Pain as Defining Feature of Health Status and Prominent Therapeutic Target in Patients with Hidradenitis Suppurativa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. HS Patients
2.3. Measures of Pain
- 1.
- Question 1 of the Skindex-17
- 2.
- Bodily Pain (BP) scale of the SF-36
- 3.
- Visual Analog Scale (VAS) of pain
2.4. Clinical Severity Measures
2.5. Statistical Analysis
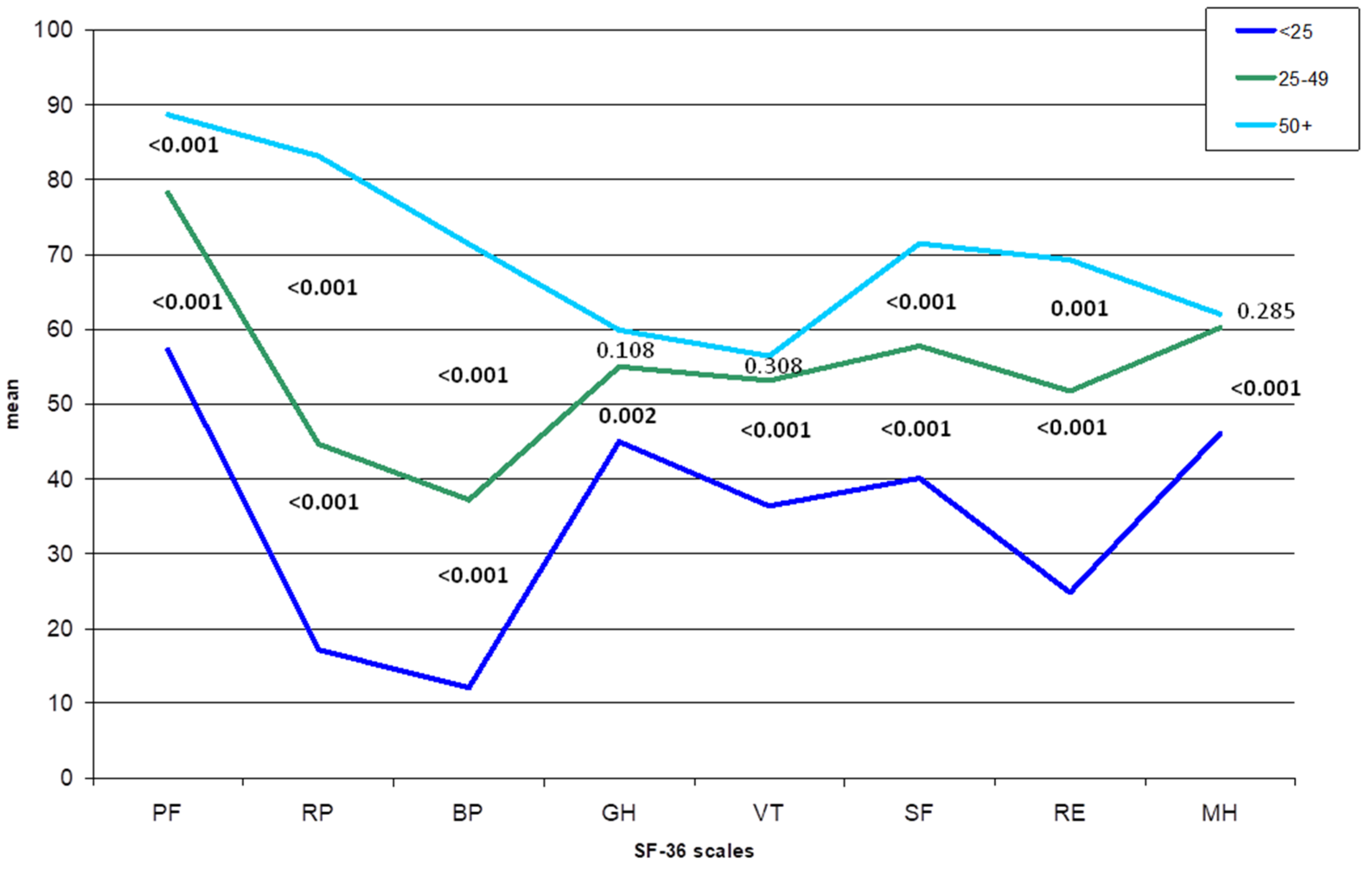
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zouboulis, C.C.; Desai, N.; Emtestam, L.; Hunger, R.E.; Ioannides, D.; Juhász, I.; Lapins, J.; Matusiak, L.; Prens, E.P.; Revuz, J.; et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 619–644. [Google Scholar] [CrossRef]
- Fania, L.; Ricci, F.; Sampogna, F.; Mazzanti, C.; Didona, B.; Pintori, G.; Abeni, D. Prevalence and incidence of hidradenitis suppurativa: An exercise on indirect estimation from psoriasis data. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e410–e411. [Google Scholar] [CrossRef]
- Jemec, G.B.E.; Heidenheim, M.; Nielsen, N.H. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J. Am. Acad. Dermatol. 1996, 35, 191–194. [Google Scholar] [CrossRef]
- Napolitano, M.; Fabbrocini, G.; Marasca, C.; Monfrecola, G. Update on pathogenesis of hidradenitis suppurativa. G. Ital. Dermatol. Venereol. 2018, 153, 3–7. [Google Scholar] [PubMed]
- Zouboulis, C.C.; Del Marmol, V.; Mrowietz, U.; Prens, E.P.; Tzellos, T.; Jemec, G.B.E. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology 2015, 231, 184–190. [Google Scholar] [CrossRef]
- Nielsen, R.M.; Lindsø Andersen, P.; Sigsgaard, V.; Theut Riis, P.; Jemec, G.B. Pain perception in patients with hidradenitis suppurativa. Br. J. Dermatol. 2020, 182, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Merskey, H.; Bogduk, N. (Eds.) Classification of Chronic Pain, 2nd ed.; IASP Press: Seattle, WA, USA, 1994. [Google Scholar]
- Smith, H.S.; Chao, J.D.; Teitelbaum, J. Painful hidradenitis suppurativa. Clin. J. Pain 2010, 26, 435–444. [Google Scholar] [CrossRef]
- Esmann, S.; Jemec, G.B.E. Psychosocial impact of hidradenitis suppurativa: A qualitative study. Acta Derm. Venereol. 2011, 91, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Von Der Werth, J.M.; Jemec, G.B.E. Morbidity in patients with hidradenitis suppurativa. Br. J. Dermatol. 2001, 144, 809–813. [Google Scholar] [CrossRef] [Green Version]
- Ring, H.C.; Theut Riis, P.; Miller, I.M.; Saunte, D.M.; Jemec, G.B. Self-reported pain management in hidradenitis suppurativa. Br. J. Dermatol. 2016, 174, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, Ł. Profound consequences of hidradenitis suppurativa: A review. Br. J. Dermatol. 2020, 183, e171–e177. [Google Scholar] [CrossRef]
- Sampogna, F.; Abeni, D.; Gieler, U.; Tomas Aragones, L.; Lien, L.; Poot, F.; Jemec, G.B.E.; Szabó, C.; Linder, D.; van Middendorp, H.; et al. Exploring the EQ-5D Dimension of Pain/Discomfort in Dermatology Outpatients from a Multicentre Study in 13 European Countries. Acta Derm. Venereol. 2020, 100, adv00120. [Google Scholar] [CrossRef] [PubMed]
- Balieva, F.; Kupfer, J.; Lien, L.; Gieler, U.; Finlay, A.Y.; Tomás-Aragonés, L.; Poot, F.; Misery, L.; Sampogna, F.; van Middendorp, H.; et al. The burden of common skin diseases assessed with the EQ5DTM: A European multicentre study in 13 countries. Br. J. Dermatol. 2017, 176, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Sampogna, F.; Fania, L.; Mazzanti, C.; Pallotta, S.; Panebianco, A.; Mastroeni, S.; Didona, B.; Abeni, D. The impact of hidradenitis suppurativa on general health is higher than that of hypertension, congestive heart failure, type 2 diabetes, myocardial infarction and depression. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e386–e388. [Google Scholar] [CrossRef] [PubMed]
- Thorlacius, L.; Ingram, J.R.; Villumsen, B.; Esmann, S.; Kirby, J.S.; Gottlieb, A.B.; Merola, J.F.; Dellavalle, R.; Nielsen, S.M.; Christensen, R.; et al. A core domain set for hidradenitis suppurativa trial outcomes: An international Delphi process. Br. J. Dermatol. 2018, 179, 642–650. [Google Scholar] [CrossRef]
- Horváth, B.; Janse, I.C.; Sibbald, G.R. Pain management in patients with hidradenitis suppurativa. J. Am. Acad. Dermatol. 2015, 73, S47–S51. [Google Scholar] [CrossRef] [PubMed]
- Jemec, G. Quality of life considerations and pain management in hidradenitis suppurativa. Semin. Cutan. Med. Surg. 2017, 36, 75–78. [Google Scholar] [CrossRef]
- Matusiak, Ł.; Szczęch, J.; Kaaz, K.; Lelonek, E.; Szepietowski, J.C. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm. Venereol. 2018, 98, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Patel, Z.S.; Hoffman, L.K.; Buse, D.C.; Grinberg, A.S.; Afifi, L.; Cohen, S.R.; Lowes, M.A.; Seng, E.K. Pain, Psychological Comorbidities, Disability, and Impaired Qualify of Life in Hidradenitis Suppurativa. Curr. Pain Headache Rep. 2017, 21, 49. [Google Scholar] [CrossRef]
- Nijsten, T.E.C.; Sampogna, F.; Chren, M.-M.; Abeni, D.D. Testing and reducing skindex-29 using Rasch analysis: Skindex-17. J. Investig. Dermatol. 2006, 126, 1244–1250. [Google Scholar] [CrossRef] [Green Version]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Hurley, H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus. Surgical approach. In Roenigk and Roenigk’s Dermatologic Surgery: Principles and Practice; Roenigk, R., Roenigk, H.J., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 623–645. [Google Scholar]
- Sartorius, K.; Emtestam, L.; Jemec, G.B.E.; Lapins, J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br. J. Dermatol. 2009, 161, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br. J. Dermatol. 2017, 177, 1401–1409. [Google Scholar] [CrossRef] [Green Version]
- Nashan, D.; Meiss, F.; Gralow, I. Pain: Basics and relevance in dermatology: Academy CME. JDDG J. Ger. Soc. Dermatol. 2009, 7, 704–716. [Google Scholar]
- Patel, Z.S.; Hoffman, L.K.; Sutton, L.; Cohen, S.R.; Lowes, M.A.; Seng, E.K. The patient experience of pain in hidradenitis suppurativa. Br. J. Dermatol. 2020. [Google Scholar] [CrossRef]
- Scheinfeld, N. Treatment of hidradenitis supprurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gabapentin, pegabalin, duloxetine, and venlafaxine. Dermatol. Online J. 2013, 19, 20616. [Google Scholar] [CrossRef]
- Gilron, I.; Baron, R.; Jensen, T. Neuropathic pain: Principles of diagnosis and treatment. Mayo Clin. Proc. 2015, 90, 532–545. [Google Scholar] [CrossRef] [Green Version]
- Krajewski, P.K.; Matusiak, Ł.; von Stebut, E.; Schultheis, M.; Kirschner, U.; Nikolakis, G.; Szepietowski, J.C. Pain in Hidradenitis Suppurativa: A Cross-sectional Study of 1795 Patients. Acta Derm. Venereol. 2021, 101, 1–4. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Matusiak, Ł.; von Stebut, E.; Schultheis, M.; Kirschner, U.; Nikolakis, G.; Szepietowski, J.C. Quality-of-Life Impairment among Patients with Hidradenitis Suppurativa: A Cross-Sectional Study of 1795 Patients. Life 2021, 11, 34. [Google Scholar] [CrossRef]
- Saunte, D.M.; Boer, J.; Stratigos, A.; Szepietowski, J.C.; Hamzavi, I.; Kim, K.H.; Zarchi, K.; Antoniou, C.; Matusiak, L.; Lim, H.W.; et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br. J. Dermatol. 2015, 173, 1546–1549. [Google Scholar] [CrossRef] [PubMed]
- Kaaz, K.; Szepietowski, J.C.; Matusiak, Ł. Influence of itch and pain on sleep quality in patients with hidradenitis suppurativa. Acta Derm. Venereol. 2018, 98, 757–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingram, J.R.; Hadjieconomou, S.; Piguet, V. Development of core outcome sets in hidradenitis suppurativa: Systematic review of outcome measure instruments to inform the process. Br. J. Dermatol. 2016, 175, 263–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarchi, K.; Yazdanyar, N.; Yazdanyar, S.; Wortsman, X.; Jemec, G.B.E. Pain and inflammation in hidradenitis suppurativa correspond to morphological changes identified by high-frequency ultrasound. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 527–532. [Google Scholar] [CrossRef] [PubMed]


| Variable | Level | N (%) | IHS4 Mean | Sartorius Mean | Hurley Mean | Nr Fistulae Mean | Nr Abscesses Mean | Nr Nodules Mean | Nr Scars Mean | |
|---|---|---|---|---|---|---|---|---|---|---|
| overall | 341 (100) | 19.8 | 58.5 | 1.7 | 2.0 | 2.1 | 7.6 | 10.0 | ||
| Skindex-17 Item 1 |
| 16 (5.1) | 6.1 | 35.2 | 1.4 | 0.3 | 0.2 | 4.7 | 9.1 | |
| 96 (30.4) | 13.2 | 46.1 | 1.6 | 1.1 | 1.4 | 5.9 | 8.3 | |||
| 204 (64.5) | 24.6 | 68.9 | 1.9 | 2.6 | 2.7 | 8.7 | 11.2 | |||
| p * | <0.001 | <0.001 | 0.001 | 0.002 | 0.011 | 0.008 | 0.315 | |||
| p ** | ||||||||||
| a–b | 0.568 | 0.823 | 0.384 | 0.760 | 0.595 | 0.862 | 0.982 | |||
| b–c | 0.001 | 0.001 | 0.007 | 0.007 | 0.039 | 0.015 | 0.295 | |||
| a–c | 0.017 | 0.053 | 0.013 | 0.082 | 0.083 | 0.160 | 0.861 | |||
| SF-36 BP scale |
| 116 (39.4) | 10.6 | 39.6 | 1.5 | 0.9 | 0.8 | 5.8 | 7.8 | |
| 87 (29.6) | 15.3 | 49.4 | 1.7 | 1.6 | 1.3 | 6.4 | 8.3 | |||
| 91 (31.0) | 30.3 | 76.4 | 2.0 | 3.6 | 3.2 | 9.5 | 11.0 | |||
| p * | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | 0.300 | |||
| p ** | ||||||||||
| d–e | 0.287 | 0.344 | 0.024 | 0.357 | 0.480 | 0.814 | 0.971 | |||
| e–f | <0.001 | 0.001 | 0.005 | 0.001 | <0.001 | 0.025 | 0.472 | |||
| d–f | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | 0.299 | |||
| VAS pain |
| 150 (44.0) | 12.4 | 42.2 | 1.6 | 1.1 | 1.2 | 5.5 | 7.2 | |
| 68 (19.9) | 16.8 | 54.0 | 1.7 | 1.6 | 1.6 | 7.6 | 9.5 | |||
| 123 (36.1) | 30.5 | 80.9 | 2.0 | 3.4 | 3.4 | 10.2 | 13.5 | |||
| p * | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| p ** | ||||||||||
| g–h | 0.435 | 0.278 | 0.441 | 0.734 | 0.765 | 0.174 | 0.560 | |||
| h–i | 0.001 | 0.003 | 0.010 | 0.007 | 0.012 | 0.063 | 0.182 | |||
| g–i | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampogna, F.; Campana, I.; Fania, L.; Mastroeni, S.; Fusari, R.; Ciccone, D.; Pallotta, S.; Abeni, D. Pain as Defining Feature of Health Status and Prominent Therapeutic Target in Patients with Hidradenitis Suppurativa. J. Clin. Med. 2021, 10, 3648. https://doi.org/10.3390/jcm10163648
Sampogna F, Campana I, Fania L, Mastroeni S, Fusari R, Ciccone D, Pallotta S, Abeni D. Pain as Defining Feature of Health Status and Prominent Therapeutic Target in Patients with Hidradenitis Suppurativa. Journal of Clinical Medicine. 2021; 10(16):3648. https://doi.org/10.3390/jcm10163648
Chicago/Turabian StyleSampogna, Francesca, Irene Campana, Luca Fania, Simona Mastroeni, Roberta Fusari, Davide Ciccone, Sabatino Pallotta, and Damiano Abeni. 2021. "Pain as Defining Feature of Health Status and Prominent Therapeutic Target in Patients with Hidradenitis Suppurativa" Journal of Clinical Medicine 10, no. 16: 3648. https://doi.org/10.3390/jcm10163648
APA StyleSampogna, F., Campana, I., Fania, L., Mastroeni, S., Fusari, R., Ciccone, D., Pallotta, S., & Abeni, D. (2021). Pain as Defining Feature of Health Status and Prominent Therapeutic Target in Patients with Hidradenitis Suppurativa. Journal of Clinical Medicine, 10(16), 3648. https://doi.org/10.3390/jcm10163648






