Outcomes of Diabetic Retinopathy Post-Bariatric Surgery in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
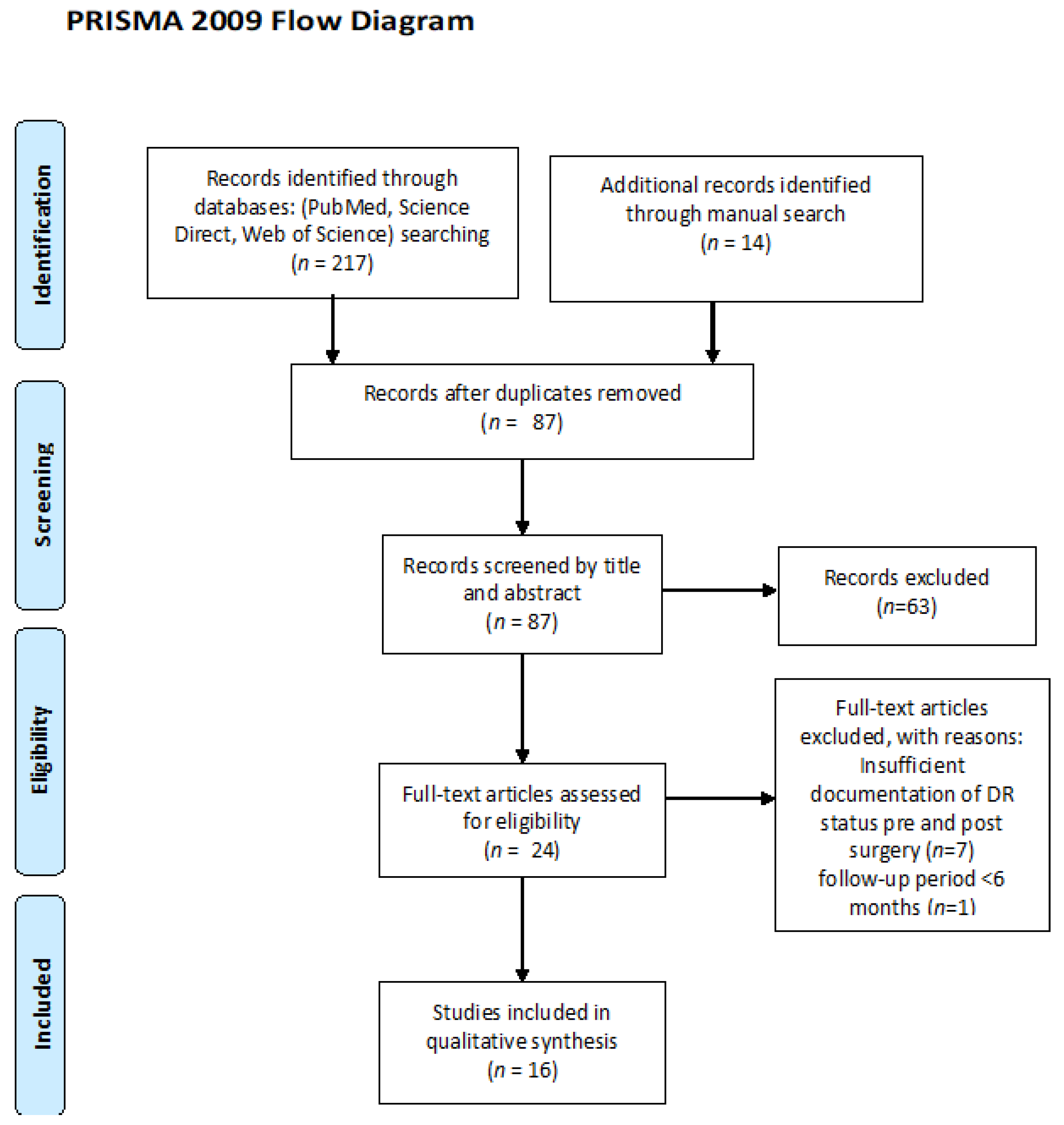
2. Materials and Methods
Data Extraction and Analysis
3. Results
3.1. Risk of Bias
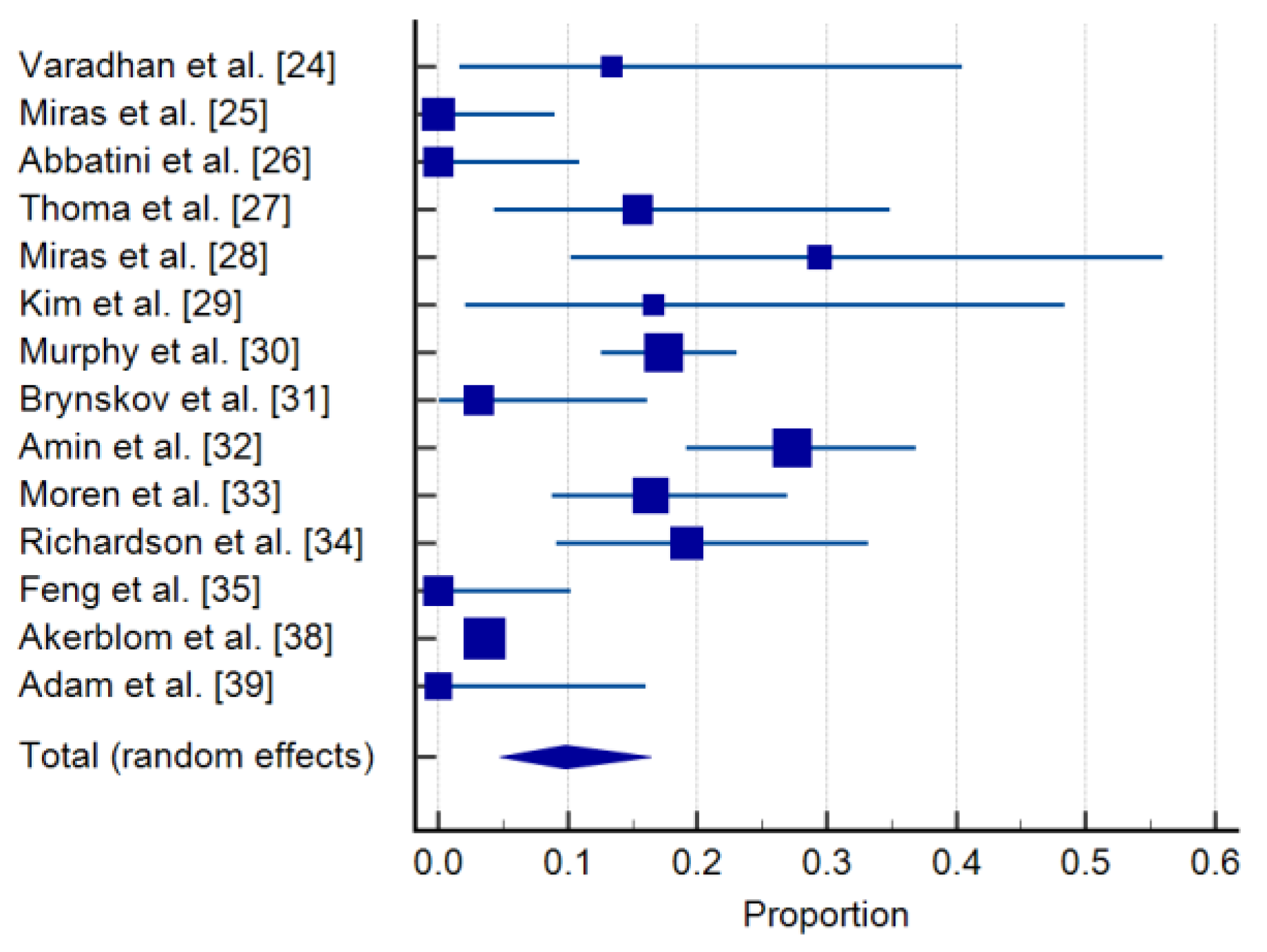
3.2. Incidence of De Novo DR in the Bariatric Surgery Group
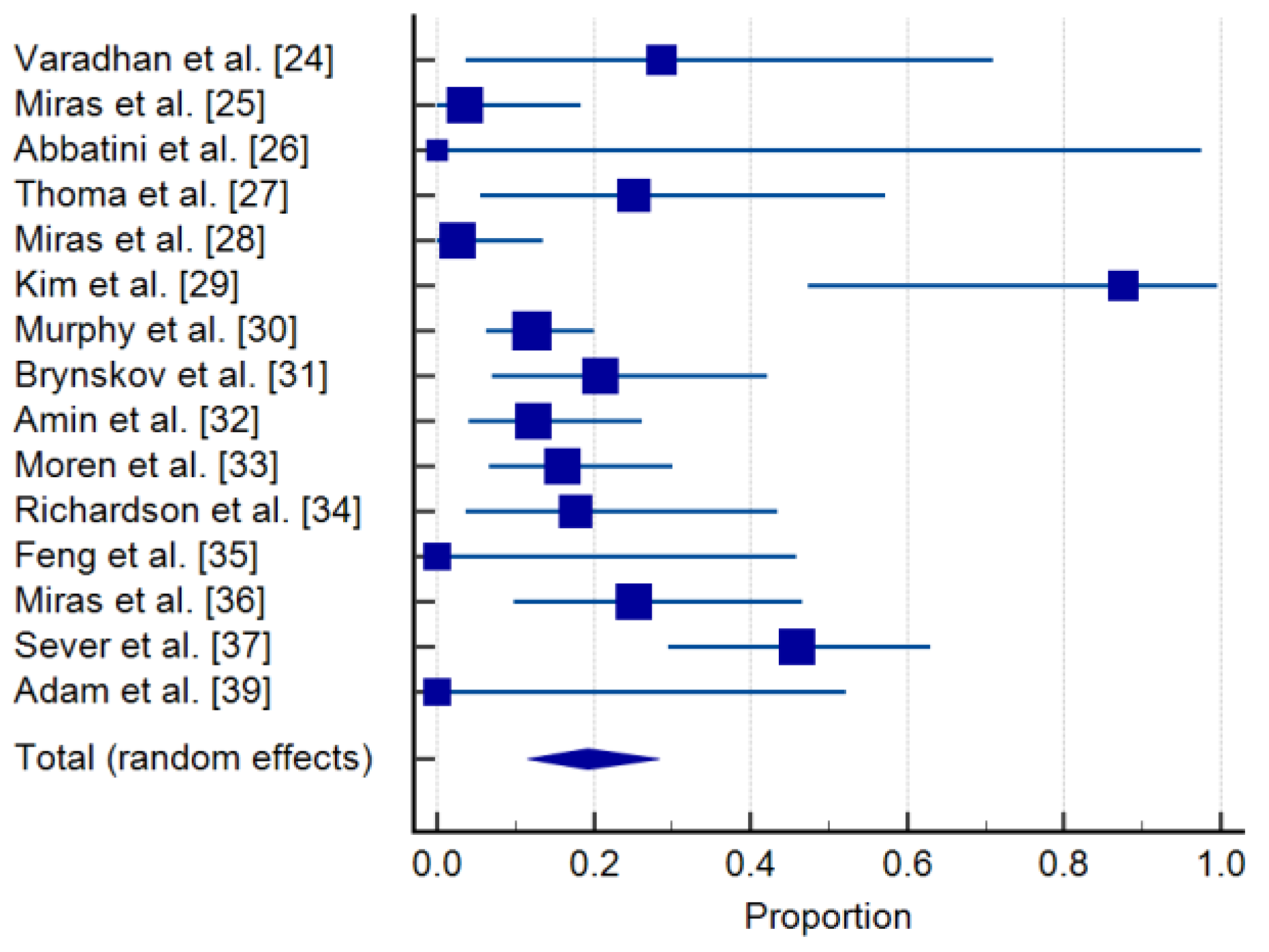
3.3. Progression of DR after Bariatric Surgery and Clinical Correlations
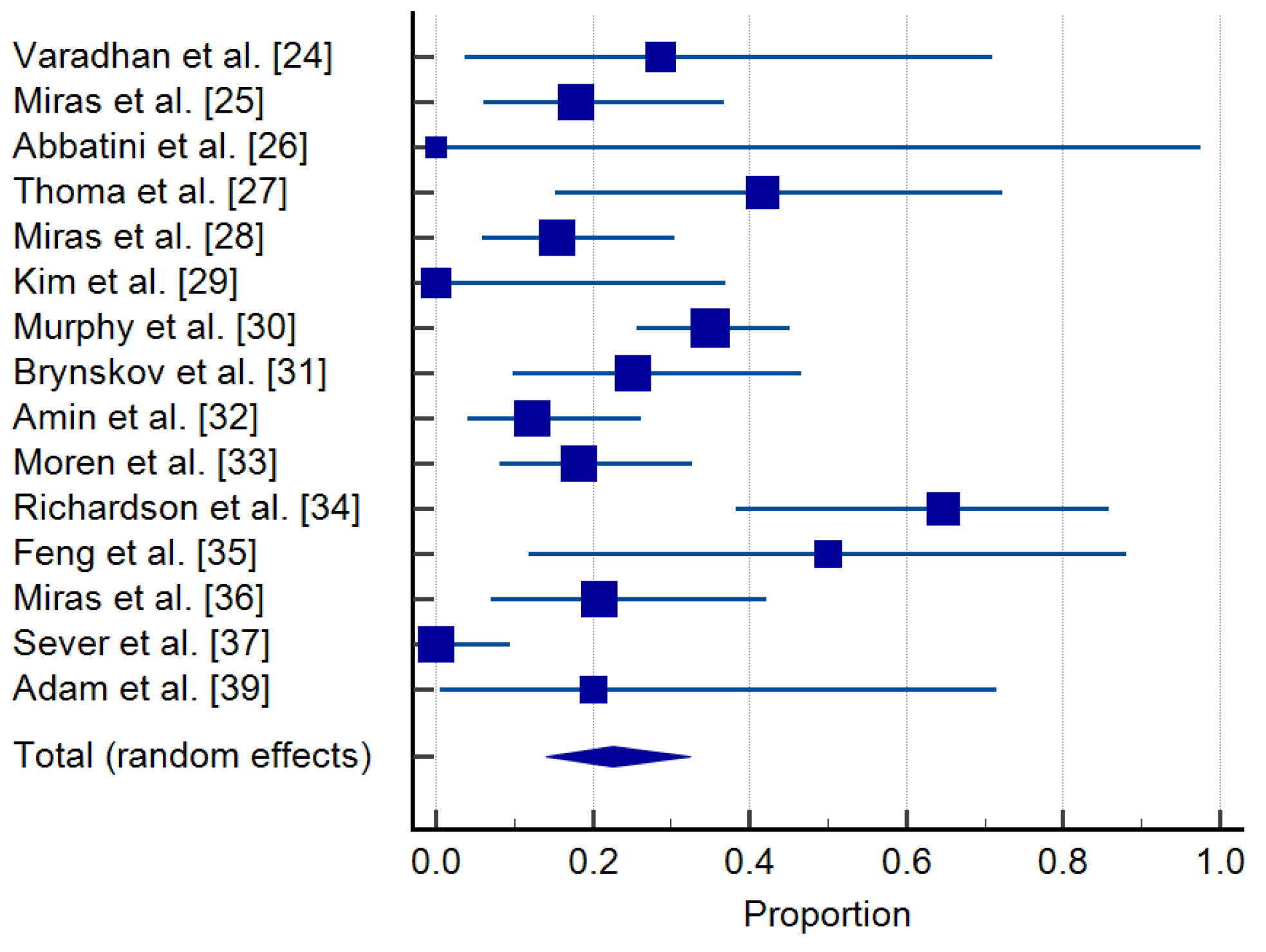
3.4. Regression of DR
3.5. The Impact of Bariatric Surgery upon Systolic Blood Pressure (SBP), Cholesterol and Serum Triglycerides (TG)
4. Discussion
4.1. Gut Hormones and Metabolic Changes after Bariatric Surgery Procedures
4.2. The Paradoxical Effect of Glucose-Lowering Therapy on Diabetic Retinopathy
4.3. DR Phenotypes and Risk for Progression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baskota, A.; Li, S.; Dhakal, N.; Liu, G.; Tian, H. Bariatric Surgery for Type 2 Diabetes Mellitus in Patients with BMI <30 kg/m2: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0132335. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Kahn, S.E.; Ferrannini, E.; Goldfine, A.B.; Nathan, D.M.; Schwartz, M.W.; Smith, R.J.; Smith, S.R. Obesity and type 2 diabetes: What can be unified and what needs to be individualized? J. Clin. Endocrinol. Metab. 2011, 96, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Glycemic Targets: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S66–S76. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.R.; Gatmaitan, P.; Brethauer, S.; Schauer, P. Bariatric surgery for type 2 diabetes: Weighing the impact for obese patients. Cleve Clin. J. Med. 2010, 77, 468–476. [Google Scholar] [CrossRef]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef]
- Park, J.Y.; Heo, Y.; Kim, Y.J.; Park, J.M.; Kim, S.M.; Park, D.J.; Lee, S.K.; Han, S.M.; Shim, K.W.; Lee, Y.J.; et al. Long-term effect of bariatric surgery versus conventional therapy in obese Korean patients: A multicenter retrospective cohort study. Ann. Surg. Treat. Res. 2019, 96, 283–289. [Google Scholar] [CrossRef]
- Stahl, J.M.; Malhotra, S. Obesity Surgery Indications And Contraindications. [Updated 2020 July 31]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513285/ (accessed on 15 June 2021).
- Vella, A. β-cell function after weight-loss induced by bariatric surgery. Physiology (Bethesda) 2014, 29, 84–85. [Google Scholar] [CrossRef]
- Souteiro, P.; Belo, S.; Neves, J.S.; Magalhães, D.; Silva, R.B.; Oliveira, S.C.; Costa, M.M.; Saavedra, A.; Oliveira, J.; Cunha, F.; et al. Preoperative Beta Cell Function Is Predictive of Diabetes Remission After Bariatric Surgery. Obes. Surg. 2017, 27, 288–294. [Google Scholar] [CrossRef] [PubMed]
- King, P.; Peacock, I.; Donnelly, R. The UK prospective diabetes study (UKPDS): Clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol. 1999, 48, 643–648. [Google Scholar] [CrossRef]
- Fong, D.S.; Aiello, L.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L.; Klein, R. Retinopathy in Diabetes. Diabetes Care 2004, 27 (Suppl. 1), s84–s87. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.; Papanas, N.; Dascalu, A.M.; Stana, D.; Nicolae, V.A.; Vancea, G.; Badiu, C.D.; Tanasescu, D.; Tudor, C.; Balasescu, S.A.; et al. Diabetic Retinopathy in Patients With Diabetic Foot Ulcer: A Systematic Review. Int. J. Low Extrem. Wounds 2021, 20, 98–103. [Google Scholar] [CrossRef]
- Chatziralli, I.P. The Role of Glycemic Control and Variability in Diabetic Retinopathy. Diabetes Ther. 2018, 9, 431–434. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [CrossRef]
- Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; Lovato, J.F.; Perdue, L.H.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef]
- Davis, M.D. Diabetic retinopathy. A clinical overview. Diabetes Care 1992, 15, 1844–1874. [Google Scholar] [CrossRef] [PubMed]
- Moskalets, E.; Galstyan, G.; Starostina, E.; Antsiferov, M.; Chantelau, E. Association of blindness to intensification of glycemic control in insulin-dependent diabetes mellitus. J. Diabetes Complicat. 1994, 8, 45–50. [Google Scholar] [CrossRef]
- The Diabetes Control and Complications Trial Research Group. Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch. Ophthalmol. 1998, 116, 874–886. [Google Scholar] [CrossRef]
- Dahl-Jorgensen, K.; Brinchmann-Hansen, O.; Hanssen, K.F.; Sandvik, L.; Aagenaes, O. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: The Oslo study. Br. Med. J. (Clin. Res. Ed.) 1985, 290, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Bain, S.C.; Klufas, M.A.; Ho, A.; Matthews, D.R. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes Obes. Metab. 2019, 21, 454–466. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Varadhan, L.; Humphreys, T.; Walker, A.B.; Cheruvu, C.V.; Varughese, G.I. Bariatric surgery and diabetic retinopathy: A pilot analysis. Obes. Surg. 2012, 22, 515–516. [Google Scholar] [CrossRef]
- Miras, A.D.; Chuah, L.L.; Lascaratos, G.; Faruq, S.; Mohite, A.A.; Shah, P.R.; Gill, M.; Jackson, S.N.; Johnston, D.G.; Olbers, T.; et al. Bariatric surgery does not exacerbate and may be beneficial for the microvascular complications of type 2 diabetes. Diabetes Care 2012, 35, e81. [Google Scholar] [CrossRef] [PubMed]
- Abbatini, F.; Capoccia, D.; Casella, G.; Soricelli, E.; Leonetti, F.; Basso, N. Long-term remission of type 2 diabetes in morbidly obese patients after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2013, 9, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.L.; Prior, S.L.; Barry, J.D.; Luzio, S.D.; Eyre, N.; Caplin, S.; Stephens, J.W.; Owens, D.R. Does bariatric surgery adversely impact on diabetic retinopathy in persons with morbid obesity and type 2 diabetes? A pilot study. J. Diabetes Complicat. 2014, 28, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Miras, A.D.; Chuah, L.L.; Khalil, N.; Nicotra, A.; Vusirikala, A.; Baqai, N.; Graham, C.; Ravindra, S.; Lascaratos, G.; Oliver, N.; et al. Type 2 diabetes mellitus and microvascular complications 1 year after Roux-en-Y gastric bypass: A case-control study. Diabetologia 2015, 58, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Seo, D.R.; Kim, M.J.; Lee, S.J.; Hur, K.Y.; Choi, K.S. Clinical course of diabetic retinopathy in Korean type 2 diabetes after bariatric surgery: A pilot study. Retina 2015, 35, 935–943. [Google Scholar] [CrossRef]
- Murphy, R.; Jiang, Y.; Booth, M.; Babor, R.; MacCormick, A.; Hammodat, H.; Beban, G.; Barnes, R.M.; Vincent, A.L. Progression of diabetic retinopathy after bariatric surgery. Diabet. Med. 2015, 32, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Brynskov, T.; Laugesen, C.S.; Svenningsen, A.L.; Floyd, A.K.; Sorensen, T.L. Monitoring of diabetic retinopathy in relation to bariatric surgery: A prospective observational study. Obes. Surg. 2016, 26, 1279–1286. [Google Scholar] [CrossRef]
- Amin, A.M.; Wharton, H.; Clarke, M.; Syed, A.; Dodson, P.; Tahrani, A.A. The impact of bariatric surgery on retinopathy in patients with type 2 diabetes: A retrospective cohort study. Surg. Obes. Relat. Dis. 2016, 12, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Moren, Å.; Sundbom, M.; Ottosson, J.; Granstam, E. Gastric bypass surgery does not increase the risk for sight-threatening diabetic retinopathy. Acta Ophthalmol. 2018, 96, 279–282. [Google Scholar] [CrossRef]
- Richardson, P.; Hulpus, A.; Idris, I. Short-Term Impact of Bariatric Surgery on Best-Corrected Distance Visual Acuity and Diabetic Retinopathy Progression. Obes. Surg. 2018, 28, 3711–3713. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Yin, T.; Chu, X.; Shan, X.; Jiang, C.; Wang, Y.; Qian, Y.; Zhu, D.; Sun, X.; Bi, Y. Metabolic effects and safety of Roux-en-Y gastric bypass surgery vs. conventional medication in obese Chinese patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2019, 35, e3138. [Google Scholar] [CrossRef] [PubMed]
- Miras, A.D.; Ravindra, S.; Humphreys, A.; Lascaratos, G.; Quartey, K.N.K.; Ahmed, A.R.; Cousins, J.; Moorthy, K.; Purkayastha, S.; Hakky, S.; et al. Metabolic Changes and Diabetes Microvascular Complications 5 Years After Obesity Surgery. Obes. Surg. 2019, 29, 3907–3911. [Google Scholar] [CrossRef]
- Sever, O.; Horozoglu, F. Bariatric surgery might aggravate proliferative diabetic retinopathy. Acta Ophthalmol. 2020, 98, e579–e584. [Google Scholar] [CrossRef]
- Åkerblom, H.; Franzén, S.; Zhou, C.; Morén, Å.; Ottosson, J.; Sundbom, M.; Eliasson, B.; Svensson, A.M.; Granstam, E. Association of Gastric Bypass Surgery With Risk of Developing Diabetic Retinopathy Among Patients with Obesity and Type 2 Diabetes in Sweden: An Observational Study. JAMA Ophthalmol. 2021, 139, 200–205. [Google Scholar] [CrossRef]
- Adam, S.; Azmi, S.; Ho, J.H.; Liu, Y.; Ferdousi, M.; Siahmansur, T.; Kalteniece, A.; Marshall, A.; Dhage, S.S.; Iqbal, Z.; et al. Improvements in Diabetic Neuropathy and Nephropathy After Bariatric Surgery: A Prospective Cohort Study. Obes. Surg. 2021, 31, 554–563. [Google Scholar] [CrossRef]
- Madura, J.A., 2nd; Dibaise, J.K. Quick fix or long-term cure? Pros and cons of bariatric surgery. F1000 Med. Rep. 2012, 4, 19. [Google Scholar] [CrossRef]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [CrossRef]
- Goh, Y.M.; Toumi, Z.; Date, R.S. Surgical cure for type 2 diabetes by foregut or hindgut operations: A myth or reality? A systematic review. Surg. Endosc. 2017, 31, 25–37. [Google Scholar] [CrossRef]
- Zakaria, A.S.; Rossetti, L.; Cristina, M.; Veronelli, A.; Lombardi, F.; Saibene, A.; Micheletto, G.; Pontiroli, A.E. LAGB10 working group. Effects of gastric banding on glucose tolerance, cardiovascular and renal function, and diabetic complications: A 13-year study of the morbidly obese. Surg. Obes. Relat. Dis. 2016, 12, 587–595. [Google Scholar] [CrossRef]
- Rubino, F.; Forgione, A.; Cummings, D.E.; Vix, M.; Gnuli, D.; Mingrone, G.; Castagneto, M.; Marescaux, J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann. Surg. 2006, 244, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.D.F.H.S.; Diniz, M.T.C.; Sanches, S.R.A.; de Almeida Salgado, P.P.C.; Valadão, M.M.A.; Freitas, C.P.; Vieira, D.J. Glycemic Control in Diabetic Patients after Bariatric Surgery. Obes Surg. 2004, 14, 1051–1055. [Google Scholar] [CrossRef]
- Singh, R.P.; Gans, R.; Kashyap, S.R.; Bedi, R.; Wolski, K.; Brethauer, S.A.; Nissen, S.E.; Bhatt, D.L.; Schauer, P. Effect of bariatric surgery versus intensive medical management on diabetic ophthalmic outcomes. Diabetes Care 2015, 38, e32–e33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Brien, R.; Johnson, E.; Haneuse, S.; Coleman, K.J.; O’Connor, P.J.; Fisher, D.P.; Sidney, S.; Bogart, A.; Theis, M.K.; Anau, J.; et al. Microvascular Outcomes in Patients With Diabetes After Bariatric Surgery Versus Usual Care: A Matched Cohort Study. Ann. Intern. Med. 2018, 169, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.L.; Blackhurst, D.W.; Latham, B.B.; Cull, D.L.; Bour, E.S.; Oliver, T.L.; Williams, B.; Taylor, S.M.; Scott, J.D. Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J. Am. Coll Surg. 2013, 216, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.M.S.; Sjöholm, K.; Karlsson, C.; Jacobson, P.; Andersson-Assarsson, J.C.; Svensson, P.A.; Larsson, I.; Hjorth, S.; Neovius, M.; Taube, M.; et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: A post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2017, 5, 271–279. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes-5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chao, S.H.; Chen, C.C.; Ser, K.H.; Chong, K.; Lu, C.H.; Hsieh, M.L.; Huang, Y.Y.; Lee, Y.C.; Hsu, C.C.; et al. The Effects of Bariatric Surgery on Renal, Neurological, and Ophthalmic Complications in Patients with Type 2 Diabetes: The Taiwan Diabesity Study. Obes. Surg. 2021, 31, 117–126. [Google Scholar] [CrossRef]
- Singer, J.R.; Bakall, B.; Gordon, G.M.; Reddy, R.K. Treatment of vitamin A deficiency retinopathy with sublingual vitamin A palmitate. Doc. Ophthalmol. 2016, 132, 137–145. [Google Scholar] [CrossRef]
- Saenz-de-Viteri, M.; Sadaba, L.M. Optical Coherence Tomography Assessment Before and After Vitamin Supplementation in a Patient With Vitamin A Deficiency: A Case Report and Literature Review. Medicine (Baltim.) 2016, 95, e2680. [Google Scholar] [CrossRef]
- Rapoport, Y.; Lavin, P.J. Nutritional Optic Neuropathy Caused by Copper Deficiency After Bariatric Surgery. J. Neuroophthalmol. 2016, 36, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Pierko, A.; Obuchowska, I.; Hady, R.H.; Mariak, Z.; Dadan, J. Nutritional optic neuropathy following bariatric surgery. Videosurgery Other Miniinvasive Tech. 2014, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Capristo, E.; Chamseddine, G.; Bornstein, S.R.; Rubino, F. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2021, 397, 293–304. [Google Scholar] [CrossRef]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Karason , K.; Lönroth, H.; et al. Association of Bariatric Surgery With Long-term Remission of Type 2 Diabetes and With Microvascular and Macrovascular Complications. JAMA 2014, 311, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Huang, R.; Ding, X.; Fu, H.; Cai, Q. Potential mechanisms of sleeve gastrectomy for reducing weight and improving metabolism in patients with obesity. Surg. Obes. Relat. Dis. 2019, 15, 1861–1871. [Google Scholar] [CrossRef]
- Pournaras, D.J.; le Roux, C.W. Ghrelin and metabolic surgery. Int. J. Pept. 2010, 2010, 217267. [Google Scholar] [CrossRef] [PubMed]
- Billeter, A.T.; Fischer, L.; Wekerle, A.L.; Senft, J.; Müller-Stich, B. Malabsorption as a Therapeutic Approach in Bariatric Surgery. Viszeralmedizin 2014, 30, 198–204. [Google Scholar] [CrossRef]
- Bjørklund, G.; Peana, M.; Pivina, L.; Dosa, A.; Aaseth, J.; Semenova, Y.; Chirumbolo, S.; Medici, S.; Dadar, M.; Costea, D.-O. Iron Deficiency in Obesity and after Bariatric Surgery. Biomolecules 2021, 11, 613. [Google Scholar] [CrossRef]
- De Silva, A.; Bloom, S.R. Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity. Gut Liver 2012, 6, 10–20. [Google Scholar] [CrossRef]
- Rubino, F.; Gagner, M.; Gentileschi, P.; Kini, S.; Fukuyama, S.; Feng, J. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann. Surg. 2004, 240, 236–242. [Google Scholar] [CrossRef]
- Kamvissi, V.; Salerno, A.; Bornstein, S.R.; Mingrone, G.; Rubino, F. Incretins or anti-incretins? A new model for the “entero-pancreatic axis”. Horm. Metab. Res. 2015, 47, 84–87. [Google Scholar] [CrossRef][Green Version]
- Hutch, C.R.; Sandoval, D. The Role of GLP-1 in the Metabolic Success of Bariatric Surgery. Endocrinology 2017, 158, 4139–4151. [Google Scholar] [CrossRef]
- Shah, M.; Vella, A. Effects of GLP-1 on appetite and weight. Rev. Endocr. Metab. Disord. 2014, 15, 181–187. [Google Scholar] [CrossRef]
- Douros, J.D.; Tong, J.; D’Alessio, D.A. The Effects of Bariatric Surgery on Islet Function, Insulin Secretion, and Glucose Control. Endocr. Rev. 2019, 40, 1394–1423. [Google Scholar] [CrossRef]
- Holter, M.M.; Dutia, R.; Stano, S.M.; Prigeon, R.L.; Homel, P.; McGinty, J.J., Jr.; Belsley, S.J.; Ren, C.J.; Rosen, D.; Laferrère, B. Glucose Metabolism After Gastric Banding and Gastric Bypass in Individuals With Type 2 Diabetes: Weight Loss Effect. Diabetes Care 2017, 40, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Knop, F.K.; Taylor, R. Mechanism of metabolic advantages after bariatric surgery: It’s all gastrointestinal factors versus it’s all food restriction. Diabetes Care 2013, 36 (Suppl. 2), S287–S291. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Prokosch, V. Energy Metabolism in the Inner Retina in Health and Glaucoma. Int. J. Mol. Sci. 2021, 22, 3689. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group and the Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Study Group. Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) follow-on study. Diabetes Care 2016, 39, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Zoungas, S.; Arima, H.; Gerstein, H.C.; Holman, R.R.; Woodward, M.; Reaven, P.; Hayward, R.A.; Craven, T.; Coleman, R.L.; Chalmers, J.; et al. Collaborators on Trials of Lowering Glucose (CONTROL) group. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: A meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017, 5, 431–437. [Google Scholar] [CrossRef]
- Neff, K.J.; Le Roux, C.W. The Effect of Metabolic Surgery on the Complications of Diabetes: What Are the Unanswered Questions? Front. Endocrinol. (Lausanne) 2020, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Gorman, D.M.; le Roux, C.W.; Docherty, N.G. The Effect of Bariatric Surgery on Diabetic Retinopathy: Good, Bad, or Both? Diabetes Metab. J. 2016, 40, 354–364. [Google Scholar] [CrossRef]
- Grunwald, J.E.; Brucker, A.J.; Schwartz, S.S.; Braunstein, S.N.; Baker, L.; Petrig, B.L.; Riva, C.E. Diabetic glycemic control and retinal blood flow. Diabetes 1990, 39, 602–607. [Google Scholar] [CrossRef]
- Jingi, A.M.; Tankeu, A.T.; Ateba, N.A.; Noubiap, J.J. Mechanism of worsening diabetic retinopathy with rapid lowering of blood glucose: The synergistic hypothesis. BMC Endocr. Disord. 2017, 17, 63. [Google Scholar]
- Poulaki, V.; Qin, W.; Joussen, A.M.; Hurlbut, P.; Wiegand, S.J.; Rudge, J.; Yancopoulos, G.D.; Adamis, A.P. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxiainducible factor-1alpha and VEGF. J. Clin. Investig. 2002, 109, 805–815. [Google Scholar] [CrossRef]
- Cho, N.H.; Kim, T.H.; Woo, S.J.; Park, K.H.; Lim, S.; Cho, Y.M.; Park, K.S.; Jang, H.C.; Choi, S.H. Optimal HbA1c cutoff for detecting diabetic retinopathy. Acta Diabetol. 2013, 50, 837–842. [Google Scholar] [CrossRef]
- Sharma, S.; Joshi, S.N.; Karki, P. HbA1c as a predictor for response of bevacizumab in diabetic macular oedema. BMJ Open Ophthalmol. 2020, 5, e000449. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Ribeiro, L.; Lobo, C. Phenotypes and biomarkers of diabetic retinopathy. Prog. Retin. Eye Res. 2014, 41, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J. Characterization and relevance of different diabetic retinopathy phenotypes. Dev. Ophthalmol. 2007, 39, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.P.; Madeira, M.H.; Messias, A.L.; Santos, T.; Martinho, A.C.; Figueira, J.; Cunha-Vaz, J. Retinopathy Phenotypes in Type 2 Diabetes with Different Risks for Macular Edema and Proliferative Retinopathy. J. Clin. Med. 2020, 9, 1433. [Google Scholar] [CrossRef] [PubMed]
| Study, Year | No of Patients (Surgical; Medical) | Type of Study | Follow-Up Period (Months) | Baseline No DR/DR | Type of Bariatric Procedure | New Onset of DR | % DR Worsening | %DR Improving | % No Change in DR Stage | Change in HbA1c (%) | Discontinuation of Oral Medication |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Varadhan et al., 2012 [24] | 22 | retrospective | 6–12 | 15/7 | SG, RYGB | 2/15 (13%) | 2/7 (9%) | 2/7 (9%) | 16/22 (73%) | 2.0% (0.3–4.2%) | No info |
| Miras et al., 2012 [25] | 67 | prospective | 12–18 | 39/28 | SG 22.6% RYGB 70.2% GB 7.1% | 0/39 | 1/28(3.6%) | 5/28 (17.8%) | 61/67 (91%) | No info | No info |
| Abbatini et al., 2013 [26] | 33 | prospective | 36–60 | 32/1 | SG | 0/32 | 0/1 | 0/1 | 33/33 | −2.0% | 76.9% |
| Thomas et al., 2014 [27] | 38 | retrospective | 12 | 26/12 | SG 35% RYBP 30% GB 10% BPD 25% | 4/26 (15%) | 3/12 (25%) | 5/12 (42%) | 26/38 (68%) | −1.5% | No info |
| Miras et al., 2015 [28] | 56; 21 | prospective | 12 | 17/39 9/12 | RYGB vs. med | 5/17; 0/9 | 1/39 (2.5%) 3/12 (25%) | 6/39 (15%) 1/12 (8.3%) | 44/56(78%) 17/21(81%) | −3.3% +0.7% | decreased medication by 41% medication vs. increased medication by 27% |
| Kim et al., 2015 [29] | 20 | retrospective | 12–46 | 12/8 | RYGB | 2/12 (16.6%) | 7/8 (87.5%) | 0% | 11/20 (55%) | −2.4% | 6(30%) remission T2DM; 3 cases experienced DR progression |
| Murphy et al., 2015 [30] | 318 | retrospective | 12 | 218/100 | RYGB 30.8% SG 65.7% DS 3.5% | 38/218 (17%) | 12/100 (12%) | 35/100 (35%) | 232/318 (73%) | −3.9% | 18% |
| Brynskov et al., 2016 [31] | 56 | prospective | 12 | 32/24 | RYGB 94% SG 6% | 1/32 (3%) | Any visit: 5/24 (21%) 12 mo: 3/24 (13%) | Any visit: 6/24 (25%) 12 mo: 4/24 (17%) | 49/56 (87%) | −1.7% | 59% |
| Amin et al., 2016 [32] | 152 | Retrospective cohort analysis | 36 | 106/41 | GB 70% RYGB 25% SG 4.6% | 29/106 (27%) | 5/41 (12%) | 5/41 (12%) | 113/152 (74%) | −0.9% | n/a |
| Moren et al., 2018 [33] | 117 | retrospective | 16 | 73/44 | RYGB | 12/73 (12%) | 7/44 (16%) | 8/44 (18%) | 90/117 (77%) | −1.9% | 66% |
| Richardson et al., 2018 [34] | 32 (64 eyes) | prospective | 36 | 47/17 | RYGB | 9/47 (19%) | 3/17 (17%) | 11/17 (64%) | 41/64 (64%) | n/a | n/a |
| Feng et al., 2019 [35] | 40; 36 | Retrospective controlled | 12 | 34/6 29/7 | RYGB Vs med | - | - | 3/34 (8%) vs. 0% | 37/40 (92%) vs. 100% 36/36 (1005) | −1.9% −0.3% | 48 vs. 3% |
| Miras et al., 2019 [36] | 24 | prospective | 60 | n/a | RYGB, SG, GB | 0/24 | 6/24 (25%) | 5/24 (20.8%) | 13/24 (54.2%) | −1.4% | 43% |
| Sever et al., 2020 [37] | 21 (37 eyes) 27 (37 eyes) | Retrospective, comparative | 12 | PDR only | n/a | Increased % of complication in surgical vs. medical group: IOH, NVG, retinal vein occlusion (21.6, 16, 8 vs. 5.4, 2.7, 0) | −1.0% −0.7% | n/a | |||
| Akerblom et al., 2021 [38] | 5321; 5321 | Retrospective database analysis, comparative | 54 | No DR only | RYGB | 188 (3.5%) 317 (5.9%) | - | - | 5133 (94.3%) 5004 (94.1%) | n/a | n/a |
| Adam et al., 2021 [39] | 26 | prospective | 12 | 21/5 (R1) | RYGB-21 (81) SG-5 (19%) | 0% | 0% | 1(20%) | 25/26 (96%) | −1.4% | n/a |
| Study | Sample Size | Proportion (%) | 95% CI | Weight (%) |
|---|---|---|---|---|
| Random | ||||
| Varadhan et al. [24] | 15 | 13.333 | 1.658 to 40.460 | 5.75 |
| Miras et al. [25] | 39 | 0.000 | 0.000 to 9.025 | 7.32 |
| Abbatini et al. [26] | 32 | 0.000 | 0.000 to 10.888 | 7.05 |
| Thomas et al. [27] | 26 | 15.385 | 4.356 to 34.868 | 6.73 |
| Miras et al. [28] | 17 | 29.412 | 10.314 to 55.958 | 5.98 |
| Kim et al. [29] | 12 | 16.667 | 2.086 to 48.414 | 5.31 |
| Murphy et al. [30] | 218 | 17.431 | 12.640 to 23.131 | 8.60 |
| Brynskov et al. [31] | 32 | 3.125 | 0.0791 to 16.217 | 7.05 |
| Amin et al. [32] | 106 | 27.358 | 19.149 to 36.874 | 8.26 |
| Moren et al. [33] | 73 | 16.438 | 8.793 to 26.954 | 7.99 |
| Richardson et al. [34] | 47 | 19.149 | 9.149 to 33.260 | 7.55 |
| Feng et al. [35] | 34 | 0.000 | 0.000 to 10.282 | 7.13 |
| Akerblom et al. [38] | 5321 | 3.533 | 3.053 to 4.065 | 8.93 |
| Adam et al. [39] | 21 | 0.000 | 0.000 to 16.110 | 6.37 |
| Total (random effects) | 5993 | 9.818 | 4.784 to 16.397 | 100.00 |
| Study | Sample Size | Proportion (%) | 95% CI | Weight (%) | |
|---|---|---|---|---|---|
| Random | |||||
| Varadhan et al. [24] | 7 | 28.571 | 3.669 to 70.958 | 4.93 | |
| Miras et al. [25] | 28 | 3.571 | 0.0904 to 18.348 | 7.86 | |
| Abbatini et al. [26] | 1 | 0.000 | 0.000 to 97.500 | 1.94 | |
| Thomas et al. [27] | 12 | 25.000 | 5.486 to 57.186 | 6.14 | |
| Miras et al. [28] | 39 | 2.564 | 0.0649 to 13.476 | 8.38 | |
| Kim et al. [29] | 8 | 87.500 | 47.349 to 99.684 | 5.23 | |
| Murphy et al. [30] | 100 | 12.000 | 6.357 to 20.024 | 9.37 | |
| Brynskov et al. [31] | 24 | 20.833 | 7.132 to 42.151 | 7.58 | |
| Amin et al. [32] | 41 | 12.195 | 4.081 to 26.204 | 8.45 | |
| Moren et al. [33] | 44 | 15.909 | 6.644 to 30.065 | 8.55 | |
| Richardson et al. [34] | 17 | 17.647 | 3.799 to 43.432 | 6.90 | |
| Feng et al. [35] | 6 | 0.000 | 0.000 to 45.926 | 4.59 | |
| Miras et al. [36] | 24 | 25.000 | 9.773 to 46.711 | 7.58 | |
| Sever et al. [37] | 37 | 45.946 | 29.487 to 63.078 | 9.31 | 8.30 |
| Adam et al. [39] | 5 | 0.000 | 0.000 to 52.182 | 1.47 | 4.20 |
| Total (random effects) | 393 | 19.231 | 11.554 to 28.315 | 100.00 | 100.00 |
| Study | Sample Size | Proportion (%) | 95% CI | Weight (%) |
|---|---|---|---|---|
| Random | ||||
| Varadhan et al. [24] | 7 | 28.571 | 3.669 to 70.958 | 5.04 |
| Miras et al. [25] | 28 | 17.857 | 6.064 to 36.893 | 7.81 |
| Abbatini et al. [26] | 1 | 0.000 | 0.000 to 97.500 | 2.04 |
| Thomas et al. [27] | 12 | 41.667 | 15.165 to 72.333 | 6.21 |
| Miras et al. [28] | 39 | 15.385 | 5.862 to 30.528 | 8.29 |
| Kim et al. [29] | 8 | 0.000 | 0.000 to 36.942 | 5.33 |
| Murphy et al. [30] | 100 | 35.000 | 25.729 to 45.185 | 9.19 |
| Brynskov et al. [31] | 24 | 25.000 | 9.773 to 46.711 | 7.56 |
| Amin et al. [32] | 41 | 12.195 | 4.081 to 26.204 | 8.36 |
| Moren et al. [33] | 44 | 18.182 | 8.192 to 32.714 | 8.44 |
| Richardson et al. [34] | 17 | 64.706 | 38.328 to 85.790 | 6.92 |
| Feng et al. [35] | 6 | 50.000 | 11.812 to 88.188 | 4.71 |
| Miras et al. [36] | 24 | 20.833 | 7.132 to 42.151 | 7.56 |
| Sever et al. [37] | 37 | 0.000 | 0.000 to 9.489 | 8.22 |
| Adam et al. [39] | 5 | 20.000 | 0.505 to 71.642 | 4.33 |
| Total (random effects) | 393 | 22.568 | 14.040 to 32.441 | 100.00 |
| Study | Preop SBP Mean ± DS (mmHg) | Postop SBP (Mean ± DS), p-Value | Preop Cholesterol (mg/dL) | Postop Cholesterol (mg/dL), p Value | Preop TG | Postop TG, p Value |
|---|---|---|---|---|---|---|
| Abbatini et al., 2013 [26] | - | % patients with hypertension decreased from 54.5% to 15.1% | - | % patients with hypercholesterolemia decreased from 21 to 9% | - | % patients with hyperTG decreased from 18 to 9% |
| Thomas et al., 2014 [27] | DR progression: 180.3 ± 32.8 DR no change:141.4 ± 17.0 DR regression: 130.5 ± 27.6 | No info | 181.7 (38.7) | 166.3 (p = 0.36) | No info | No info |
| Miras et al., 2015 [28] | 143 ± 2 | 130 ± 3 (p < 0.001) | No info | No info | No info | No info |
| Feng et al., 2019 [35] | 134.0 ± 3.6 | 123.1 ± 2.9 (p < 0.05) complete remission in 14/24 (58%) | 193.35 ± 7.73 | 154.68 ± 7.73, p < 0.001 | 265.7 ± 35.43 | 97.43 ± 8.857 p < 0.001 |
| Miras et al., 2019 [36] | 142 (103–195) | 128 (104–196) p < 0.0001 | Total C: 181.75 HDL-C: 42.54 LDL-C: 100.54 | Total C: 170.15, p = 0.21 HDL-C: 54.14, p < 0.0001 LDL-C: 88.94, p = 0.16 | 159.43 (53.14–655.4) | 115.15 (35.43–389.7), p < 0.0001 |
| Adam et al., 2021 [39] | 134 ± 15 | 119 ± 15, p < 0.001 | Total C: 144 ± 28.6 HDL-C: 33.2 (29.7–39.0) LDL-C: 81.9 ± 23.9 | Total C: 162 ± 36.7, p = 0.035 HDL-C: 44.0 (38.6–50.6), p < 0.001 LDL-C: 93.8 ± 35.1, p = 0.38 | 134 (81.4–165) | 100 (77.0–132), p = 0.071 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dascalu, A.M.; Stoian, A.P.; Cherecheanu, A.P.; Serban, D.; Costea, D.O.; Tudosie, M.S.; Stana, D.; Tanasescu, D.; Sabau, A.D.; Gangura, G.A.; et al. Outcomes of Diabetic Retinopathy Post-Bariatric Surgery in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2021, 10, 3736. https://doi.org/10.3390/jcm10163736
Dascalu AM, Stoian AP, Cherecheanu AP, Serban D, Costea DO, Tudosie MS, Stana D, Tanasescu D, Sabau AD, Gangura GA, et al. Outcomes of Diabetic Retinopathy Post-Bariatric Surgery in Patients with Type 2 Diabetes Mellitus. Journal of Clinical Medicine. 2021; 10(16):3736. https://doi.org/10.3390/jcm10163736
Chicago/Turabian StyleDascalu, Ana Maria, Anca Pantea Stoian, Alina Popa Cherecheanu, Dragos Serban, Daniel Ovidiu Costea, Mihail Silviu Tudosie, Daniela Stana, Denisa Tanasescu, Alexandru Dan Sabau, Gabriel Andrei Gangura, and et al. 2021. "Outcomes of Diabetic Retinopathy Post-Bariatric Surgery in Patients with Type 2 Diabetes Mellitus" Journal of Clinical Medicine 10, no. 16: 3736. https://doi.org/10.3390/jcm10163736
APA StyleDascalu, A. M., Stoian, A. P., Cherecheanu, A. P., Serban, D., Costea, D. O., Tudosie, M. S., Stana, D., Tanasescu, D., Sabau, A. D., Gangura, G. A., Costea, A. C., Nicolae, V. A., & Smarandache, C. G. (2021). Outcomes of Diabetic Retinopathy Post-Bariatric Surgery in Patients with Type 2 Diabetes Mellitus. Journal of Clinical Medicine, 10(16), 3736. https://doi.org/10.3390/jcm10163736








