.; Donswijk, M.L.; van der Poel, H.G.; Horenblas, S.; van Leeuwen, F.W.B.; Brouwer, O.R. Hybrid Indocyanine Green–99mTc-nanocolloid for Single-photon Emission Computed Tomography and Combined Radio- and Fluorescence-guided Sentinel Node Biopsy in Penile Cancer: Results of 740 Inguinal Basins Assessed at a Single Institution. Eur. Urol. 2020, 78, 865–872. [Google Scholar] [CrossRef]
- Hekman, M.C.; Rijpkema, M.; Muselaers, C.H.; Oosterwijk, E.; Hulsbergen-Van de Kaa, C.A.; Boerman, O.C.; Oyen, W.J.; Langenhuijsen, J.F.; Mulders, P.F. Tumor-targeted dual-modality imaging to improve intraoperative visualization of clear cell renal cell carcinoma: A first in man study. Theranostics 2018, 8, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liang, X.; Li, Y.; Sun, T.; Jin, Z.; Xue, H.; Tian, J. Nuclear and Fluorescent Labeled PD-1-Liposome-DOX-64Cu/IRDye800CW Allows Improved Breast Tumor Targeted Imaging and Therapy. Mol. Pharm. 2017, 14, 3978–3986. [Google Scholar] [CrossRef] [PubMed]
- Elekonawo, F.M.K.; De Gooyer, J.M.; Bos, D.L.; Goldenberg, D.M.; Boerman, O.C.; Brosens, L.A.A.; Bremers, A.J.A.; De Wilt, J.H.W.; Rijpkem, M. Ex vivo assessment of tumor-targeting fluorescent tracers for image-guided surgery. Cancers 2020, 12, 987. [Google Scholar] [CrossRef] [PubMed]
| Patient Number | Age | Gender | Tumor Location | Tumor Type | Administered Activity (MBq) | Specimen Activity (kBq) |
|---|---|---|---|---|---|---|
| 1 | 60 | Male | Floor of mouth | SCC | 297 | 3482 |
| 2 | 54 | Male | Tongue | SCC | 300 | 137 |
| 3 | 78 | Male | Scalp | SCC | 479 | 310 |
| 4 | 62 | Male | Nose | Angiosarcoma | 306 | 215 |
| 5 | 79 | Male | Scalp | SCC | 377 | 90 |
| Preauricular | SCC | |||||
| Preauricular | BCC | |||||
| 6 | 85 | Male | Ear | SCC | 340 | 94 |
| Preauricular | SCC | |||||
| 7 | 28 | Female | Thyroid | Medullary Carcinoma | 257 | 85 |
| 8 | 60 | Female | Tongue | SCC | 221 | 48 |
| Patient Number | Specimen Number | Time of Anesthesia after Injection (min) A | Time of Resection (min) B | Time of PET/CT (min) C |
|---|---|---|---|---|
| 1 | 1 | 32 | 132 | 146 |
| 2 | 2 | 20 | 129 | 135 |
| 3 | 3 | 77 | 137 | 179 |
| 4 | 4 | 34 | 74 | 92 |
| 5 | 5 | 99 | 139 | 183 |
| 6 | 159 | |||
| 7 | 169 | |||
| 6 | 8 | 40 | 80 | 98 |
| 9 | 165 | |||
| 7 | 10 | 50 | 130 | 140 |
| 8 | 11 | 33 | 115 | 137 |
| Average | 48 | 130 | 139 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debacker, J.M.; Schelfhout, V.; Brochez, L.; Creytens, D.; D’Asseler, Y.; Deron, P.; Keereman, V.; Van de Vijver, K.; Vanhove, C.; Huvenne, W. High-Resolution 18F-FDG PET/CT for Assessing Three-Dimensional Intraoperative Margins Status in Malignancies of the Head and Neck, a Proof-of-Concept. J. Clin. Med. 2021, 10, 3737. https://doi.org/10.3390/jcm10163737
Debacker JM, Schelfhout V, Brochez L, Creytens D, D’Asseler Y, Deron P, Keereman V, Van de Vijver K, Vanhove C, Huvenne W. High-Resolution 18F-FDG PET/CT for Assessing Three-Dimensional Intraoperative Margins Status in Malignancies of the Head and Neck, a Proof-of-Concept. Journal of Clinical Medicine. 2021; 10(16):3737. https://doi.org/10.3390/jcm10163737
Chicago/Turabian StyleDebacker, Jens M., Vanessa Schelfhout, Lieve Brochez, David Creytens, Yves D’Asseler, Philippe Deron, Vincent Keereman, Koen Van de Vijver, Christian Vanhove, and Wouter Huvenne. 2021. "High-Resolution 18F-FDG PET/CT for Assessing Three-Dimensional Intraoperative Margins Status in Malignancies of the Head and Neck, a Proof-of-Concept" Journal of Clinical Medicine 10, no. 16: 3737. https://doi.org/10.3390/jcm10163737
APA StyleDebacker, J. M., Schelfhout, V., Brochez, L., Creytens, D., D’Asseler, Y., Deron, P., Keereman, V., Van de Vijver, K., Vanhove, C., & Huvenne, W. (2021). High-Resolution 18F-FDG PET/CT for Assessing Three-Dimensional Intraoperative Margins Status in Malignancies of the Head and Neck, a Proof-of-Concept. Journal of Clinical Medicine, 10(16), 3737. https://doi.org/10.3390/jcm10163737






