Imaging Tests in the Early Diagnosis of Giant Cell Arteritis
Abstract
:1. Introduction
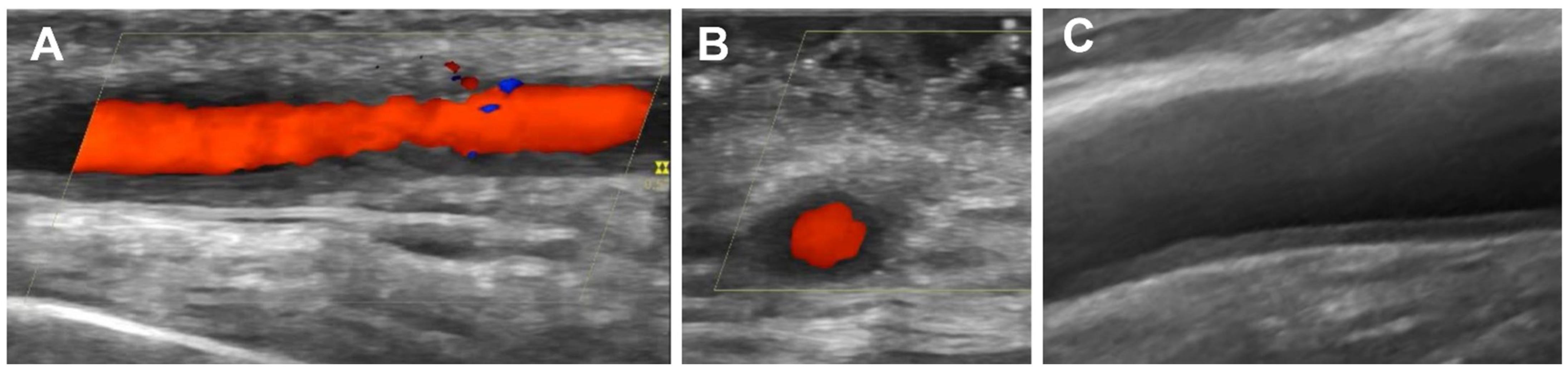
2. Ultrasound for GCA Diagnosis
3. Magnetic Resonance Imaging for GCA Diagnosis
4. Computed Tomography Angiography for GCA Diagnosis
5. 18F-FDG (Fluorodeoxyglucose)-Positron Emission Tomography (PET)/Computed Tomography (CT) for GCA Diagnosis
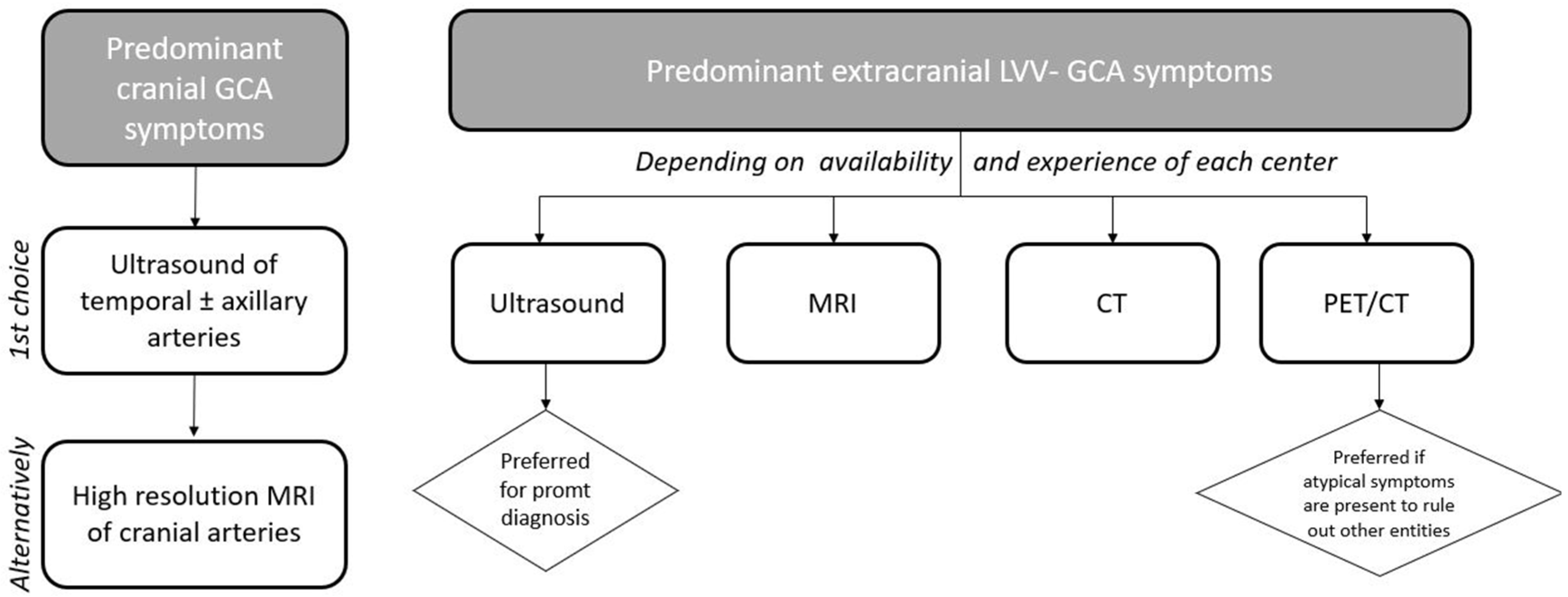
6. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| GCA | giant cell arteritis |
| IMT | intima-media thickness |
| MRI | magnetic resonance imaging |
| PET | positron emission tomography |
| LVV | large vessel vasculitis |
| US | ultrasound |
References
- González, M.A.; Vazquez-Rodriguez, T.R.; Lopez-Diaz, M.J. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum 2009, 61, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mohammad, A.J.; Turesson, C. Incidence and prevalence of giant cell arteritis and polymyalgia rheumatica: A systematic literature review. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 2020; Volume 50, pp. 1040–1048. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A. The diagnosis and management of patients with giant cell arteritis. J. Rheumatol. 2005, 32, 1186–1188. [Google Scholar] [PubMed]
- Gonzalez-Gay, M.A.; Barros, S.; Lopez-Diaz, M.J.; Garcia-Porrua, C.; Sanchez-Andrade, A.; Llorca, J. Giant cell arteritis: Disease patterns of clinical presentation in a series of 240 patients. Medicine 2005, 84, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gay, M.A.; Castañeda, S.; Llorca, J. Giant Cell Arteritis: Visual Loss Is Our Major Concern. J. Rheumatol. 2016, 43, 1458–1461. [Google Scholar] [CrossRef] [Green Version]
- Arend, W.P.; Michel, B.A.; Bloch, D.A.; Hunder, G.G.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; Lightfoot, R.W., Jr.; et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990, 33, 1129–1134. [Google Scholar] [CrossRef]
- Quinn, K.A.; Grayson, P.C. The Role of Vascular Imaging to Advance Clinical Care and Research in Large-Vessel Vasculitis. Curr. Treat. Options Rheumatol. 2019, 5, 20–35. [Google Scholar] [CrossRef]
- Duftner, C.; Dejaco, C.; Sepriano, A.; Falzon, L.; Schmidt, W.A.; Ramiro, S. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: A systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open 2018, 4, e000612. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, V.S.; Jin, L.; Schmidt, W.A. Imaging for Diagnosis, Monitoring, and Outcome Prediction of Large Vessel Vasculitides. Curr. Rheumatol. Rep. 2020, 22, 76. [Google Scholar] [CrossRef]
- González-Gay, M.A.; Prieto-Peña, D.; Martínez-Rodríguez, I.; Calderon-Goercke, M.; Banzo, I.; Blanco, R.; Castañeda, S. Early large vessel systemic vasculitis in adults. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101424. [Google Scholar] [CrossRef]
- Van Der Geest, K.S.; Sandovici, M.; van Sleen, Y.; Sanders, J.S.; Bos, N.A.; Abdulahad, W.H.; Stegeman, C.A.; Heeringa, P.; Rutgers, A.; Kallenberg, C.G.; et al. Review: What Is the Current Evidence for Disease Subsets in Giant Cell Arteritis? Arthritis Rheumatol. 2018, 70, 1366–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejaco, C.; Duftner, C.; Buttgereit, F.; Matteson, E.L.; Dasgupta, B. The spectrum of giant cell arteritis and polymyalgia rheumatica: Revisiting the concept of the disease. Rheumatology 2017, 56, 506–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Gay, M.A.; Prieto-Peña, D.; Calderón-Goercke, M.; Atienza-Mateo, B.; Castañeda, S. Giant cell arteritis: More than a cranial disease. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S124), 15–17. [Google Scholar] [PubMed]
- Emamifar, A.; Ellingsen, T.; Hess, S.; Gerke, O.; Hviid Larsen, R.; Ahangarani Farahani, Z.; Syrak Hansen, P.; Jensen Hansen, I.M.; Petersen, H.; Marcussen, N.; et al. The Utility of 18F-FDG PET/CT in Patients with Clinical Suspicion of Polymyalgia Rheumatica and Giant Cell Arteritis: A Prospective, Observational, and Cross-sectional Study. ACR Open Rheumatol. 2020, 2, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Peña, D.; Martínez-Rodríguez, I.; Loricera, J.; Banzo, I.; Calderón-Goercke, M.; Calvo-Río, V.; González-Vela, C.; Corrales, A.; Castañeda, S.; Blanco, R.; et al. Predictors of positive 18F-FDG PET/CT-scan for large vessel vasculitis in patients with persistent polymyalgia rheumatica. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 2019; Volume 48, pp. 720–727. [Google Scholar] [CrossRef] [Green Version]
- Dejaco, C.; Ramiro, S.; Duftner, C.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Brouwer, E.; Cimmino, M.A.; Clark, E.; Dasgupta, B.; et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann. Rheum. Dis. 2018, 77, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.A.; Kraft, H.E.; Vorpahl, K.; Völker, L.; Gromnica-Ihle, E.J. Color duplex ultrasonography in the diagnosis of temporal arteritis. N. Engl. J. Med. 1997, 337, 1336–1342. [Google Scholar] [CrossRef]
- Karassa, F.B.; Matsagas, M.I.; Schmidt, W.A.; Ioannidis, J.P.A. Meta-analysis: Test performance of ultrasonography for giant-cell arteritis. Ann. Intern. Med. 2005, 142, 359–369. [Google Scholar] [CrossRef]
- De Miguel, E.; Castillo, C.; Rodríguez, A.; De Agustín, J.J.; Working Group Ultrasound Giant Cell Arteritis. Learning and reliability of colour Doppler ultrasound in giant cell arteritis. Clin. Exp. Rheumatol. 2009, 27, S53–S58. [Google Scholar]
- Vicente-Rabaneda, E.F.; Acebes, C.; Castañeda, S. Usefulness of extra-articular ultrasound applied to systemic inflammatory diseases in clinical practice. Reumatol. Clin. (Engl. Ed.) 2021, 17, 229–236, (In English and Spanish). [Google Scholar] [CrossRef]
- Arida, A.; Kyprianou, M.; Kanakis, M.; Sfikakis, P.P. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: A second meta-analysis. BMC Musculoskelet. Disord. 2010, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Buttgereit, F.; Dejaco, C.; Matteson, E.L.; Dasgupta, B. Polymyalgia Rheumatica and Giant Cell Arteritis: A Systematic Review. JAMA 2016, 315, 2442–2458. [Google Scholar] [CrossRef]
- Luqmani, R.; Lee, E.; Singh, S.; Gillett, M.; Schmidt, W.A.; Bradburn, M.; Dasgupta, B.; Diamantopoulos, A.P.; Forrester-Barker, W.; Hamilton, W.; et al. The Role of Ultrasound Compared to Biopsy of Temporal Arteries in the Diagnosis and Treatment of Giant Cell Arteritis (TABUL): A diagnostic accuracy and cost-effectiveness study. Health Technol. Assess. (Winch. Engl.) 2016, 20, 1–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranda-Valera, I.C.; García Carazo, S.; Monjo Henry, I.; De Miguel Mendieta, E. Diagnostic validity of Doppler ultrasound in giant cell arteritis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. S103), 123–127. [Google Scholar]
- Schmidt, W.A. Ultrasound in the diagnosis and management of giant cell arteritis. Rheumatology 2018, 57, ii22–ii31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrysidis, S.; Duftner, C.; Dejaco, C.; Schäfer, V.S.; Ramiro, S.; Carrara, G.; Scirè, C.A.; Hocevar, A.; Diamantopoulos, A.P.; Iagnocco, A.; et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: A study from the OMERACT Large Vessel Vasculitis Ultrasound Working Group. RMD Open 2018, 4, e000598. [Google Scholar] [CrossRef] [Green Version]
- Kopterides, P.; Moyssakis, I.; Margos, P.; Sipsas, N.V. Echocardiographic findings in patients with temporal arteritis: Apropos of one case of temporal arteritis-associated verrucous (Libman-Sachs) endocarditis. Clin. Exp. Rheumatol. 2006, 24, S35–S37. [Google Scholar]
- Aschwanden, M.; Daikeler, T.; Kesten, F.; Baldi, T.; Benz, D.; Tyndall, A.; Imfeld, S.; Staub, D.; Hess, C.; Jaeger, K.A. Temporal artery compression sign—A novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall Med.-Eur. J. Ultrasound 2013, 34, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, V.S.; Juche, A.; Ramiro, S.; Krause, A.; Schmidt, W.A. Ultrasound cut-off values for intima-media thickness of temporal, facial and axillary arteries in giant cell arteritis. Rheumatology 2017, 56, 1479–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Geest, K.S.; Borg, F.; Kayani, A.; Paap, D.; Gondo, P.; Schmidt, W.; Luqmani, R.A.; Dasgupta, B. Novel ultrasonographic Halo Score for giant cell arteritis: Assessment of diagnostic accuracy and association with ocular ischaemia. Ann. Rheum. Dis. 2020, 79, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, A.; van der Geest, K.S.; Coath, F.; Gondo, P.; Kayani, A.; Mackerness, C.; Hadebe, B.; Innes, S.; Jackson, J.; Dasgupta, B. Halo score (temporal artery, its branches and axillary artery) as a diagnostic, prognostic and disease monitoring tool for Giant Cell Arteritis (GCA). BMC Rheumatol. 2020, 4, 35. [Google Scholar] [CrossRef]
- Czihal, M.; Schröttle, A.; Baustel, K.; Lottspeich, C.; Dechant, C.; Treitl, K.M.; Treitl, M.; Schulze-Koops, H.; Hoffmann, U. B-mode sonography wall thickness assessment of the temporal and axillary arteries for the diagnosis of giant cell arteritis: A cohort study. Clin. Exp. Rheumatol. 2017, 35, 128–133. [Google Scholar]
- Czihal, M.; Köhler, A.; Lottspeich, C.; Prearo, I.; Hoffmann, U.; Schulze-Koops, H.; Priglinger, S.G.; Mackert, M.J.; Dechant, C. Temporal artery compression sonography for the diagnosis of giant cell arteritis in elderly patients with acute ocular arterial occlusions. Rheumatology 2021, 60, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, E.; Beltran, L.M.; Monjo, I.; Deodati, F.; Schmidt, W.A.; Garcia-Puig, J. Atherosclerosis as a potential pitfall in the diagnosis of giant cell arteritis. Rheumatology 2018, 57, 318–321. [Google Scholar] [CrossRef] [Green Version]
- Diamantopoulos, A.P.; Haugeberg, G.; Lindland, A.; Myklebust, G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: Towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology 2016, 55, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Patil, P.; Williams, M.; Maw, W.W.; Achilleos, K.; Elsideeg, S.; Dejaco, C.; Borg, F.; Gupta, S.; Dasgupta, B. Fast track pathway reduces sight loss in giant cell arteritis: Results of a longitudinal observational cohort study. Clin. Exp. Rheumatol. 2015, 33, S103–S106. [Google Scholar]
- Bley, T.A.; Uhl, M.; Carew, J.; Markl, M.; Schmidt, D.; Peter, H.H.; Langer, M.; Wieben, O. Diagnostic value of high-resolution MR imaging in giant cell arteritis. AJNR Am. J. Neuroradiol. 2007, 28, 1722–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, J.; Bley, T.; Uhl, M.; Frydrychowicz, A.; Langer, M.; Markl, M. Diagnostic value of T2-weighted imaging for the detection of superficial cranial artery inflammation in giant cell arteritis. J. Magn. Reson. Imaging 2010, 31, 470–474. [Google Scholar] [CrossRef]
- Klink, T.; Geiger, J.; Both, M.; Ness, T.; Heinzelmann, S.; Reinhard, M.; Holl-Ulrich, K.; Duwendag, D.; Vaith, P.; Bley, T.A. Giant cell arteritis: Diagnostic accuracy of MR imaging of superficial cranial arteries in initial diagnosis-results from a multicenter trial. Radiology 2014, 273, 844–852. [Google Scholar] [CrossRef]
- Klink, T.; Geiger, J.; Both, M.; Ness, T.; Heinzelmann, S.; Reinhard, M.; Holl-Ulrich, K.; Duwendag, D.; Vaith, P.; Bley, T.A. High-Resolution Magnetic Resonance Imaging of Scalp Arteries for the Diagnosis of Giant Cell Arteritis: Results of a Prospective Cohort Study. Arthritis Rheumatol. 2017, 69, 161–168. [Google Scholar] [CrossRef]
- Yip, A.; Jernberg, E.T.; Bardi, M.; Geiger, J.; Lohne, F.; Schmidt, W.A.; Myklebust, G.; Diamantopoulos, A.P. Magnetic resonance imaging compared to ultrasonography in giant cell arteritis: A cross-sectional study. Arthritis Res. Ther. 2020, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Sommer, N.N.; Treitl, K.M.; Coppenrath, E.; Kooijman, H.; Dechant, C.; Czihal, M.; Kolben, T.M.; Beyer, S.E.; Sommer, W.H.; Saam, T. Three-Dimensional High-Resolution Black-Blood Magnetic Resonance Imaging for Detection of Arteritic Anterior Ischemic Optic Neuropathy in Patients With Giant Cell Arteritis. Investig. Radiol. 2018, 53, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Mournet, S.; Sené, T.; Charbonneau, F.; Poillon, G.; Vignal, C.; Clavel, G.; Zuber, K.; Savatovsky, J.; Lecler, A. High-resolution MRI demonstrates signal abnormalities of the 3rd cranial nerve in giant cell arteritis patients with 3rd cranial nerve impairment. Eur. Radiol. 2021, 31, 4472–4480. [Google Scholar] [CrossRef]
- Lariviere, D.; Benali, K.; Coustet, B.; Pasi, N.; Hyafil, F.; Klein, I.; Chauchard, M.; Alexandra, J.F.; Goulenok, T.; Dossier, A.; et al. Positron emission tomography and computed tomography angiography for the diagnosis of giant cell arteritis: A real-life prospective study. Medicine 2016, 95, e4146. [Google Scholar] [CrossRef]
- De Boysson, H.; Dumont, A.; Liozon, E.; Lambert, M.; Boutemy, J.; Maigné, G.; Silva, N.M.; Sultan, A.; Ly, K.H.; Aide, N.; et al. Giant-cell arteritis: Concordance study between aortic CT angiography and FDG-PET/CT in detection of large-vessel involvement. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Hommada, M.; Mekinian, A.; Brillet, P.Y.; Abad, S.; Larroche, C.; Dhôte, R.; Fain, O.; Soussan, M. Aortitis in giant cell arteritis: Diagnosis with FDG PET/CT and agreement with CT angiography. Autoimmun. Rev. 2017, 16, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Vaidyanathan, S.; Chattopadhyay, A.; Mackie, S.L.; Scarsbrook, A.F. Comparative effectiveness of 18F-FDG PET-CT and contrast-enhanced CT in the diagnosis of suspected large-vessel vasculitis. Br. J. Radiol. 2018, 91, 20180247. [Google Scholar] [CrossRef] [Green Version]
- Solanes, M.M.; Magarolas, M.A.; Miramon, J.M.; Aróztegui, A.C.; Jiménez, M.M.; Morera, J.O.; Martín, C.D.; Revuelto, A.R.; Ferrer, Z.B.; Roqueta, L.B. Comparative study of 18F-FDG PET/CT and CT angiography in detection of large vessel vasculitis. Rev. Española Med. Nucl. Imagen Mol. (Engl. Ed.) 2019, 38, 280–289. [Google Scholar] [CrossRef]
- Riemer, H.J.A.S.; Writing Group; Reviewer Group; Members of EANM Cardiovascular; Members of EANM Infection & Inflammation; Members of Committees; SNMMI Cardiovascular; Members of Council; PET Interest Group; Members of ASNC; et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: Joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1250–1269. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Rodríguez, I.; Del Castillo-Matos, R.; Quirce, R.; Banzo, I.; Jiménez-Bonilla, J.; Martínez-Amador, N.; Ibáñez-Bravo, S.; Lavado-Pérez, C.; Bravo-Ferrer, Z.; Carril, J.M. Aortic 18F-FDG PET/CT uptake pattern at 60 min (early) and 180 min (delayed) acquisition in a control population: A visual and semiquantitative comparative analysis. Nucl. Med. Commun. 2013, 34, 926–930. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, I.; Martínez-Amador, N.; Banzo, I.; Quirce, R.; Jiménez-Bonilla, J.; De Arcocha-Torres, M.; Ibáñez-Bravo, S.; Lavado-Pérez, C.; Bravo-Ferrer, Z.; Blanco, R.; et al. Assessment of aortitis by semiquantitative analysis of 180-min 18F-FDG PET/CT acquisition images. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2319–2324. [Google Scholar] [CrossRef]
- Rosenblum, J.S.; Quinn, K.A.; Rimland, C.A.; Mehta, N.N.; Ahlman, M.A.; Grayson, P.C. Clinical Factors Associated with Time-Specific Distribution of 18F-Fluorodeoxyglucose in Large-Vessel Vasculitis. Sci. Rep. 2019, 9, 15180. [Google Scholar] [CrossRef]
- Besson, F.L.; Parienti, J.J.; Bienvenu, B.; Prior, J.O.; Costo, S.; Bouvard, G.; Agostini, D. Diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1764–1772. [Google Scholar] [CrossRef]
- Soussan, M.; Nicolas, P.; Schramm, C.; Katsahian, S.; Pop, G.; Fain, O.; Mekinian, A. Management of large-vessel vasculitis with FDG-PET: A systematic literature review and meta-analysis. Medicine 2015, 94, e622. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Diagnostic accuracy of 18F-FDG PET or PET/CT for large vessel vasculitis: A meta-analysis. Z. Rheumatol. 2016, 75, 924–931. [Google Scholar] [CrossRef]
- Dunphy, M.P.; Freiman, A.; Larson, S.M.; Strauss, H.W. Association of vascular 18F-FDG uptake with vascular calcification. J. Nucl. Med. 2005, 46, 1278–1284. [Google Scholar]
- Ben-Haim, S.; Kupzov, E.; Tamir, A.; Israel, O. Evaluation of 18F-FDG uptake and arterial wall calcifications using 18F-FDG PET/CT. J. Nucl. Med. 2004, 45, 1816–1821. [Google Scholar] [PubMed]
- Nielsen, B.D.; Hansen, I.T.; Kramer, S.; Haraldsen, A.; Hjorthaug, K.; Bogsrud, T.V.; Ejlersen, J.A.; Stolle, L.B.; Keller, K.K.; Therkildsen, P.; et al. Simple dichotomous assessment of cranial artery inflammation by conventional 18F-FDG PET/CT shows high accuracy for the diagnosis of giant cell arteritis: A case-control study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Sammel, A.M.; Hsiao, E.; Schembri, G.; Nguyen, K.; Brewer, J.; Schrieber, L.; Janssen, B.; Youssef, P.; Fraser, C.L.; Bailey, E.; et al. Diagnostic Accuracy of Positron Emission Tomography/Computed Tomography of the Head, Neck, and Chest for Giant Cell Arteritis: A Prospective, Double-Blind, Cross-Sectional Study. Arthritis Rheumatol. 2019, 71, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.D.; Gormsen, L.C.; Hansen, I.T.; Keller, K.K.; Therkildsen, P.; Hauge, E.-M. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Nienhuis, P.H.; van Praagh, G.D.; Glaudemans, A.W.J.M.; Brouwer, E.; Slart, R.H.J.A. A Review on the Value of Imaging in Differentiating between Large Vessel Vasculitis and Atherosclerosis. J. Pers. Med. 2021, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, M.D.; Brouwer, E.; Lensen, K.J.D.; Rutgers, A.; Arends, S.; Van Der Geest, K.S.; Glaudemans, A.W.; Slart, R.H. Different Scoring Methods of FDG PET/CT in Giant Cell Arteritis: Need for Standardization. Medicine 2015, 94, e1542. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gay, M.A.; Garcia-Porrua, C.; Miranda-Filloy, J.A. Giant cell arteritis: Diagnosis and therapeutic management. Curr. Rheumatol. Rep. 2006, 8, 299–302. [Google Scholar] [CrossRef] [PubMed]
- González-Gay, M.A.; Matteson, E.L.; Castañeda, S. Polymyalgia rheumatica. Lancet 2017, 390, 1700–1712. [Google Scholar] [CrossRef]
- Czihal, M.; Lottspeich, C.; Bernau, C.; Henke, T.; Prearo, I.; Mackert, M.; Priglinger, S.; Dechant, C.; Schulze-Koops, H.; Hoffmann, U. A Diagnostic Algorithm Based on a Simple Clinical Prediction Rule for the Diagnosis of Cranial Giant Cell Arteritis. J. Clin. Med. 2021, 10, 1163. [Google Scholar] [CrossRef]
- Sebastian, A.; Tomelleri, A.; Kayani, A.; Prieto-Pena, D.; Ranasinghe, C.; Dasgupta, B. Probability-based algorithm using ultrasound and additional tests for suspected GCA in a fast-track clinic. RMD Open 2020, 6, e001297. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto-Peña, D.; Castañeda, S.; Martínez-Rodríguez, I.; Atienza-Mateo, B.; Blanco, R.; González-Gay, M.A. Imaging Tests in the Early Diagnosis of Giant Cell Arteritis. J. Clin. Med. 2021, 10, 3704. https://doi.org/10.3390/jcm10163704
Prieto-Peña D, Castañeda S, Martínez-Rodríguez I, Atienza-Mateo B, Blanco R, González-Gay MA. Imaging Tests in the Early Diagnosis of Giant Cell Arteritis. Journal of Clinical Medicine. 2021; 10(16):3704. https://doi.org/10.3390/jcm10163704
Chicago/Turabian StylePrieto-Peña, Diana, Santos Castañeda, Isabel Martínez-Rodríguez, Belén Atienza-Mateo, Ricardo Blanco, and Miguel A. González-Gay. 2021. "Imaging Tests in the Early Diagnosis of Giant Cell Arteritis" Journal of Clinical Medicine 10, no. 16: 3704. https://doi.org/10.3390/jcm10163704
APA StylePrieto-Peña, D., Castañeda, S., Martínez-Rodríguez, I., Atienza-Mateo, B., Blanco, R., & González-Gay, M. A. (2021). Imaging Tests in the Early Diagnosis of Giant Cell Arteritis. Journal of Clinical Medicine, 10(16), 3704. https://doi.org/10.3390/jcm10163704







