Risks of Recurrent Cardiovascular Events and Mortality in 1-Year Survivors of Acute Myocardial Infarction Implanted with Newer-Generation Drug-Eluting Stents
Abstract
1. Introduction
2. Materials and Methods
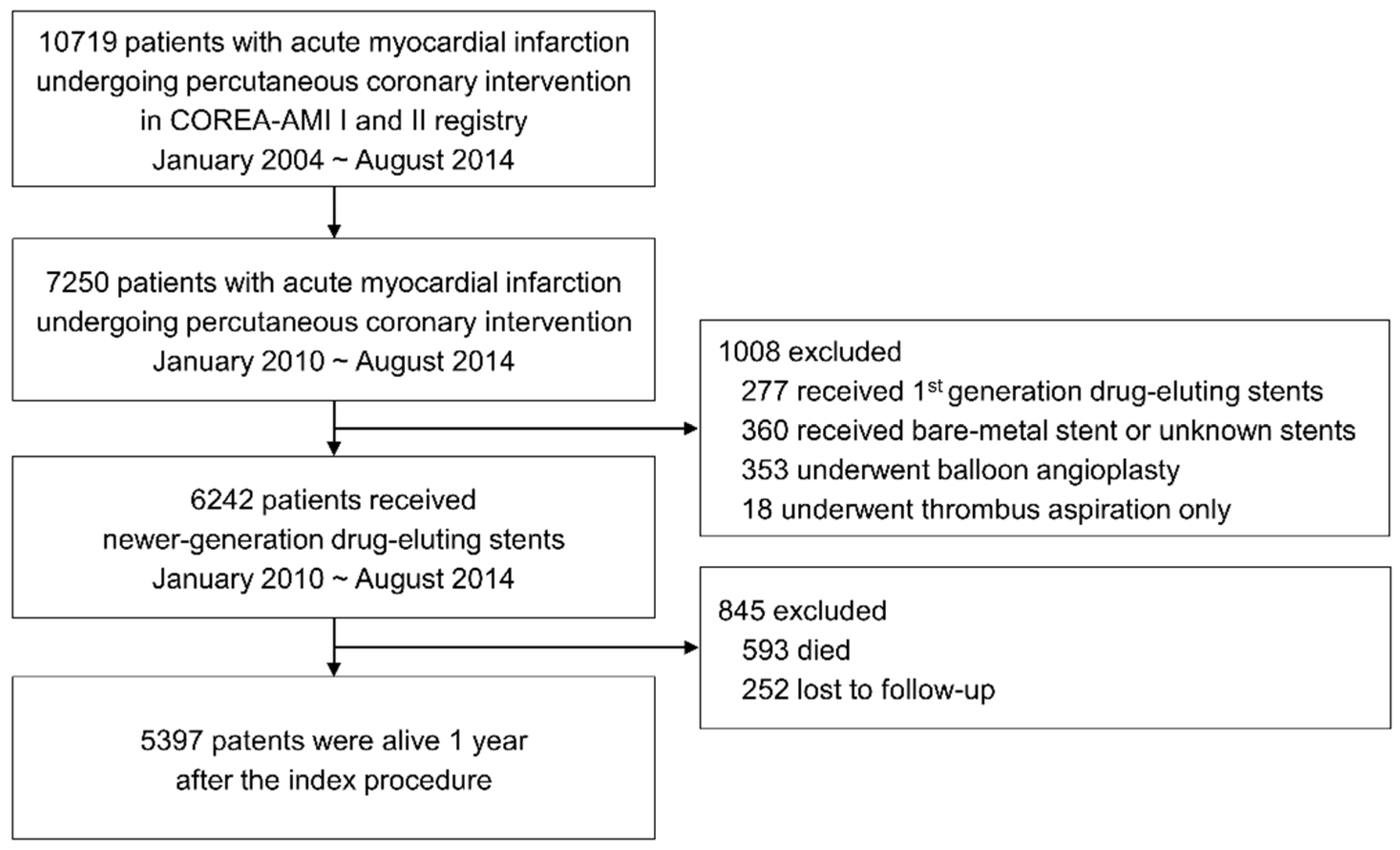
2.1. Study Population
2.2. Treatment and Data Collection
2.3. Endpoints and Definitions
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
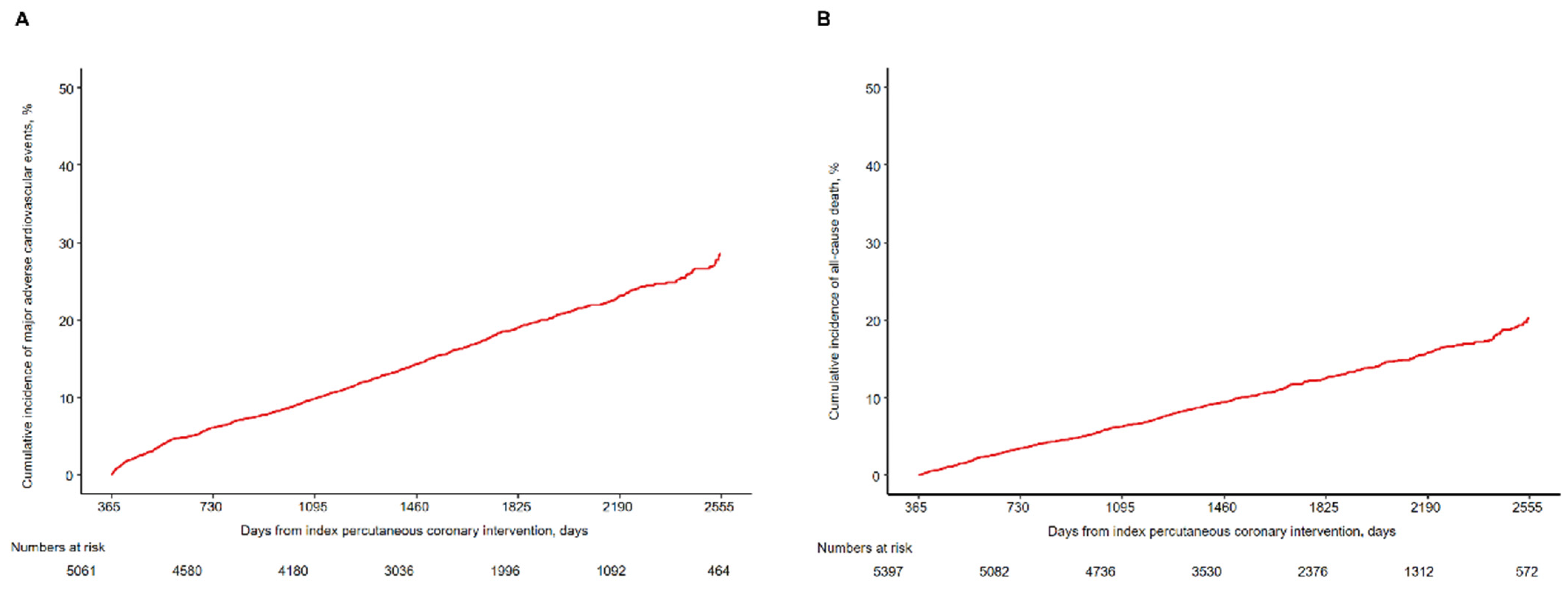
3.2. Clinical Outcomes beyond 1 Year
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Nabel, E.G.; Braunwald, E. A tale of coronary artery disease and myocardial infarction. N. Engl. J. Med. 2012, 366, 54–63. [Google Scholar] [CrossRef]
- Ford, E.S.; Ajani, U.A.; Croft, J.B.; Critchley, J.A.; Labarthe, D.R.; Kottke, T.E.; Giles, W.H.; Capewell, S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N. Engl. J. Med. 2007, 356, 2388–2398. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Stone, G.W.; Witzenbichler, B.; Guagliumi, G.; Peruga, J.Z.; Brodie, B.R.; Dudek, D.; Kornowski, R.; Hartmann, F.; Gersh, B.J.; Pocock, S.J.; et al. Bivalirudin during primary PCI in acute myocardial infarction. N. Engl. J. Med. 2008, 358, 2218–2230. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Keeley, E.C.; Boura, J.A.; Grines, C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet 2003, 361, 13–20. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Kereiakes, D.J.; Yeh, R.W.; Driscoll-Shempp, P.; Cutlip, D.E.; Steg, P.G.; Normand, S.L.; Braunwald, E.; Wiviott, S.D.; Cohen, D.J.; et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 2014, 371, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef]
- Hall, M.; Dondo, T.B.; Yan, A.T.; Goodman, S.G.; Bueno, H.; Chew, D.P.; Brieger, D.; Timmis, A.; Batin, P.D.; Deanfield, J.E.; et al. Association of Clinical Factors and Therapeutic Strategies with Improvements in Survival Following Non-ST-Elevation Myocardial Infarction, 2003–2013. JAMA 2016, 316, 1073–1082. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, M.H.; Yang, D.H.; Park, H.S.; Cho, Y.; Jeong, M.H.; Kim, Y.J.; Kim, K.S.; Hur, S.H.; Seong, I.W.; et al. Contemporary Trends of Optimal Evidence-Based Medical Therapy at Discharge for Patients Surviving Acute Myocardial Infarction fom the Korea Acute Myocardial Infarction Registry. Clin. Cardiol. 2015, 38, 350–356. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Thygesen, K.; Alpert, J.S.; White, H.D.; Jaffe, A.S.; et al. Third universal definition of myocardial infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef]
- Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Blömstrom-Lundqvist, C.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G.; Fernandez-Aviles, F.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef]
- Hamm, C.W.; Bassand, J.P.; Agewall, S.; Bax, J.; Boersma, E.; Bueno, H.; Caso, P.; Dudek, D.; Gielen, S.; Huber, K.; et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 2999–3054. [Google Scholar]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, 2354–2394. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Danchin, N.; Popovic, B.; Puymirat, E.; Goldstein, P.; Belle, L.; Cayla, G.; Roubille, F.; Lemesle, G.; Ferrières, J.; Schiele, F.; et al. Five-year outcomes following timely primary percutaneous intervention, late primary percutaneous intervention, or a pharmaco-invasive strategy in ST-segment elevation myocardial infarction: The FAST-MI programme. Eur. Heart J. 2020, 41, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Özcan, C.; Deleskog, A.; Schjerning Olsen, A.M.; Nordahl Christensen, H.; Lock Hansen, M.; Hilmar Gislason, G. Coronary artery disease severity and long-term cardiovascular risk in patients with myocardial infarction: A Danish nationwide register-based cohort study. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Rapsomaniki, E.; Thuresson, M.; Yang, E.; Blin, P.; Hunt, P.; Chung, S.C.; Stogiannis, D.; Pujades-Rodriguez, M.; Timmis, A.; Denaxas, S.C.; et al. Using big data from health records from four countries to evaluate chronic disease outcomes: A study in 114 364 survivors of myocardial infarction. Eur. Heart J. Qual. Care Clin. Outcomes 2016, 2, 172–183. [Google Scholar] [CrossRef]
- Jernberg, T.; Hasvold, P.; Henriksson, M.; Hjelm, H.; Thuresson, M.; Janzon, M. Cardiovascular risk in post-myocardial infarction patients: Nationwide real world data demonstrate the importance of a long-term perspective. Eur. Heart J. 2015, 36, 1163–1170. [Google Scholar] [CrossRef]
- Yamaji, K.; Natsuaki, M.; Morimoto, T.; Ono, K.; Furukawa, Y.; Nakagawa, Y.; Kadota, K.; Ando, K.; Shirai, S.; Watanabe, H.; et al. Long-Term Outcomes After Coronary Stent Implantation in Patients Presenting with Versus Without Acute Myocardial Infarction (An Observation from Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2). Am. J. Cardiol. 2015, 116, 15–23. [Google Scholar] [CrossRef]
- Hahn, J.Y.; Song, Y.B.; Oh, J.H.; Cho, D.K.; Lee, J.B.; Doh, J.H.; Kim, S.H.; Jeong, J.O.; Bae, J.H.; Kim, B.O.; et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): A randomised, open-label, non-inferiority trial. Lancet 2018, 391, 1274–1284. [Google Scholar] [CrossRef]
- Sabaté, M.; Brugaletta, S.; Cequier, A.; Iñiguez, A.; Serra, A.; Jiménez-Quevedo, P.; Mainar, V.; Campo, G.; Tespili, M.; den Heijer, P.; et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet 2016, 387, 357–366. [Google Scholar] [CrossRef]
- de Waha, A.; King, L.A.; Stefanini, G.G.; Byrne, R.A.; Serruys, P.W.; Meier, B.; Jüni, P.; Kastrati, A.; Windecker, S. Long-term outcomes of biodegradable versus durable polymer drug-eluting stents in patients with acute ST-segment elevation myocardial infarction: A pooled analysis of individual patient data from three randomised trials. EuroIntervention 2015, 10, 1425–1431. [Google Scholar] [CrossRef]
- Holmvang, L.; Kelbæk, H.; Kaltoft, A.; Thuesen, L.; Lassen, J.F.; Clemmensen, P.; Kløvgaard, L.; Engstrøm, T.; Bøtker, H.E.; Saunamäki, K.; et al. Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: 5 years follow-up from the randomized DEDICATION trial (Drug Elution and Distal Protection in Acute Myocardial Infarction). JACC Cardiovasc. Interv. 2013, 6, 548–553. [Google Scholar]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef]
- Xie, X.; Atkins, E.; Lv, J.; Bennett, A.; Neal, B.; Ninomiya, T.; Woodward, M.; MacMahon, S.; Turnbull, F.; Hillis, G.S.; et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: Updated systematic review and meta-analysis. Lancet 2016, 387, 435–443. [Google Scholar] [CrossRef]
- Lopes, R.D.; Subherwal, S.; Holmes, D.N.; Thomas, L.; Wang, T.Y.; Rao, S.V.; Magnus Ohman, E.; Roe, M.T.; Peterson, E.D.; Alexander, K.P. The association of in-hospital major bleeding with short-, intermediate-, and long-term mortality among older patients with non-ST-segment elevation myocardial infarction. Eur. Heart J. 2012, 33, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, J.Y.; Ahn, J.M.; Song, H.; Kim, W.J.; Yun, S.C.; Park, D.W.; Kang, S.J.; Lee, S.W.; Whan Lee, C.; et al. Impact of bleeding on subsequent early and late mortality after drug-eluting stent implantation. JACC Cardiovasc. Interv. 2011, 4, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.S.; Elliott, L.; Gallup, D.; Roe, M.; Granger, C.B.; Armstrong, P.W.; Simes, R.J.; White, H.D.; Van de Werf, F.; Topol, E.J.; et al. Sex differences in mortality following acute coronary syndromes. JAMA 2009, 302, 874–882. [Google Scholar] [CrossRef]
- Nauta, S.T.; Deckers, J.W.; van Domburg, R.T.; Akkerhuis, K.M. Sex-related trends in mortality in hospitalized men and women after myocardial infarction between 1985 and 2008: Equal benefit for women and men. Circulation 2012, 126, 2184–2189. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Variables | At Index Procedure (n = 6242) | At 1 Year (n = 5397) |
|---|---|---|
| Age, years | 63.78 ± 12.63 | 62.84 ± 12.39 |
| <55 | 1640 (26.3) | 1530 (28.3) |
| 55–64 | 1514 (24.3) | 1367 (25.3) |
| 65–74 | 1688 (27.0) | 1448 (26.8) |
| ≥75 | 1400 (22.4) | 1052 (19.5) |
| Men | 4508 (72.2) | 3975 (73.7) |
| Body mass index, kg/m2 | 24.11 ± 3.27 | 24.22 ± 3.21 |
| Body mass index ≥ 25 | 2236 (36.8) | 2013 (37.9) |
| Hypertension | 3291 (52.7) | 2770 (51.3) |
| Diabetes mellitus | 1985 (31.8) | 1641 (30.4) |
| Dyslipidemia | 1196 (19.2) | 1082 (20.0) |
| Current smoker | 2477 (39.7) | 2211 (41.0) |
| Family history of coronary artery disease | 184 (2.9) | 163 (3.0) |
| Previous myocardial infarction | 213 (3.4) | 169 (3.1) |
| Previous percutaneous coronary intervention | 402 (6.4) | 325 (6.0) |
| Previous coronary artery bypass graft | 25 (0.4) | 18 (0.3) |
| Previous cerebrovascular accident | 470 (7.5) | 368 (6.8) |
| Atrial fibrillation | 283 (4.5) | 210 (3.9) |
| Clinical presentation | ||
| STEMI | 3243 (52.0) | 2756 (51.1) |
| NSTEMI | 2999 (48.0) | 2641 (48.9) |
| Killip classification III–IV | 965 (15.8) | 663 (12.5) |
| Left ventricular ejection fraction, % | 61.12 | 62.89 |
| Left ventricular ejection fraction < 40 | 695 (11.7) | 510 (9.7) |
| Hemoglobin, g/dL | 13.66 ± 2.14 | 13.81 ± 2.05 |
| Anemia * | 1788 (28.7) | 1388 (25.7) |
| Estimated glomerular filtration rate, mL/min/m2 | 76.58 ± 25.96 | 79.14 ± 24.61 |
| Renal insufficiency † | 1568 (25.2) | 1149 (21.3) |
| Number of coronary arteries involved | ||
| One | 2806 (45.0) | 2489 (46.1) |
| Two | 2108 (33.8) | 1817 (33.7) |
| Three | 1328 (21.3) | 1091 (20.2) |
| Multivessel coronary artery disease | 3436 (55.0) | 2908 (53.9) |
| Culprit coronary artery | ||
| Left anterior descending | 2999 (48.0) | 2578 (47.8) |
| Left circumflex | 1056 (16.9) | 952 (17.6) |
| Right coronary | 1951 (31.3) | 1695 (31.4) |
| Left main | 233 (3.7) | 169 (3.1) |
| Left anterior descending artery or left main culprit vessel | 3232 (51.8) | 2747 (50.9) |
| Number of coronary arteries treated ≥2 | 1841 (29.5) | 1597 (29.6) |
| Drug-eluting stents | ||
| Everolimus-eluting stents | 3262 (52.3) | 2771 (51.3) |
| Zotarolimus-eluting stents | 1364 (21.9) | 1189 (22.0) |
| Biolimus-eluting stents | 1175 (18.8) | 1053 (19.5) |
| Mixed stents | 441 (7.1) | 384 (7.1) |
| Total number of stents | 1.58 ± 0.83 | 1.59 ± 0.84 |
| ≥3 stents | 851 (13.6) | 745 (13.8) |
| Mean stent diameter, mm | 2.75 ± 0.85 | 2.71 ± 0.85 |
| <3 mm | 1843 (29.5) | 1543 (28.6) |
| Total stent length, mm | 33.55 ± 20.06 | 33.71 ± 20.30 |
| >60 mm | 629 (10.1) | 554 (10.3) |
| Uncontrolled systolic blood pressure within 1 year | 812 (14.5) | 705 (13.4) |
| Uncontrolled LDL-cholesterol within 1 year | 1066 (25.4) | 1039 (25.3) |
| Outcomes | 0–1 Year | 1–7 Years | 1–2 Years | 2–3 Years | 3–4 Years | 4–5 Years | 5–6 Years | 6–7 Years |
|---|---|---|---|---|---|---|---|---|
| Major adverse cardiovascular event * | 862/6242 (14.1 (13.2–15.0)) | 928/5061 (28.5 (26.4–30.5)) | 301/5061 (6.1 (5.4–6.8)) | 177/4580 (3.9 (3.4–4.5)) | 181/4180 (4.9 (4.2–5.6)) | 140/3036 (5.5 (4.6–6.4)) | 78/1996 (5.0 (3.9–6.2)) | 51/1092 (7.1 (5.1–9.0)) |
| All-cause death | 593/6242 (9.6 (8.9–10.4)) | 684/5397 (20.2 (18.5–21.9)) | 181/5397 (3.4 (2.9–3.9)) | 146/5082 (2.9 (2.4–3.4)) | 140/4736 (3.3 (2.8–3.9)) | 101/3530 (3.4 (2.7–4.0)) | 70/2376 (3.8 (2.9–4.7)) | 46/1312 (5.3 (3.8–6.8)) |
| Cardiovascular death | 507/6242 (8.3 (7.6–8.9)) | 494/5397 (15.4 (13.8–17.0)) | 123/5397 (2.3 (1.9–2.7)) | 107/5082 (2.1 (1.7–2.5)) | 106/4736 (2.6 (2.1–3.0)) | 69/3530 (2.3 (1.8–2.9)) | 53/2376 (2.9 (2.1–3.7)) | 36/1312 (4.2 (2.8–5.6)) |
| Non-fatal myocardial infarction | 92/6242 (1.6 (1.3–1.9)) | 130/5317 (4.1 (3.2–4.9)) | 40/5317 (0.8 (0.5–1.0)) | 21/4971 (0.4 (0.2–0.6)) | 21/4614 (0.5 (0.3–0.7)) | 28/3420 (0.9 (0.6–1.3)) | 16/2295 (0.9 (0.5–1.4)) | 4/1264 (0.5 (0.0–1.0)) |
| Non-fatal ischemic stroke | 66/6242 (1.1 (0.9–1.4)) | 80/5339 (2.4 (1.8–3.0)) | 20/5339 (0.4 (0.2–0.6)) | 13/5013 (0.3 (0.1–0.4)) | 21/4660 (0.5 (0.3–0.7)) | 15/3465 (0.5 (0.3–0.8)) | 8/2324 (0.5 (0.1–0.8)) | 3/1277 (0.3 (0.0–0.6)) |
| Any revascularization | 280/6242 (5.0 (4.4–5.5)) | 433/5140 (12.9 (11.4–14.3)) | 179/5140 (3.6 (3.1–4.1)) | 70/4673 (1.5 (1.2–1.9)) | 70/4280 (1.9 (1.4–2.3)) | 72/3118 (2.8 (2.1–3.4)) | 25/2051 (1.5 (0.9–2.1)) | 17/1127 (2.3 (1.2–3.4)) |
| Stent thrombosis † | 58/6242 (1.0 (0.7–1.2)) | 28/5363 (0.2 (0.1–0.2)) | 6/5363 (0.1 (0.0–0.2)) | 5/5047 (0.1 (0.0–0.2)) | 6/4700 (0.2 (0.0–0.3)) | 10/3498 (0.3 (0.1–0.5)) | 1/2353 (0.1 (0.0–0.2)) | 1/1299 (0.1 (0.0–0.3)) |
| BARC 2, 3, and 5 bleeding | 406/6242 (6.9 (6.2–7.5)) | 225/5085 (6.6 (5.6–7.6)) | 86/5085 (1.7 (1.4–2.1)) | 43/4726 (1.0 (0.7–1.2)) | 42/4384 (1.0 (0.7–1.3)) | 29/3284 (1.1 (0.7–1.5)) | 18/2223 (1.1 (0.6–1.6)) | 7/1231 (0.9 (0.2–1.5)) |
| Variables | Major Adverse Cardiovascular Event | Variables | ALL-Cause Death | ||
|---|---|---|---|---|---|
| Adjusted HR * (95% CI) | p Value | Adjusted HR * (95% CI) | p Value | ||
| Age, years | Age, years | ||||
| <55 | 1 | <55 | 1 | ||
| 55–64 | 1.32 (1.03–1.69) | 0.028 | 55–64 | 2.08 (1.21–3.59) | 0.008 |
| 65–74 | 1.39 (1.07–1.80) | 0.015 | 65–74 | 5.03 (3.02–8.39) | <0.001 |
| ≥75 | 2.02 (1.50–2.72) | <0.001 | ≥75 | 9.12 (5.34–15.6) | <0.001 |
| Hypertension | 1.20 (1.00–1.44) | 0.046 | Male sex | 1.71 (1.30–2.24) | <0.001 |
| Diabetes mellitus | 1.24 (1.04–1.48) | 0.016 | Diabetes mellitus | 1.35 (1.06–1.72) | 0.016 |
| Current smoker | 1.23 (1.01–1.49) | 0.043 | Left ventricular ejection fraction < 40% | 2.24 (1.68–2.99) | <0.001 |
| Previous percutaneous coronary intervention | 1.44 (1.01–2.04) | 0.043 | Anemia | 1.39 (1.08–1.78) | 0.011 |
| Left ventricular ejection fraction < 40% | 1.41 (1.10–1.81) | 0.006 | Renal insufficiency | 2.07 (1.60–2.68) | <0.001 |
| Anemia | 1.23 (1.02–1.48) | 0.030 | Multivessel coronary artery disease | 1.29 (1.00–1.66) | 0.048 |
| Renal insufficiency | 1.44 (1.18–1.76) | <0.001 | Uncontrolled systolic blood pressure | 2.17 (1.65–2.86) | <0.001 |
| Multivessel coronary artery disease | 1.42 (1.19–1.68) | <0.001 | Uncontrolled LDL-cholesterol | 1.36 (1.06–1.74) | 0.014 |
| Mean stent diameter < 3 mm | 1.26 (1.05–1.50) | 0.011 | |||
| Uncontrolled systolic blood pressure | 1.42 (1.12–1.78) | 0.003 | |||
| Uncontrolled LDL-cholesterol | 1.38 (1.17–1.65) | <0.001 | |||
| Non-Fatal Events | Events | Unadjusted HR (95% CI) | p Value | Adjusted HR * (95% CI) | p Value |
|---|---|---|---|---|---|
| Myocardial infarction | 80 (1.5) | 2.23 (1.44–3.44) | <0.001 | 1.64 (1.02–2.65) | 0.042 |
| Ischemic stroke | 58 (1.1) | 2.28 (1.37–3.81) | 0.002 | 1.81 (1.01–3.23) | 0.046 |
| BARC 2, 3, and 5 bleeding | 312 (5.8) | 2.44 (1.92–3.09) | <0.001 | 1.56 (1.19–2.04) | 0.001 |
| Study | Year of Publication | Study Design with Trial Name | Patients | Number of Patients | Year of Enrollment | Age (Mean Years) | Men (%) | Period of Events | Clinical Outcomes | Clinical Outcomes of the Present Registry during the Same Period |
|---|---|---|---|---|---|---|---|---|---|---|
| N Danchin et al. [22] | 2020 | FAST-MI 2005 and 2010 | STEMI with pPCI | timely pPCI 1288 late pPCI 830 | 2005 and 2010 | 61 | 78.1 | 0–5 years | CE (AD, MI, or stroke) 15.7% and 23.5%, AD 11.8% and 20.5% in timely pPCI and in late pPCI, respectively | CE (AD, MI, or stroke) 25.6%, AD 20.9% |
| C Özcan et al. [23] | 2018 | Danish national registry | AMI | 43,045 | 2004–2010 | 68 | 65.4 | 1–5 years | CE (CD, MI, or stroke) 21.7%, CD 9.4%, MI 8.4%, stroke 3.9%. | CE (CD, MI, or stroke) 12.6%, CD 9.0%, MI 3.0%, stroke 2.5%. |
| E Rapsomaniki et al. [24] | 2016 | 4 national health record data | AMI | 114,364 | 2002–2011 | 77.5~78.6 | 50.8~58.5 | 1–4 years | CE (AD, MI, or stroke) 26.0–36.2%. | CE (AD, MI, or stroke) 11.6%. |
| T Jernberg et al. [25] | 2015 | Swedish health record data | AMI | 76,687 | 2006–2011 | 71.5 | 63.3 | 1–4 years | CD 11.0%, MI 9.2%, stroke 3.7%, AD 20.1%. | CD 6.9%, MI 1.9%, stroke 1.8%, AD 9.4%. |
| K Yamaji et al. [26] | 2015 | CREDO-Kyoto cohort-2 | AMI with DES | 820 | 2005–2007 | 67.5 | 73 | 0–5 years | CD 10.0%, MI 5.2%, AD 18.0%. | CD 16.5%, MI 4.8%, AD 20.9%. |
| J-Y Hahn et al. [27] | 2018 | RCT, SMART-DATE | ACS with DES (AMI 69%) | 2712 | 2012–2015 | 62.0~62.2 | 74.9~75.9 | 0–1.5 years | CE (AD, MI, or stroke) 4.2–4.7%, CD 1.4–1.8%, MI 0.8–1.8%, stroke 0.8–0.9%, AD 2.6–2.9%. | CE (AD, MI, or stroke) 13.8%, CD 9.3%, MI 2.3%, stroke 1.4%, AD 11.1%. |
| M Sabeté et al. [28] | 2016 | RCT, EXAMINATION | STEMI with DES | 751 | 2008–2010 | 60.8~61.6 | 82~84 | 0–5 years | CE (AD, MI, or revascularization) 21%, CE (CD, target vessel MI, TLR) 12%, CD 6%, MI 5%, revascularization 12%. | CE (AD, MI, or revascularization) 30.7%, CE (CD, target vessel MI, TLR) 20.9%, CD 16.5%, MI 4.8%, revascularization 13.4%. |
| MP Bonaca et al. [13] | 2015 | RCT, PEGASUS-TIMI 54 | AMI with high risk | 21,162 | 2010–2013 | 65.2~65.4 | 75.7~76.4 | 1–4 (to 3–6) years | CE (CD, MI, or stroke) 7.8–9.0%, CD 2.9–3.4%, MI 4.4–5.3%, stroke 1.5–1.9%, AD 4.7–5.2%. | CE (CD, MI, or stroke) 9.2%, CD 6.9%, MI 1.9%, stroke 1.8%, AD 9.4%. |
| A de Waha et al. [29] | 2015 | RCT, ISAR-TEST-4 and LEADERS | STEMI with DES | 497 | 2006–2008 | 62.5~63.1 | 72.3~73.5 | 1–4 years | CE (CD, MI, or TLR) 5.2–8.6%, CD 2.3–3.9%, MI 0.8–2.4%, AD 4.0–6.0%. | CE (CD, MI, or TLR) 9.2%, CD 6.9%, MI 1.9%, AD 9.4%. |
| L Holmvang et al. [30] | 2013 | RCT, DEDICATION | STEMI with DES | 313 | 2005–2006 | 62~63 | 73~74 | 8 months–5 years | CE (CD, MI, or TLR) 7.7%, CD 3.5%, MI 3.8%, AD 11.2%. | CE (CD, MI, or TLR) 14.3%, CD 10.7%, MI 3.6%, AD 14.5%. |
| The present study | COREA-AMI registry | AMI with newer-generation DES | 5397 | 2010–2014 | 63.8 | 72.2 | 1–7 years | CE (CD, MI, stroke, or revascularization) 28.5%, AD 20.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.; Choo, E.H.; Choi, I.J.; Lee, K.Y.; Lee, S.N.; Hwang, B.-H.; Kim, C.J.; Park, M.-W.; Lee, J.-M.; Park, C.S.; et al. Risks of Recurrent Cardiovascular Events and Mortality in 1-Year Survivors of Acute Myocardial Infarction Implanted with Newer-Generation Drug-Eluting Stents. J. Clin. Med. 2021, 10, 3642. https://doi.org/10.3390/jcm10163642
Lim S, Choo EH, Choi IJ, Lee KY, Lee SN, Hwang B-H, Kim CJ, Park M-W, Lee J-M, Park CS, et al. Risks of Recurrent Cardiovascular Events and Mortality in 1-Year Survivors of Acute Myocardial Infarction Implanted with Newer-Generation Drug-Eluting Stents. Journal of Clinical Medicine. 2021; 10(16):3642. https://doi.org/10.3390/jcm10163642
Chicago/Turabian StyleLim, Sungmin, Eun Ho Choo, Ik Jun Choi, Kwan Yong Lee, Su Nam Lee, Byung-Hee Hwang, Chan Joon Kim, Mahn-Won Park, Jong-Min Lee, Chul Soo Park, and et al. 2021. "Risks of Recurrent Cardiovascular Events and Mortality in 1-Year Survivors of Acute Myocardial Infarction Implanted with Newer-Generation Drug-Eluting Stents" Journal of Clinical Medicine 10, no. 16: 3642. https://doi.org/10.3390/jcm10163642
APA StyleLim, S., Choo, E. H., Choi, I. J., Lee, K. Y., Lee, S. N., Hwang, B.-H., Kim, C. J., Park, M.-W., Lee, J.-M., Park, C. S., Kim, H.-Y., Yoo, K.-D., Jeon, D. S., Youn, H. J., Chung, W. S., Kim, M. C., Jeong, M. H., Yim, H. W., Ahn, Y., & Chang, K. (2021). Risks of Recurrent Cardiovascular Events and Mortality in 1-Year Survivors of Acute Myocardial Infarction Implanted with Newer-Generation Drug-Eluting Stents. Journal of Clinical Medicine, 10(16), 3642. https://doi.org/10.3390/jcm10163642






