Low Preoperative Mean Platelet Volume/Platelet Count Ratio Indicates Worse Prognosis in Non-Metastatic Renal Cell Carcinoma
Abstract
:1. Introduction
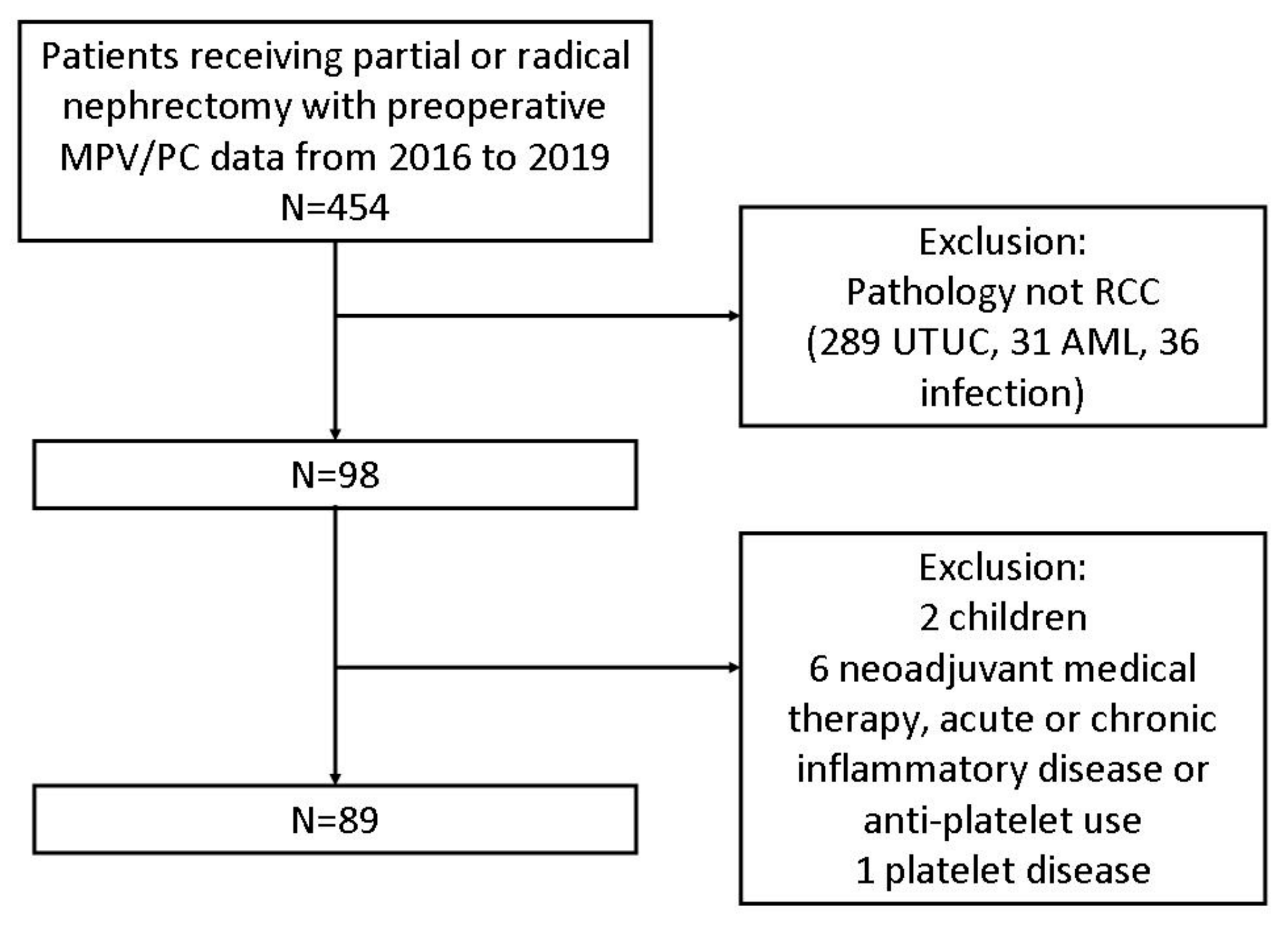
2. Materials and Methods
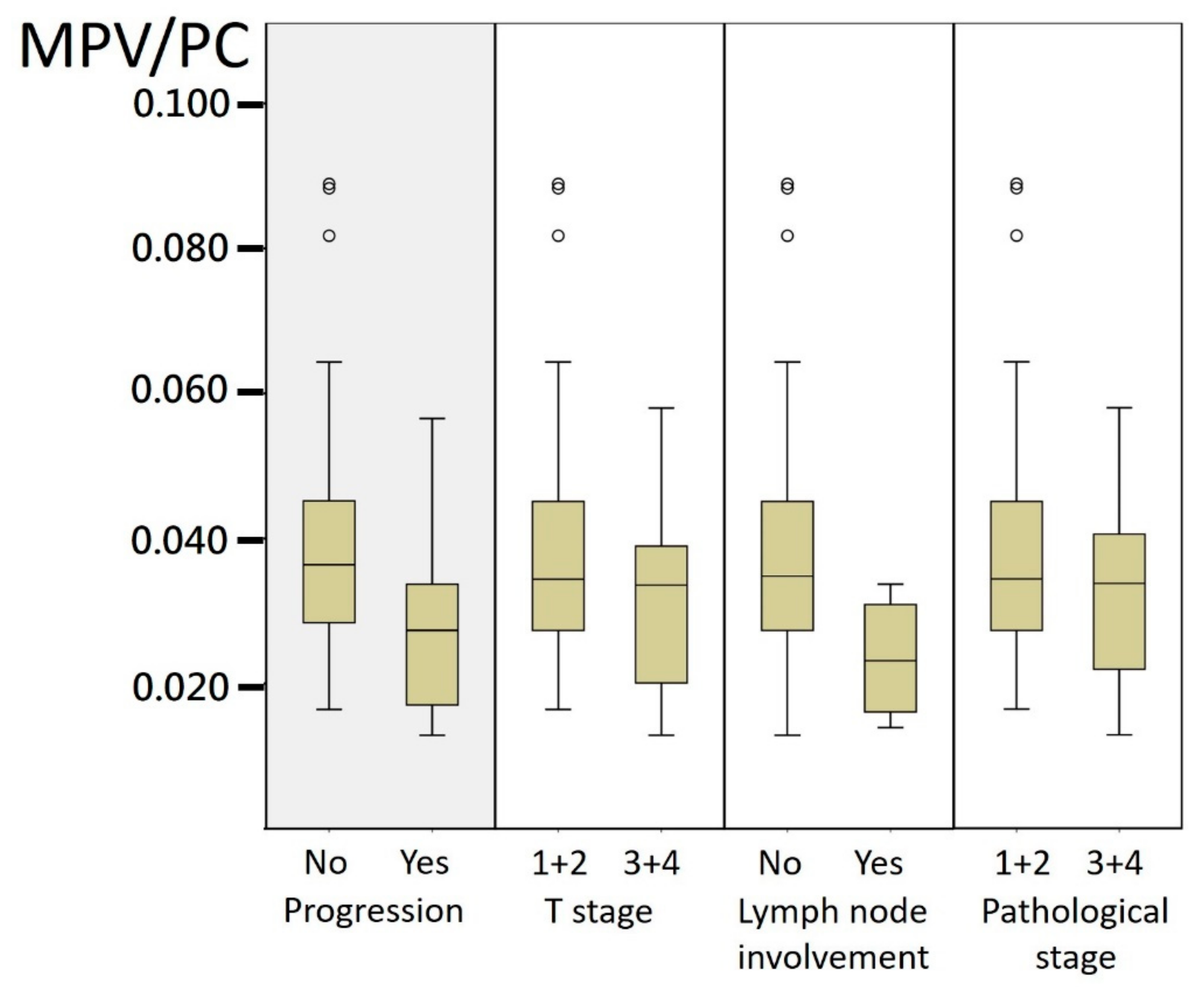
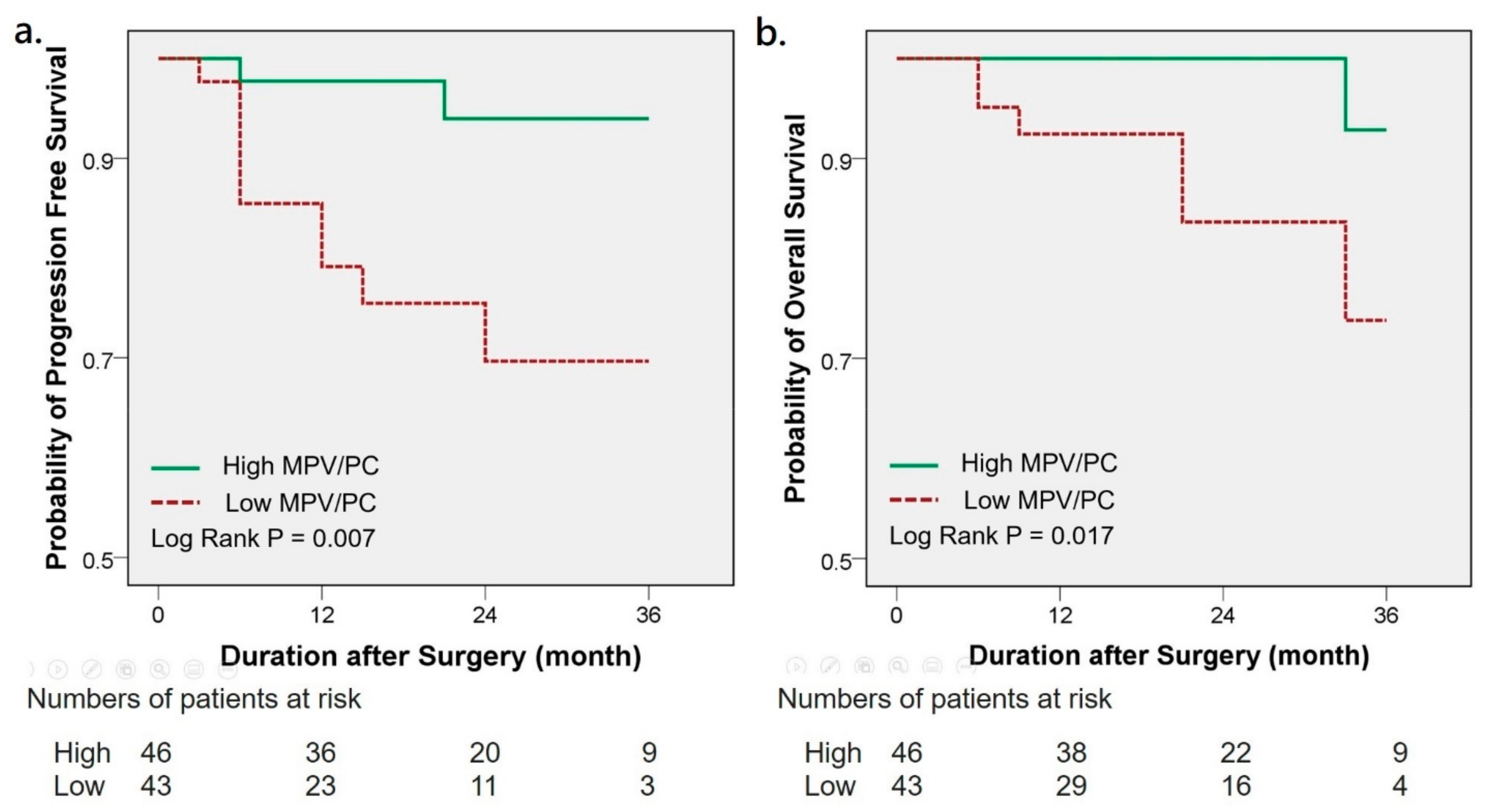
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.-H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Goubran, H.A.; Stakiw, J.; Radosevic, M.; Burnouf, T. Platelet-cancer interactions. Semin. Thromb. Hemost. 2014, 40, 296–305. [Google Scholar] [PubMed]
- Bai, Y.Y.; Du, L.; Jing, L.; Tian, T.; Liang, X.; Jiao, M.; Nan, K.-J.; Guo, H.; Ruan, Z.-P. Clinicopathological and prognostic significance of pretreatment thrombocytosis in patients with endometrial cancer: A meta-analysis. Cancer Manag. Res. 2019, 11, 4283–4295. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Deng, S.J.; Yang, Y.D.; Yao, N.; Zhao, J.M.; Min, G.T.; Wang, J.; Xu, T.-F.; Zhao, P.-Y.; Wang, H.-P.; et al. The pretreatment thrombocytosis may predict prognosis of patients with colorectal cancer: A systematic review and meta-analysis. Biomark. Med. 2017, 11, 195–210. [Google Scholar] [CrossRef]
- Gucer, F.; Moser, F.; Tamussino, K.; Reich, O.; Haas, J.; Arikan, G.; Petru, E.; Winter, R. Thrombocytosis as a prognostic factor in endometrial carcinoma. Gynecol. Oncol. 1998, 70, 210–214. [Google Scholar] [CrossRef]
- Cohen, J.G.; Tran, A.Q.; Rimel, B.J.; Cass, I.; Walsh, C.S.; Karlan, B.Y.; Li, A.J. Thrombocytosis at secondary cytoreduction for recurrent ovarian cancer predicts suboptimal resection and poor survival. Gynecol. Oncol. 2014, 132, 556–559. [Google Scholar] [CrossRef]
- Avecilla, S.T.; Hattori, K.; Heissig, B.; Tejada, R.; Liao, F.; Shido, K.; Jin, D.K.; Dias, S.; Zhang, F.; Hartman, T.; et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 2004, 10, 64–71. [Google Scholar] [CrossRef]
- Kaser, A.; Brandacher, G.; Steurer, W.; Kaser, S.; Offner, F.A.; Zoller, H.; Theurl, I.; Widder, W.; Molnar, C.; Ludwiczek, O.; et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: Role in inflammatory thrombocytosis. Blood 2001, 98, 2720–2725. [Google Scholar] [CrossRef] [Green Version]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupaimoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Seretis, C.; Youssef, H.; Chapman, M. Hypercoagulation in colorectal cancer: What can platelet indices tell us? Platelets 2015, 26, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Seles, M.; Posch, F.; Pichler, G.P.; Gary, T.; Pummer, K.; Zigeuner, R.; Hutterer, G.C.; Pichler, M. Blood Platelet Volume Represents a Novel Prognostic Factor in Patients with Nonmetastatic Renal Cell Carcinoma and Improves the Predictive Ability of Established Prognostic Scores. J. Urol. 2017, 198, 1247–1252. [Google Scholar] [CrossRef]
- Prokopowicz, G.; Życzkowski, M.; Nowakowski, K.; Bogacki, R.; Bryniarski, P.; Paradysz, A. Basic parameters of blood count as prognostic factors for renal cell carcinoma. BioMed Res. Int. 2016, 2016, 8687575. [Google Scholar] [CrossRef]
- Tuncel, T.; Ozgun, A.; Emirzeoglu, L.; Celik, S.; Bilgi, O.; Karagoz, B. Mean platelet volume as a prognostic marker in metastatic colorectal cancer patients treated with bevacizumab-combined chemotherapy. Asian Pac. J. Cancer Prev. 2014, 15, 6421–6423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, S.; Tokuno, J.; Ueda, Y.; Marumo, S.; Shoji, T.; Nishimura, T.; Fukui, M.; Huang, C.-L. Prognostic significance of preoperative mean platelet volume in resected non-small-cell lung cancer. Mol. Clin. Oncol. 2015, 3, 197–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kılınçalp, S.; Ekiz, F.; Başar, Ö.; Ayte, M.R.; Çoban, Ş.; Yılmaz, B.; Altınbaş, A.; Basar, N.; Aktas, B.; Tuna, Y.; et al. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets 2014, 25, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, Z.; Wang, P.; Hu, X.; Gao, Y.; He, J. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol. 2016, 37, 9323–9331. [Google Scholar] [CrossRef] [Green Version]
- Yun, Z.Y.; Zhang, X.; Liu, Y.S.; Liu, T.; Liu, Z.P.; Wang, R.T.; Yu, K.-J. Lower mean platelet volume predicts poor prognosis in renal cell carcinoma. Sci. Rep. 2017, 7, 6700. [Google Scholar] [CrossRef] [Green Version]
- Gu, M.; Zhai, Z.; Huang, L.; Zheng, W.; Zhou, Y.; Zhu, R.; Shen, F.; Yuan, C. Pre-treatment mean platelet volume associates with worse clinicopathologic features and prognosis of patients with invasive breast cancer. Breast Cancer 2016, 23, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Biricik, S.; Narci, H.; Dundar, G.A.; Ayrik, C.; Turkmenoglu, M.O. Mean platelet volume and the ratio of mean platelet volume to platelet count in the diagnosis of acute appendicitis. Am. J. Emerg. Med. 2019, 37, 411–414. [Google Scholar] [CrossRef]
- Inagaki, N.; Kibata, K.; Tamaki, T.; Shimizu, T.; Nomura, S. Prognostic impact of the mean platelet volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer 2014, 83, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Qin, Y.Y.; Chen, M.; Wu, Y.Y.; Lin, F.Q. Combined Use of Mean Platelet Volume/Platelet Count Ratio and Platelet Distribution Width to Distinguish Between Patients with Nasopharyngeal Carcinoma, Those with Benign Tumors of the Nasopharynx, and Healthy Subjects. Cancer Manag. Res. 2019, 11, 10375–10382. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Brummel-Ziedins, K.E.; Bouchard, B.A.; Holmes, C.E. Platelets in tumor progression: A host factor that offers multiple potential targets in the treatment of cancer. J. Cell Physiol. 2014, 229, 1005–1015. [Google Scholar] [CrossRef]
- Schlesinger, M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 2018, 11, 125. [Google Scholar] [CrossRef]
- Yan, M.; Jurasz, P. The role of platelets in the tumor microenvironment: From solid tumors to leukemia. Biochim. Biophys. Acta 2016, 1863, 392–400. [Google Scholar] [CrossRef]
- Guo, Y.; Cui, W.; Pei, Y.; Xu, D. Platelets promote invasion and induce epithelial to mesenchymal transition in ovarian cancer cells by TGF-beta signaling pathway. Gynecol. Oncol. 2019, 153, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Sierko, E.; Hempel, D.; Tucker, S.C.; Honn, K.V. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017, 36, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Kaushansky, K. Growth factors and hematopoietic cell fate. A new feature: Controversies in hematology. Blood 1998, 92, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.Y.; Ayvazyan, L.; Mikhailidis, D.P.; Kitas, G.D. Mean platelet volume: A link between thrombosis and inflammation? Curr. Pharm. Des. 2011, 17, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Aktas, G.; Kocak, M.; Duman, T.; Erkus, E.; Atak, B.; Sit, M.; Savli, H. Mean Platelet Volume (MPV) as an inflammatory marker in type 2 diabetes mellitus and obesity. Bali Med. J. 2018, 7, 650–953. [Google Scholar] [CrossRef]
- Karagoz, I.; Aktas, G.; Yoldas, H.; Yildiz, I.; Ogun, M.N.; Bilgi, M.; Demirhan, A. Association Between Hemogram Parameters and Survival of Critically Ill Patients. J. Intensive Care Med. 2019, 34, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Aktas, G.; Sit, M.; Tekce, H.; Alcelik, A.; Savli, H.; Simsek, T.; Ozmen, E.; Isci, A.; Apuhan, T. Mean platelet volume in nasal polyps. West Indian Med. J. 2013, 62, 515–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.Y.; Yang, J.J.; You, E.; Kim, B.H.; Shim, J.; Lee, H.J.; Lee, W.-I.; Suh, J.-T.; Park, T.S. Mean platelet volume/platelet count ratio in hepatocellular carcinoma. Platelets 2013, 24, 375–377. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Wu, X.; Lee, J.; Tan, M.; Hagenbeek, T.; Qu, X.; Yu, L.; Ross, J.; Korsisaari, N.; Cao, T.; et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 21248–21255. [Google Scholar] [CrossRef] [Green Version]
- Cheville, J.C.; Lohse, C.M.; Zincke, H.; Weaver, A.L.; Blute, M.L. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am. J. Surg. Pathol. 2003, 27, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, W.; Le, X.; Li, Z.; Ge, P. Preoperative Neutrophil-to-Lymphocyte Ratio Was a Predictor of Overall Survival in Small Renal Cell Carcinoma: An Analysis of 384 Consecutive Patients. Biomed Res. Int. 2020, 2020, 8051210. [Google Scholar] [CrossRef] [Green Version]
| Total (%) | Low (%) | High (%) | p Value | |
|---|---|---|---|---|
| Age (years) | 0.377 | |||
| <65 | 32 (36.0) | 30 (69.8) | 27 (58.7) | |
| ≧65 | 57 (64.0) | 13 (30.2) | 19 (41.3) | |
| Gender | 0.821 | |||
| Female | 29 (32.6) | 15 (34.9) | 14 (30.4) | |
| Male | 61 (67.4) | 28 (65.1) | 33 (69.6) | |
| BMI (kg/m2) | 0.389 | |||
| <24 | 33 (37.1) | 18 (41.9) | 15 (32.6) | |
| ≧24 | 56 (62.9) | 25 (58.1) | 31 (67.4) | |
| Hypertension | 0.821 | |||
| No | 29 (32.6) | 15 (34.9) | 14 (30.9) | |
| Yes | 60 (67.4) | 28 (65.1) | 32 (69.6) | |
| Diabetes mellitus | 0.827 | |||
| No | 59 (66.3) | 28 (65.1) | 31 (67.4) | |
| Yes | 30 (33.7) | 15 (34.9) | 15 (32.6) | |
| CKD stage | 1.00 | |||
| 1 + 2 | 71 (79.8) | 34 (79.1) | 37 (80.4) | |
| 3 + 4 + 5 | 18 (20.2) | 9 (20.9) | 9 (19.6) | |
| Leukocytosis 1 | 0.083 | |||
| Yes | 9 (10.1) | 7 (16.3) | 2 (4.3) | |
| No | 80 (89.9) | 36 (83.7) | 44 (95.7) | |
| Neutrophilia 2 | 0.217 | |||
| Yes | 12 (15.8) | 8 (21.6) | 4 (10.3) | |
| No | 64 (84.2) | 29 (78.4) | 35 (89.7) | |
| NLR | 0.360 | |||
| ≧3 | 33 (42.3) | 18 (48.6) | 15 (36.6) | |
| <3 | 45 (57.7) | 19 (51.4) | 26 (63.4) | |
| Anemia 3 | 0.361 | |||
| No | 61 (68.5) | 27 (62.8) | 34 (73.9) | |
| Yes | 28 (31.5) | 16 (37.2) | 13 (26.1) | |
| ECOG PS | 0.109 | |||
| 0 | 86 (96.6) | 40 (93) | 46 (100) | |
| 1 | 3 (3.4) | 3 (7) | 0 (0) | |
| Nephrectomy | 0.289 | |||
| Radical | 48 (53.9) | 26 (60.5) | 22 (47.8) | |
| Partial | 41 (46.1) | 17 (39.5) | 24 (51.1) | |
| Smoking | 1.00 | |||
| No | 62 (69.7) | 30 (69.8) | 32 (69.6) | |
| Yes | 27 (30.3) | 13 (30.2) | 14 (30.4) | |
| T stage | 0.584 | |||
| T1 + T2 | 73 (82) | 34 (79.1) | 39 (84.8) | |
| T3 + T4 | 16 (18) | 9 (20.9) | 7 (15.2) | |
| LN involvement | 0.051 | |||
| No | 85 (95.5) | 39 (90.7) | 46 (100) | |
| Yes | 4 (4.5) | 4 (9.3) | 0 (0) | |
| Pathological stage | 0.600 | |||
| 1 + 2 | 71 (79.8) | 33 (76.7) | 38 (82.6) | |
| 3 + 4 | 18 (20.2) | 10 (23.3) | 8 (17.4) | |
| Histology | 0.818 | |||
| Clear cell | 62 (69.7) | 29 (67.4) | 33 (71.7) | |
| Other | 27 (30.3) | 14 (32.6) | 13 (28.3) | |
| Fuhrman grade 4 | 0.637 | |||
| 1 + 2 | 56 (68.3) | 26 (65.0) | 30 (71.4) | |
| 3 + 4 | 26 (31.7) | 14 (35.0) | 12 (28.6) | |
| Intratumor Hemorrhage | 0.389 | |||
| No | 33 (37.1) | 18 (41.9) | 15 (32.6) | |
| Yes | 56 (62.9) | 25 (58.1) | 31 (67.4) | |
| Intratumor Necrosis | 0.525 | |||
| No | 41 (46.1) | 18 (41.9) | 23 (50) | |
| Yes | 48 (53.9) | 25 (58.1) | 23 (50) |
| Progression-Free Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| Hazard Ratio | p Value | Hazard Ratio | p Value | Hazard Ratio | p Value | Hazard Ratio | p Value | |
| Age (years) | 1.352 | 0.607 | 1.324 | 0.661 | 4.161 | 0.089 | 7.277 | 0.040 * |
| ≧65 vs. <65 | (0.429–4.264) | (0.377–4.645) | (0.806–21.479) | (1.092–48.479) | ||||
| Gender | 1.393 | 0.619 | 0.977 | 0.978 | ||||
| Male vs. Female | (0.377–5.148) | (0.189–5.055) | ||||||
| BMI (kg/m2) | 0.614 | 0.399 | 0.246 | 0.093 | ||||
| ≧24 vs. <24 | (0.198–1.906) | (0.048–1.266) | ||||||
| Hypertension | 1.111 | 0.864 | 3.599 | 0.236 | ||||
| Yes vs. No | (0.333–3.700) | (0.432–29.975) | ||||||
| Diabetes mellitus | 0.997 | 0.997 | 2.475 | 0.236 | ||||
| Yes vs. No | (0.300–3.320) | (0.553–11.084) | ||||||
| CKD stage | 1.332 | 0.667 | 1.597 | 0.577 | ||||
| ≧3 vs. <3 | (0.360–4.928) | (0.309–8.254) | ||||||
| Smoking | 1.044 | 0.943 | 0.972 | 0.973 | ||||
| Yes vs. No | (0.314–3.472) | (0.188–5.043) | ||||||
| Leukocytosis 1 | 2.871 | 0.114 | 1.367 | 0.772 | ||||
| Yes vs. No | (0.776–10.625) | (0.164–11.363) | ||||||
| Neutrophilia 2 | 2.261 | 0.238 | 3.279 | 0.193 | ||||
| Yes vs. No | (0.583–8.772) | (0.548–19.634) | ||||||
| NLR | 1.555 | 0.467 | 1.122 | 0.889 | ||||
| ≧3 vs. <3 | (0.474–5.098) | (0.224–5.632) | ||||||
| Anemia 3 | 2.343 | 0.141 | 3.136 | 0.136 | ||||
| Yes vs. No | (0.753–7.289) | (0.699–14.069) | ||||||
| MPV/PC ratio | 6.521 | 0.016 * | 5.391 | 0.044 * | 7.946 | 0.055 | 25.285 | 0.015 * |
| Low vs. High | (1.424–29.854) | (1.049–27.700) | (0.953–66.232) | (1.880–340.158) | ||||
| Histology | 0.781 | 0.711 | 1.004 | 0.996 | ||||
| Other vs. Clear cell | (0.211–2.888) | (0.194–5.181) | ||||||
| Pathological stage | 29.882 | <0.001 * | 18.261 | <0.001 * | 11.496 | 0.004 * | 5.908 | 0.045 * |
| ≧3 vs. <3 | (6.440–138.665) | (3.769–88.475) | (2.220–59.517) | (1.044–33.437) | ||||
| Tumor grade 4 | 3.705 | 0.027 * | 3.166 | 0.081 | 6.923 | 0.022 * | 6.755 | 0.050 * |
| ≧3 vs. <3 | (1.161–11.821) | (0.868–11.555) | (1.330–36.030) | (1.004–45.469) | ||||
| Intratumor Hemorrhage | 0.834 | 0.425 | ||||||
| Yes vs. No | (0.264–2.631) | 0.756 | (0.095–1.901) | 0.263 | ||||
| Intratumor Necrosis | 4.677 | 2.038 | 2.063 | |||||
| Yes vs. No | (1.023–21.375) | 0.047 * | (0.385–10.799) | 0.403 | (0.40–10.636) | 0.387 | ||
| MPV | |||
|---|---|---|---|
| Study | Cancer Type | Cut-off Value (fl) | Coefficient of Variation |
| Seles et al. [6] | Renal Cell Carcinoma | 9.5 | 0.12138 |
| Tuncel et al. [8] | Colorectal cancer | 7.89 | |
| Kumagai et al. [9] | Lung cancer | 8.5 | |
| Kilincalp et al. [10] | Gastric cancer | 8.25 | |
| Zhang et al. [11] | Esophageal cancer | 10.6 | |
| Yun et al. [12] | Renal Cell Carcinoma | 7.5 | |
| Gu et al. [13] | Breast cancer | 8.45 | |
| MPV/PC Ratio | |||
| Study | Cancer Type | Cut-off Value | Coefficient of Variation |
| Zhang et al. [16] | Nasopharyngeal cancer | 0.040 | 0.09876 |
| Inagaki et al. [15] | Lung cancer | 0.041 | |
| Our study | Renal Cell Carcinoma | 0.034 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-C.; Jan, H.-C.; Ou, H.-Y.; Ou, C.-H.; Hu, C.-Y. Low Preoperative Mean Platelet Volume/Platelet Count Ratio Indicates Worse Prognosis in Non-Metastatic Renal Cell Carcinoma. J. Clin. Med. 2021, 10, 3676. https://doi.org/10.3390/jcm10163676
Lin Y-C, Jan H-C, Ou H-Y, Ou C-H, Hu C-Y. Low Preoperative Mean Platelet Volume/Platelet Count Ratio Indicates Worse Prognosis in Non-Metastatic Renal Cell Carcinoma. Journal of Clinical Medicine. 2021; 10(16):3676. https://doi.org/10.3390/jcm10163676
Chicago/Turabian StyleLin, Yu-Chiao, Hau-Chern Jan, Horng-Yih Ou, Chien-Hui Ou, and Che-Yuan Hu. 2021. "Low Preoperative Mean Platelet Volume/Platelet Count Ratio Indicates Worse Prognosis in Non-Metastatic Renal Cell Carcinoma" Journal of Clinical Medicine 10, no. 16: 3676. https://doi.org/10.3390/jcm10163676
APA StyleLin, Y.-C., Jan, H.-C., Ou, H.-Y., Ou, C.-H., & Hu, C.-Y. (2021). Low Preoperative Mean Platelet Volume/Platelet Count Ratio Indicates Worse Prognosis in Non-Metastatic Renal Cell Carcinoma. Journal of Clinical Medicine, 10(16), 3676. https://doi.org/10.3390/jcm10163676






