Protocol Biopsies on de novo Renal-Transplants at 3 Months after Surgery: Impact on 5-Year Transplant Survival
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Kidney Biopsies
2.3. Immunosuppressive Regimen
2.4. Propensity Score
2.5. Endpoints
2.6. Statistical Analyses
3. Results
3.1. Recipients’ Characteristics
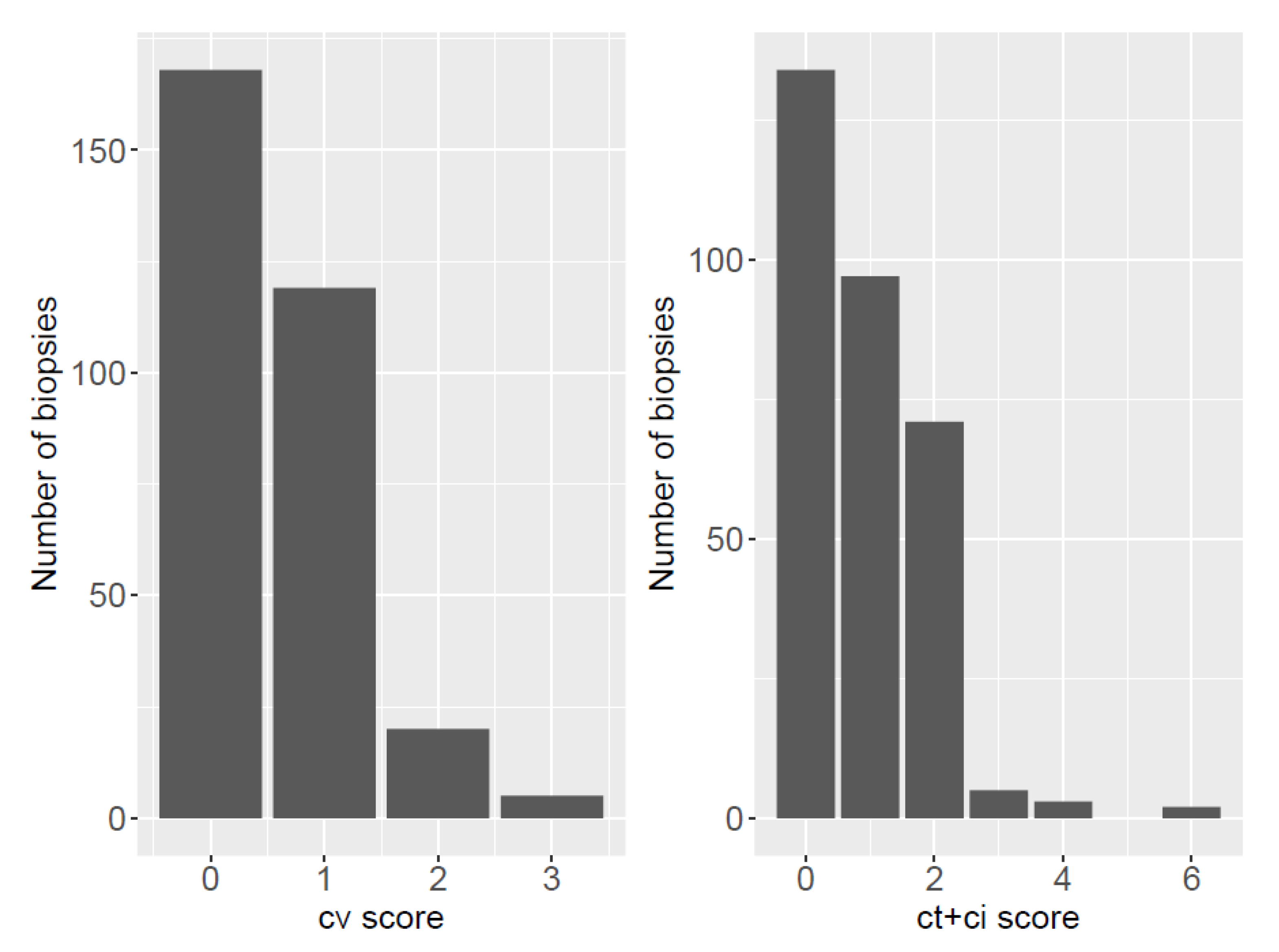
3.2. Biopsy Characteristics: Banff Classification and Chronic Lesions
3.3. Chronic Lesions
3.4. Management of Subclinical Rejections
3.5. Propensity Score Coefficients
3.6. One-Year Biopsy Proven Acute-Rejection Rates
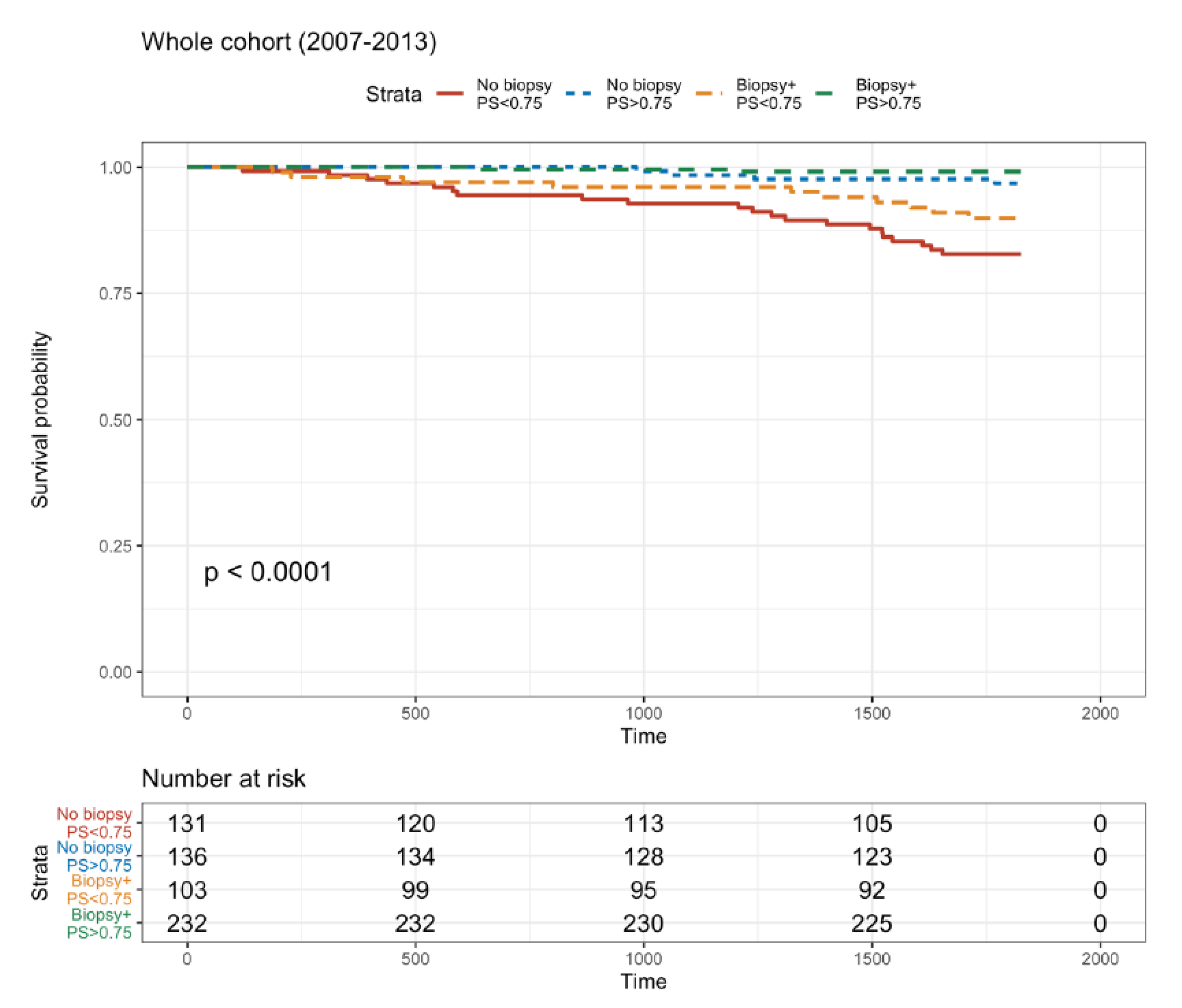
3.7. Five-Year Death-Censored Graft Survival Rate with or without a Biopsy
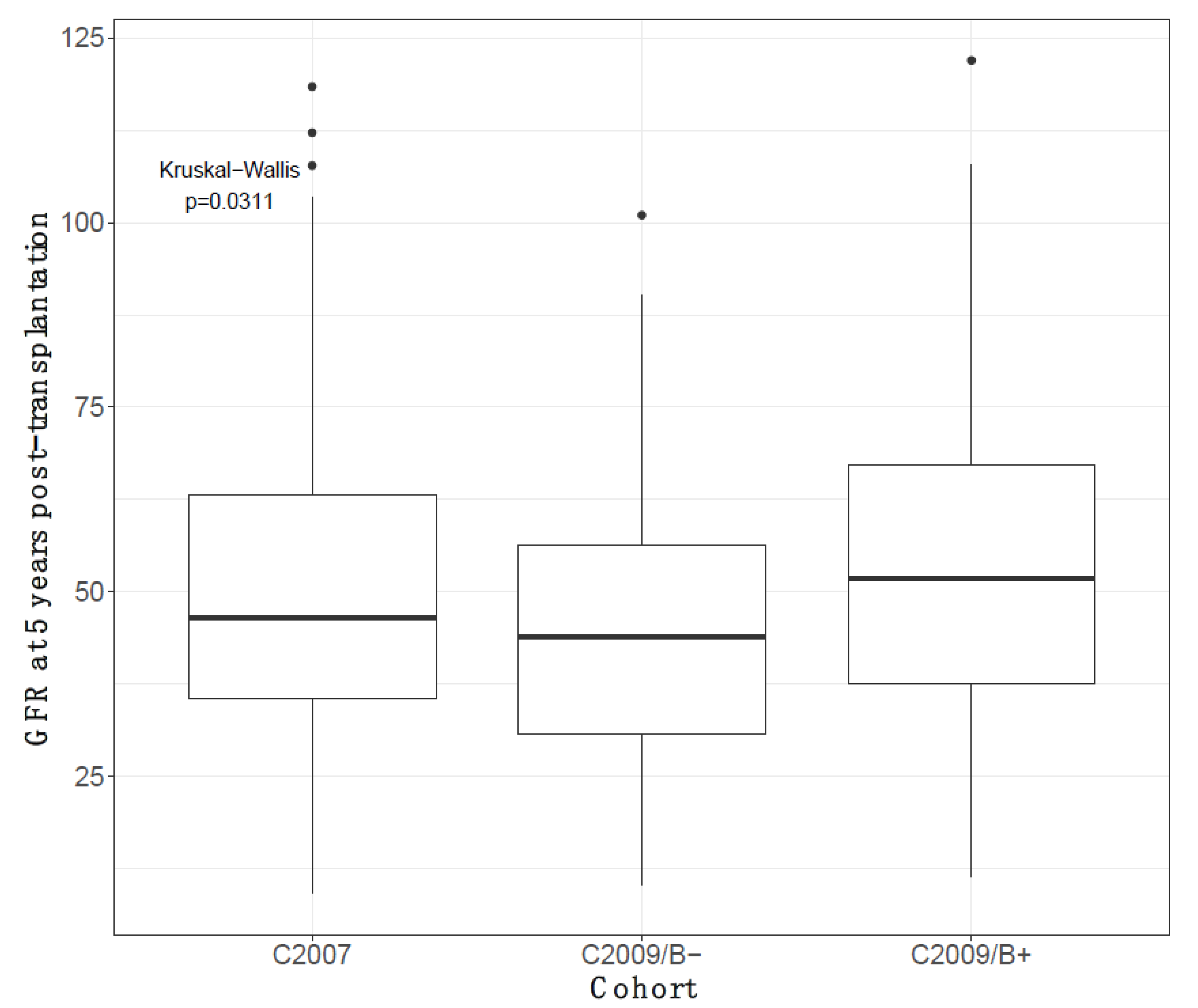
3.8. Five-Year Decline in eGFR According to Having or Not Having a Biopsy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PKB | Protocol kidney biopsy |
| DCGS | Death-censored graft survival |
| PS | Propensity score |
| CKD | Chronic kidney disease |
| ESRD | End-stage renal disease |
| KTx | Kidney transplantation |
| BKV | BK polyomavirus |
| TKB | Transplant kidney biopsy |
| SCR | Subclinical rejection |
| IF/TA | Interstitial fibrosis and tubular atrophy |
| CNI | Calcineurin inhibitor |
| RBC+ | Red-blood-cell transfusion |
| DGF | Delayed graft function |
| BMI | Body-mass index |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration equation |
| GFR | Glomerular filtration rate |
| CMV | Cytomegalovirus |
| EBV | Epstein–Barr virus |
| MMF | Mycophenolate mofetil |
| MN | Membranous nephropathy |
| MPGN | Membranoproliferative glomerulonephritis |
| s-ABMR | Subclinical antibody-mediated rejection |
| TCMR | T-cell-mediated rejection |
References
- World Kidney Day: Chronic Kidney Disease. 2015. Available online: http://www.worldkidneyday.org/faqs/chronic-kidney-disease/ (accessed on 16 August 2021).
- Evans, R.W.; Manninen, D.L.; Garrison, L.P., Jr.; Hart, L.G.; Blagg, C.R.; Gutman, R.A.; Hull, A.R.; Lowrie, E.G. The quality of life of patients with end-stage renal disease. N. Engl. J. Med. 1985, 312, 553–559. [Google Scholar] [CrossRef]
- Matas, A.J.; Smith, J.M.; Skeans, M.A.; Thompson, B.; Gustafson, S.K.; Stewart, D.E.; Cherikh, W.S.; Wainright, J.L.; Boyle, G.J.; Snyder, J.; et al. OPTN/SRTR 2013. Annual Data Report: Kidney. Am. J. Transplant. 2015, 15 (Suppl. 2), 1–34. [Google Scholar] [CrossRef]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.A.; Lamb, K.E.; Meier-Kriesche, H.U. Solid organ allograft survival improvement in the United States: The long-term does not mirror the dramatic short-term success. Am. J. Transplant. 2011, 11, 1226–1235. [Google Scholar] [CrossRef]
- Chapman, J.R. What are the key challenges we face in kidney transplantation today? Transplant. Res. 2013, 2, S1. [Google Scholar] [CrossRef]
- Sellares, J.; de Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am. J. Transplant. 2012, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Legendre, C.; Thervet, E.; Skhiri, H.; Mamzer-Bruneel, M.F.; Cantarovich, F.; Noël, L.H.; Kreis, H. Histologic features of chronic allograft nephropathy revealed by protocol biopsies in kidney transplant recipients. Transplantation 1998, 65, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- Nankivell, B.J.; Borrows, R.J.; Fung, C.L.S.; O’Connell, P.J.; Allen, R.D.M.; Chapman, J.R. Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation 2004, 78, 242–249. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Fenton-Lee, C.A.; Kuypers, D.R.; Cheung, E.; Allen, R.D. Effect of histological damage on long-term kidney transplant outcome. Transplantation 2001, 71, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Rush, D.N.; Jeffery, J.R.; Gough, J. Sequential protocol biopsies in renal transplant patients. Transplantation 1995, 59, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Anil Kumar, M.S.; Irfan Saeed, M.; Ranganna, K.; Malat, G.; Sustento-Reodica, N.; Kumar, A.M.S.; Meyers, W.C. Comparison of four different immunosuppression protocols without long-term steroid therapy in kidney recipients monitored by surveillance biopsy: Five-year outcomes. Transplant. Immunol. 2008, 20, 32–42. [Google Scholar] [CrossRef]
- Choi, B.S.; Shin, M.J.; Shin, S.J.; Kim, Y.S.; Choi, Y.J.; Kim, Y.S.; Moon, I.S.; Kim, S.Y.; Koh, Y.B.; Bang, B.K.; et al. Clinical significance of an early protocol biopsy in living-donor renal transplantation: Ten-year experience at a single center. Am. J. Transplant. 2005, 5, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Nankivell, B.J.; Chapman, J.R. The significance of subclinical rejection and the value of protocol biopsies. Am. J. Transplant. 2006, 6, 2006–2012. [Google Scholar] [CrossRef]
- Mengel, M.; Chapman, J.R.; Cosio, F.G.; Cavaillé-Coll, M.W.; Haller, H.; Halloran, P.F.; Kirk, A.D.; Mihatsch, M.J.; Nankivell, B.J.; Racusen, L.C.; et al. Protocol biopsies in renal transplantation: Insights into patient management and pathogenesis. Am. J. Transplant. 2007, 7, 512–517. [Google Scholar] [CrossRef]
- Buchmann, T.N.; Wolff, T.; Bachmann, A.; Guerke, L.; Steiger, J.; Mihatsch, M.J.; Dickenmann, M. Repeat true surveillance biopsies in kidney transplantation. Transplantation 2012, 93, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A. Protocol transplant biopsies: Are they really needed? Clin. J. Am. Soc. Nephrol. 2006, 1, 130–137. [Google Scholar] [CrossRef]
- Huang, Y.; Farkash, E. Protocol biopsies: Utility and limitations. Adv. Chronic Kidney Dis. 2016, 23, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.K.; Nankivell, B.J.; Chapman, J.R. Surveillance Protocol Kidney Transplant Biopsies: Their Evolving Role in Clinical Practice. Am. J. Transplant. 2011, 11, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Chen, J.; Shou, Z.; Wu, J.; Wang, H.; He, Q. Clinical significance of protocol biopsy at one-month posttransplantation in deceased-donor renal transplantation. Transplant. Immunol. 2007, 17, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T. The value of long-term protocol biopsies after kidney transplantation. Nephrology 2014, 19, 2–5. [Google Scholar] [CrossRef]
- Andrassy, K.M. Comments on “KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease”. Kidney Int. 2013, 84, 622–623. [Google Scholar] [CrossRef]
- Solez, K.; Colvin, R.B.; Racusen, L.C.; Haas, M.; Sis, B.; Mengel, M.; Halloran, P.F.; Baldwin, W.; Banfi, G.; Collins, A.B.; et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am. J. Transplant. 2008, 8, 753–760. [Google Scholar] [CrossRef]
- Rush, D.N.; Nickerson, P.; Jeffery, J.R.; McKenna, R.M.; Grimm, P.C.; Gough, J. Protocol biopsies in renal transplantation: Research tool or clinically useful? Curr. Opin. Nephrol. Hypertens. 1998, 7, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Kurtkoti, J.; Sakhuja, V.; Sud, K.; Minz, M.; Nada, R.; Kohli, H.S.; Gupta, K.L.; Joshi, K.; Jha, V. The utility of 1- and 3-month protocol biopsies on renal allograft function: A randomized controlled study. Am. J. Transplant. 2008, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Gwinner, W.; Hiss, M.; Radermacher, J.; Mengel, M.; Haller, H. Safety and adequacy of renal transplant protocol biopsies. Am. J. Transplant. 2005, 5, 1992–1996. [Google Scholar] [CrossRef]
- Wilczek, H.E. Percutaneous needle biopsy of the renal allograft. A clinical safety evaluation of 1129 biopsies. Transplantation 1990, 50, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.A.; Chandran, S.; Burger, I.M.; Zhang, C.A.; Goldstein, R.B. Complications of Ultrasound-Guided Renal Transplant Biopsies. Am. J. Transplant. 2016, 16, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Furness, P.N.; Philpott, C.M.; Chorbadjian, M.T.; Nicholson, M.L.; Bosmans, J.L.; Corthouts, B.L.; Bogers, J.J.; Schwarz, A.; Gwinner, W.; Haller, H.; et al. Protocol biopsy of the stable renal transplant: A multicenter study of methods and complication rates. Transplantation 2003, 76, 969–973. [Google Scholar] [CrossRef]
- Gloor, J.M.; Cohen, A.J.; Lager, D.J.; Grande, J.P.; Fidler, M.E.; Velosa, J.A.; Larson, T.S.; Schwab, T.R.; Griffin, M.D.; Prieto, M.; et al. Subclinical rejection in tacrolimus-treated renal transplant recipients. Transplantation 2002, 73, 1965–1968. [Google Scholar] [CrossRef]
- Moreso, F.; Seron, D.; Carrera, M.; Gil-Vernet, S.; Cruzado, J.M.; Hueso, M.; Fulladosa, X.; Ramos, R.; Ibernon, M.; Castelao, A.M.; et al. Baseline immunosuppression is associated with histological findings in early protocol biopsies. Transplantation 2004, 78, 1064–1068. [Google Scholar] [CrossRef]
- Mehta, R.; Sood, P.; Hariharan, S. Subclinical Rejection in Renal Transplantation: Reappraised. Transplantation 2016, 100, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Gotti, E.; Perico, N.; Perna, A.; Gaspari, F.; Cattaneo, D.; Caruso, R.; Ferrari, S.; Stucchi, N.; Marchetti, G.; Abbate, M.; et al. Renal transplantation: Can we reduce calcineurin inhibitor/stop steroids? Evidence based on protocol biopsy findings. J. Am. Soc. Nephrol. 2003, 14, 755–766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wehmeier, C.; Hirt-Minkowski, P.; Amico, P.; Georgalis, A.; Hopfer, H.; Steiger, J.; Dickenmann, M.; Schaub, S. Successful steroid withdrawal guided by surveillance biopsies-A single-center experience. Clin. Transplant. 2018, 32, e13181. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.C.; Kammer, M.; Kainz, A. Steroids and ciclosporin withdrawal after renal transplantation: A retrospective cohort study. BMC Med. 2017, 15, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernández, D.; Alonso-Titos, J.; Vázquez, T.; León, M.; Caballero, A.; Cobo, M.A.; Sola, E.; López, V.; Ruiz-Esteban, P.; Cruzado, J.M.; et al. Clinical Relevance of Corticosteroid Withdrawal on Graft Histological Lesions in Low-Immunological-Risk Kidney Transplant Patients. J. Clin. Med. 2021, 10, 2005. [Google Scholar] [CrossRef]
- Cosio, F.G.; El Ters, M.; Cornell, L.D.; Schinstock, C.A.; Stegall, M.D. Changing Kidney Allograft Histology Early Posttransplant: Prognostic Implications of 1-Year Protocol Biopsies. Am. J. Transplant. 2016, 16, 194–203. [Google Scholar] [CrossRef]
- Nanmoku, K.; Kurosawa, A.; Kubo, T.; Shinzato, T.; Shimizu, T.; Kimura, T.; Yagisawa, T. Effective and Safe Reduction of Conventional Immunosuppressants Using Everolimus in Maintenance Kidney Transplant Recipients. Transplant. Proc. 2017, 49, 1724–1728. [Google Scholar] [CrossRef]
- Wojciechowski, D.; Chandran, S.; Vincenti, F. Early post-transplant conversion from tacrolimus to belatacept for prolonged delayed graft function improves renal function in kidney transplant recipients. Clin. Transplant. 2017, 31, 5. [Google Scholar] [CrossRef]
- Kuss, O.; Blettner, M.; Börgermann, J. Propensity Score: An Alternative Method of Analyzing Treatment Effects. Dtsch. Ärzteblatt Int. 2016, 113, 597–603. [Google Scholar] [CrossRef]
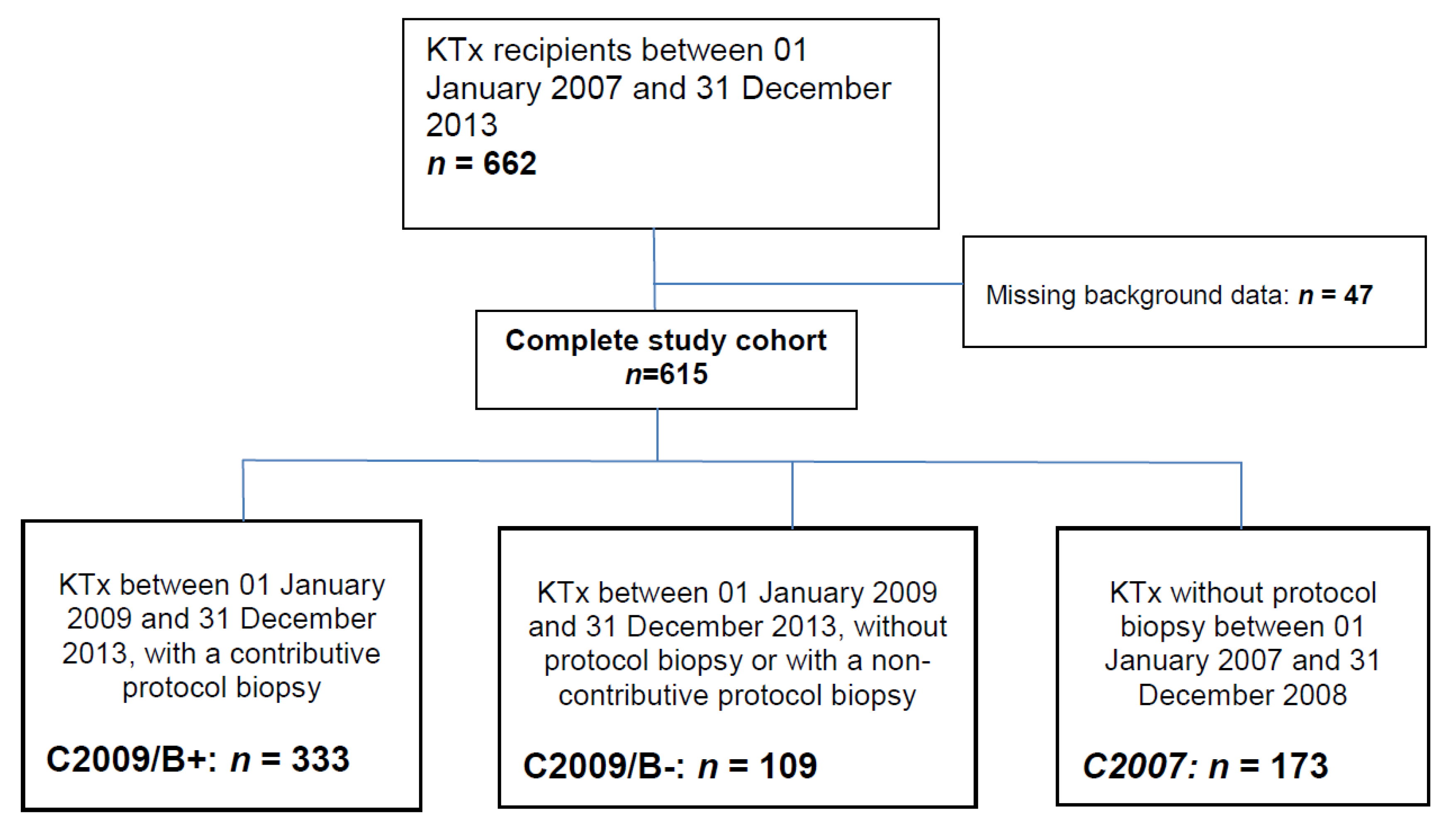
| C2007 1 (n = 173) | C2009/B− 2 (n = 109) | C2009/B+ 3 (n = 333) | Total (n = 615) | p | |
|---|---|---|---|---|---|
| Recipient age, year | 0.001 | ||||
| Mean (SD) | 50.9 (14.6) | 57.1 (12.5) | 51.8 (14.0) | 52.5 (14.1) | |
| HBP history | 138 (80%) | 73 (67%) | 236 (70%) | 447 (72%) | 0.031 |
| Diabetes history | 19 (11%) | 16 (15%) | 47 (14%) | 82 (13%) | 0.565 |
| BMI, kg/m2 | 0.738 | ||||
| Mean (SD) | 24.3 (4.1) | 24.6 (4.2) | 24.8 (4.9) | 24.6 (4.6) | |
| PreTx HLA sensitization | 40 (23%) | 36 (33%) | 83 (25%) | 159 (26%) | 0.149 |
| Dialysis vintage, year | 0.210 | ||||
| Mean (SD) | 3.5 (5.1) | 4.4 (5.7) | 3.0 (3.3) | 3.4 (4.4) | |
| Donor age, year | <0.001 | ||||
| Mean (SD) | 47.7 (16.7) | 56.9 (15.0) | 52.0 (15.5) | 51.6 (16.1) | |
| Donor type | 0.017 | ||||
| Living | 14 (8%) | 9 (8%) | 38 (11%) | 61 (10%) | |
| BD | 159 (92%) | 98 (90%) | 281 (84%) | 538 (87%) | |
| DCD | 0 | 2 (2%) | 16 (5%) | 18 (3%) | |
| Steroids on day 7 postTx | <0.001 | ||||
| Mean (SD) | 6.5 (4.8) | 8.4 (5.8) | 9.3 (12.9) | 8.3 (10.2) | |
| ATG induction | 159 (92%) | 95 (87%) | 276 (82%) | 530 (86%) | 0.013 |
| Early (first 3 months) blood transfusion | 47 (27%) | 48 (44%) | 69 (21%) | 164 (27%) | <0.001 |
| Estimate | Std. Error | z Value | Pr (>|z|) | |
|---|---|---|---|---|
| (Intercept) | 1.75 | 0.17 | 10.06 | <0.001 |
| Recipients’ age (years) | −0.036 | 0.015 | −2.45 | 0.014 |
| Donors’ age (years) | 0.0011 | 0.012 | 0.087 | 0.93 |
| Graft rank | −0.89 | 0.26 | −3.47 | <0.001 |
| Early RBC transfusion (yes/no) | −0.88 | 0.26 | −3.39 | <0.001 |
| Pre-immunization (yes/no) | 0.12 | 0.30 | 0.41 | 0.68 |
| Delayed graft function (yes/no) | −1.18 | 0.37 | −3.19 | 0.001 |
| BMI (kg/m2) | 0.026 | 0.027 | 0.98 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrec, F.; Noble, J.; Naciri-Bennani, H.; Malvezzi, P.; Janbon, B.; Emprou, C.; Giovannini, D.; Rostaing, L.; Jouve, T. Protocol Biopsies on de novo Renal-Transplants at 3 Months after Surgery: Impact on 5-Year Transplant Survival. J. Clin. Med. 2021, 10, 3635. https://doi.org/10.3390/jcm10163635
Terrec F, Noble J, Naciri-Bennani H, Malvezzi P, Janbon B, Emprou C, Giovannini D, Rostaing L, Jouve T. Protocol Biopsies on de novo Renal-Transplants at 3 Months after Surgery: Impact on 5-Year Transplant Survival. Journal of Clinical Medicine. 2021; 10(16):3635. https://doi.org/10.3390/jcm10163635
Chicago/Turabian StyleTerrec, Florian, Johan Noble, Hamza Naciri-Bennani, Paolo Malvezzi, Bénédicte Janbon, Camille Emprou, Diane Giovannini, Lionel Rostaing, and Thomas Jouve. 2021. "Protocol Biopsies on de novo Renal-Transplants at 3 Months after Surgery: Impact on 5-Year Transplant Survival" Journal of Clinical Medicine 10, no. 16: 3635. https://doi.org/10.3390/jcm10163635
APA StyleTerrec, F., Noble, J., Naciri-Bennani, H., Malvezzi, P., Janbon, B., Emprou, C., Giovannini, D., Rostaing, L., & Jouve, T. (2021). Protocol Biopsies on de novo Renal-Transplants at 3 Months after Surgery: Impact on 5-Year Transplant Survival. Journal of Clinical Medicine, 10(16), 3635. https://doi.org/10.3390/jcm10163635








