Microbiome Metabolites and Thyroid Dysfunction
Abstract
:1. Introduction
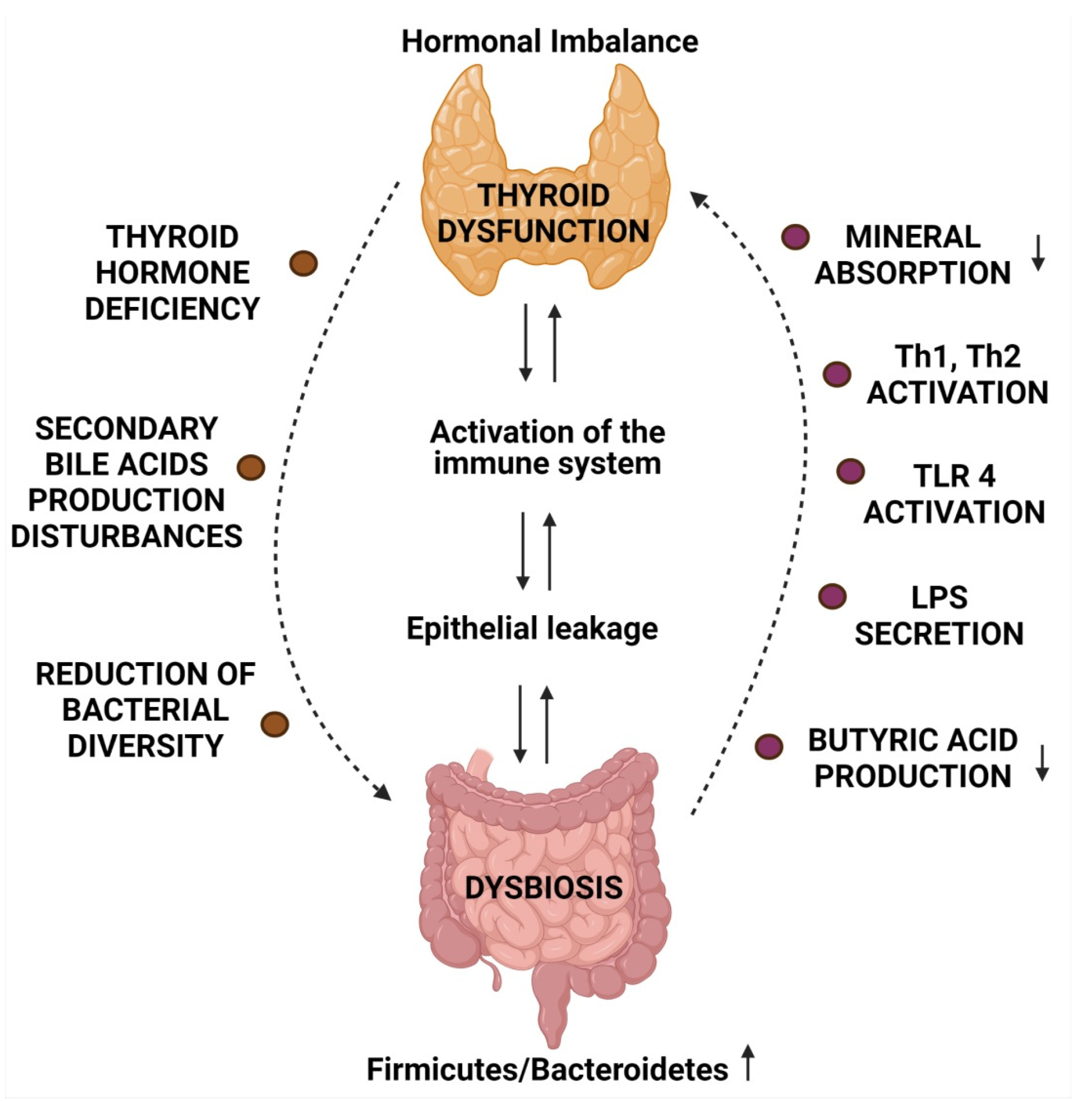
2. Microbiome and Thyroid Diseases
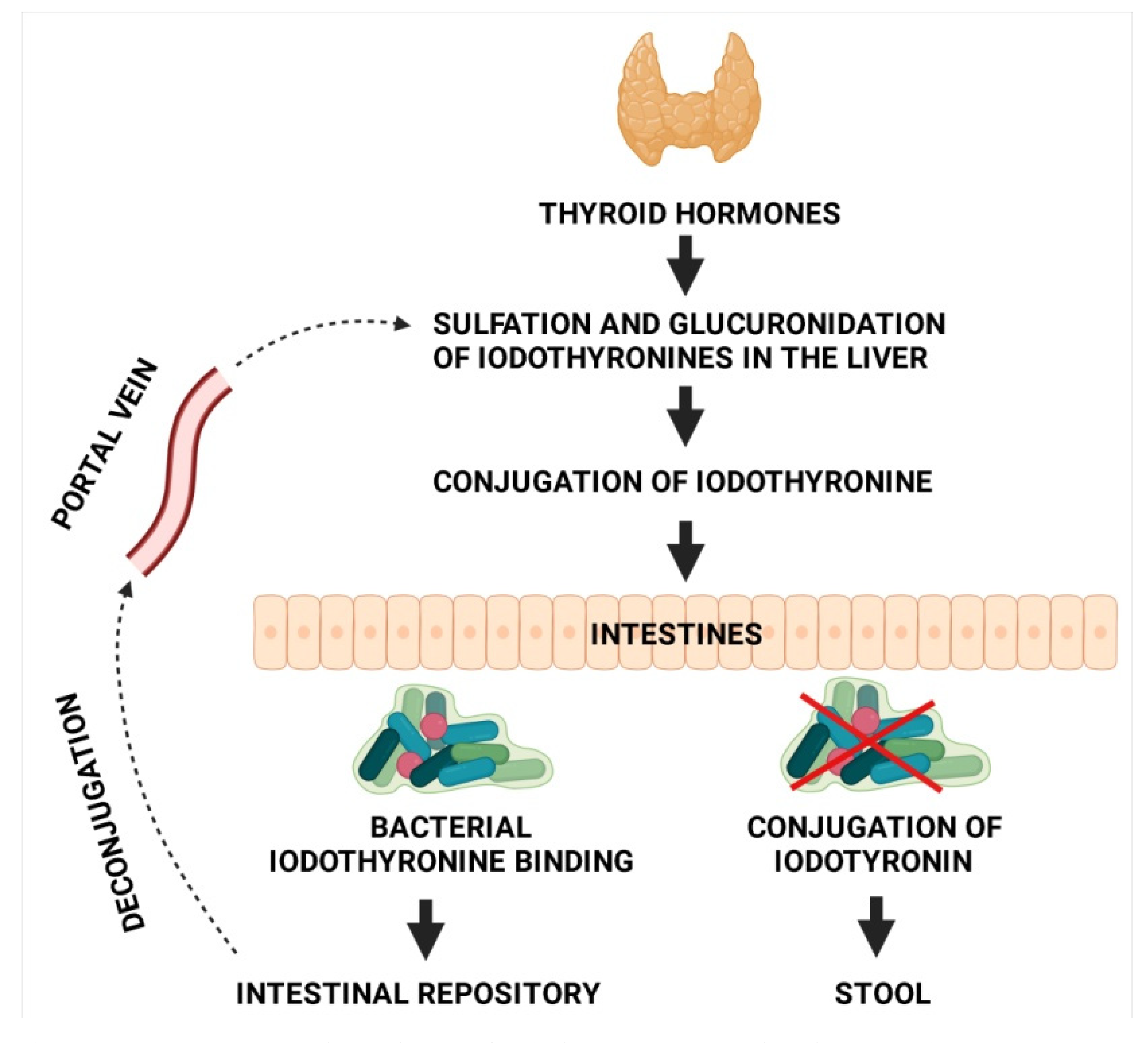
3. Microbiota and Thyroid Hormone Metabolism
4. Mineral Absorption and Microbiome
4.1. Iodine
4.2. Iron
4.3. Selenium
4.4. Zinc
4.5. Copper
5. The Impact of Oral Thyroid Hormone Supplementation on Microbiome
6. Microbiota and Immune Response in Autoimmune Diseases
6.1. Mechanism Contributing to the Development of AITD in the Course of Infection
6.2. Alterations in Gut Microbiome Leading to Augmented Immune Response
7. Short-Chain Fatty Acids
8. Secondary Bile Acids
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cuan-Baltazar, Y.; Soto-Vega, E. Microorganisms associated to thyroid autoimmunity. Autoimmun. Rev. 2020, 19, 102614. [Google Scholar] [CrossRef]
- Vanderpump, M.P.J. The epidemiology of thyroid disease. Br. Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weetman, A.; DeGroot, L.J. Autoimmunity to the Thyroid Gland. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.C.; Paquette, I.M. Surveillance, Epidemiology, and End Results (SEER) and SEER-Medicare Databases: Use in Clinical Research for Improving Colorectal Cancer Outcomes. Clin. Colon Rectal Surg. 2019, 32, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Marlicz, W.; Yung, D.E.; Skonieczna-Żydecka, K.; Loniewski, I.; van Hemert, S.; Loniewska, B.; Koulaouzidis, A. From clinical uncertainties to precision medicine: The emerging role of the gut barrier and microbiome in small bowel functional diseases. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 961–978. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Nakagawa, Y.; Ozaki, H. Does the gut microbiota trigger Hashimoto’s thyroiditis? Discov. Med. 2012, 14, 321–326. [Google Scholar] [PubMed]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 2019, 76, 3917–3937. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the microbiota and the immune system. Genes Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Zhao, F.; Feng, J.; Li, J.; Zhao, L.; Liu, Y.; Chen, H.; Jin, Y.; Zhu, B.; Wei, Y. Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid 2018, 28, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Hasler, W.L. Rome IV—Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R.; Hernandez-Sanabria, E.; Calatayud Arroyo, M.; Props, R.; Van de Wiele, T. Propionate-Producing Consortium Restores Antibiotic-Induced Dysbiosis in a Dynamic in vitro Model of the Human Intestinal Microbial Ecosystem. Front. Microbiol. 2019, 10, 1206. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020, 20, e15. [Google Scholar] [CrossRef]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef] [Green Version]
- Berding, K.; Donovan, S.M. Diet Can Impact Microbiota Composition in Children With Autism Spectrum Disorder. Front. Neurosci. 2018, 12, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G675–G685. [Google Scholar] [CrossRef]
- Hoskins, L.C.; Agustines, M.; McKee, W.B.; Boulding, E.T.; Kriaris, M.; Niedermeyer, G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J. Clin. Investig. 1985, 75, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, H.M.; Mohammad, I.S.; Guo, H.; Shahzad, M.; Hou, Y.J.; Ma, C.; Naseem, Z.; Wu, X.; Shi, P.; Xu, J. Molecular estimation of alteration in intestinal microbial composition in Hashimoto’s thyroiditis patients. Biomed. Pharmacother. 2017, 95, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Smyk, D.S.; Koutsoumpas, A.L.; Mytilinaiou, M.G.; Rigopoulou, E.I.; Sakkas, L.I.; Bogdanos, D.P. Helicobacter pylori and autoimmune disease: Cause or bystander. World J. Gastroenterol. WJG 2014, 20, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Aghili, R.; Jafarzadeh, F.; Ghorbani, R.; Khamseh, M.E.; Salami, M.A.; Malek, M. The association of Helicobacter pylori infection with Hashimoto’s thyroiditis. Acta Med. Iran. 2013, 51, 293–296. [Google Scholar]
- Bertalot, G.; Montresor, G.; Tampieri, M.; Spasiano, A.; Pedroni, M.; Milanesi, B.; Favret, M.; Manca, N.; Negrini, R. Decrease in thyroid autoantibodies after eradication of Helicobacter pylori infection. Clin. Endocrinol. 2004, 61, 650–652. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, W.; Zhang, C.; Wang, T.; Guo, X.; Wu, L.; Qin, L.; Liu, T. Meta-analysis of the correlation between Helicobacter pylori infection and autoimmune thyroid diseases. Oncotarget 2017, 8, 115691–115700. [Google Scholar] [CrossRef] [Green Version]
- Koppel, N.; Rekdal, V.M.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, eaag2770. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.N.; Turnbaugh, P.J. Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J. Clin. Investig. 2014, 124, 4173–4181. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 479–490. [Google Scholar] [CrossRef] [Green Version]
- DiStefano, J.J.; de Luze, A.; Nguyen, T.T. Binding and degradation of 3,5,3′-triiodothyronine and thyroxine by rat intestinal bacteria. Am. J. Physiol. Endocrinol. Metab. 1993, 264, E966–E972. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virili, C.; Centanni, M. Does microbiota composition affect thyroid homeostasis? Endocrine 2014, 49, 583–587. [Google Scholar] [CrossRef]
- Nguyen, T.T.; DiStefano, J.J.; Yamada, H.; Yen, Y.M. Steady state organ distribution and metabolism of thyroxine and 3,5,3′-triiodothyronine in intestines, liver, kidneys, blood, and residual carcass of the rat in vivo. Endocrinology 1993, 133, 2973–2983. [Google Scholar] [CrossRef]
- Sabatino, L.; Iervasi, G.; Ferrazzi, P.; Francesconi, D.; Chopra, I.J. A study of iodothyronine 5′-monodeiodinase activities in normal and pathological tissues in man and their comparison with activities in rat tissues. Life Sci. 2000, 68, 191–202. [Google Scholar] [CrossRef]
- Stuss, M.; Michalska-Kasiczak, M.; Sewerynek, E. The role of selenium in thyroid gland pathophysiology. Endokrynol. Pol. 2017, 68, 440–465. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, V.; Tafaro, E.; Giagulli, V.A.; Sabbà, C.; Resta, F.; Licchelli, B.; Guastamacchia, E. Role of iodine, selenium and other micronutrients in thyroid function and disorders. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 277–294. [Google Scholar] [CrossRef]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef]
- Vought, R.L.; Brown, F.A.; Sibinovic, K.H.; Mc Daniel, E.G. Effect of Changing Intestinal Bacterial Flora on Thyroid Function in the Rat. Horm. Metab. Res. 1972, 4, 43–47. [Google Scholar] [CrossRef]
- Navarro, A.M.; Suen, V.M.M.; Souza, I.M.; De Oliveira, J.E.D.; Marchini, J.S. Patients with severe bowel malabsorption do not have changes in iodine status. Nutrition 2005, 21, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Michalaki, M.; Volonakis, S.; Mamali, I.; Kalfarentzos, F.; Vagenakis, A.G.; Markou, K.B. Dietary iodine absorption is not influenced by malabsorptive bariatric surgery. Obes. Surg. 2014, 24, 1921–1925. [Google Scholar] [CrossRef]
- Virili, C.; Centanni, M. “With a little help from my friends”-The role of microbiota in thyroid hormone metabolism and enterohepatic recycling. Mol. Cell. Endocrinol. 2017, 458, 39–43. [Google Scholar] [CrossRef]
- Skrypnik, K.; Suliburska, J. Association between the gut microbiota and mineral metabolism. J. Sci. Food Agric. 2018, 98, 2449–2460. [Google Scholar] [CrossRef]
- Ren, G.; Yu, M.; Li, K.; Hu, Y.; Wang, Y.; Xu, X.; Qu, J. Seleno-lentinan prevents chronic pancreatitis development and modulates gut microbiota in mice. J. Funct. Foods 2016, 22, 177–188. [Google Scholar] [CrossRef]
- Lavu, R.V.S.; Van De Wiele, T.; Pratti, V.L.; Tack, F.; Du Laing, G. Selenium bioaccessibility in stomach, small intestine and colon: Comparison between pure Se compounds, Se-enriched food crops and food supplements. Food Chem. 2016, 197, 382–387. [Google Scholar] [CrossRef]
- Sandström, B.; Cederblad, A. Zinc absorption from composite meals II. Influence of the main protein source. Am. J. Clin. Nutr. 1980, 33, 1778–1783. [Google Scholar] [CrossRef]
- August, D.; Janghorbani, M.; Young, V.R. Determination of zinc and copper absorption at three dietary Zn-Cu ratios by using stable isotope methods in young adult and elderly subjects. Am. J. Clin. Nutr. 1989, 50, 1457–1463. [Google Scholar] [CrossRef]
- Iftikhar, A.; Khaliq, T.; Khan, J.; Rahman, Z.; Rahman, S.; Anwar, H.; Javed, I.; Muzaffar, H.; Mahmood, A. Efficacy of Vitamins, Probiotics and Protein Supplementation on Serum Health Biomarkers of Molted Male Layer Breeders. Pak. Vet. J. 2015, 35, 519–521. [Google Scholar]
- Reed, S.; Neuman, H.; Moscovich, S.; Glahn, R.P.; Koren, O.; Tako, E. Chronic Zinc Deficiency Alters Chick Gut Microbiota Composition and Function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yang, X.; Yuan, Y.; Jia, Y.; Liu, G.; Lin, N.; Xiao, H.; Zhang, L.; Chen, J. Toxicity, gut microbiota and metabolome effects after copper exposure during early life in SD rats. Toxicology 2020, 433–434, 152395. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.M.; Verdu, E.F. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacol. Res. 2013, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Virili, C.; Bassotti, G.; Santaguida, M.G.; Iuorio, R.; Del Duca, S.C.; Mercuri, V.; Picarelli, A.; Gargiulo, P.; Gargano, L.; Centanni, M. Atypical Celiac Disease as Cause of Increased Need for Thyroxine: A Systematic Study. J. Clin. Endocrinol. Metab. 2012, 97, E419–E422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santaguida, M.G.; Virili, C.; Del Duca, S.C.; Cellini, M.; Gatto, I.; Brusca, N.; De Vito, C.; Gargano, L.; Centanni, M. Thyroxine softgel capsule in patients with gastric-related T4 malabsorption. Endocrine 2015, 49, 51–57. [Google Scholar] [CrossRef]
- Brechmann, T.; Sperlbaum, A.; Schmiegel, W. Levothyroxine therapy and impaired clearance are the strongest contributors to small intestinal bacterial overgrowth: Results of a retrospective cohort study. World J. Gastroenterol. 2017, 23, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhao, M.; Gong, Y.; Chen, W.; Wang, Q.; Fu, Y.; Guo, T.; Zhao, J.; Gao, L.; Bo, T. Relation of Gut Microbes and L-Thyroxine Through Altered Thyroxine Metabolism in Subclinical Hypothyroidism Subjects. Front. Cell. Infect. Microbiol. 2020, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Evans, A.T.; Costall, B.; Smythe, J.W. Thyroid hormones, brain function and cognition: A brief review. Neurosci. Biobehav. Rev. 2002, 26, 45–60. [Google Scholar] [CrossRef]
- Fu The Synthetic Thyroid Hormone, Levothyroxine, Protects Cholinergic Neurons in the Hippocampus of Naturally Aged Mice. Available online: https://www.nrronline.org/article.asp?issn=1673-5374;year=2014;volume=9;issue=8;spage=864;epage=871;aulast=Fu (accessed on 17 June 2021).
- Virili, C.; Fallahi, P.; Antonelli, A.; Benvenga, S.; Centanni, M. Gut microbiota and Hashimoto’s thyroiditis. Rev. Endocr. Metab. Disord. 2018, 19, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef]
- Sasso, F.C.; Carbonara, O.; Torella, R.; Mezzogiorno, A.; Esposito, V.; Demagistris, L.; Secondulfo, M.; Carratu’, R.; Iafusco, D.; Cartenì, M. Ultrastructural changes in enterocytes in subjects with Hashimoto’s thyroiditis. Gut 2004, 53, 1878–1880. [Google Scholar] [CrossRef] [Green Version]
- Ercolini, A.M.; Miller, S.D. The role of infections in autoimmune disease. Clin. Exp. Immunol. 2009, 155, 1–15. [Google Scholar] [CrossRef]
- Benvenga, S.; Guarneri, F. Molecular mimicry and autoimmune thyroid disease. Rev. Endocr. Metab. Disord. 2016, 17, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Santarpia, L.; Trimarchi, F.; Guarneri, F. Human Thyroid Autoantigens and Proteins of Yersinia and Borrelia Share Amino Acid Sequence Homology That Includes Binding Motifs to HLA-DR Molecules and T-Cell Receptor. Thyroid 2006, 16, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobaru, M.; Ashida, K.; Yoshinobu, S.; Nagayama, A.; Kabashima, M.; Iwata, S.; Hasuzawa, N.; Tsuruta, M.; Wada, N.; Nakayama, H.; et al. Human Leukocyte Antigen (HLA) Subtype-Dependent Development of Myasthenia Gravis, Type-1 Diabetes Mellitus, and Hashimoto Disease: A Case Report of Autoimmune Polyendocrine Syndrome Type 3. Am. J. Case Rep. 2019, 20, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Morrison, K.E.; Bale, T.L. Sex differences in the gut microbiome–brain axis across the lifespan. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150122. [Google Scholar] [CrossRef] [Green Version]
- Covelli, D.; Ludgate, M. The thyroid, the eyes and the gut: A possible connection. J. Endocrinol. Investig. 2017, 40, 567–576. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Ahmed, A.; Wu, D.; Liu, L.; Qiu, J.; Yan, Y.; Jin, M.; Xin, Y. Gut microbe analysis between hyperthyroid and healthy individuals. Curr. Microbiol. 2014, 69, 675–680. [Google Scholar] [CrossRef]
- Hosseini, A.M.; Majidi, J.; Baradaran, B.; Yousefi, M. Toll-Like Receptors in the Pathogenesis of Autoimmune Diseases. Adv. Pharm. Bull. 2015, 5, 605–614. [Google Scholar] [CrossRef]
- Wallukat, G.; Schimke, I. Lethal immunoglobulins: Autoantibodies and sudden cardiac death. Autoimmun. Rev. 2019, 18, 749–750. [Google Scholar] [CrossRef]
- Dvornikova, K.A.; Bystrova, E.Y.; Platonova, O.N.; Churilov, L.P. Polymorphism of toll-like receptor genes and autoimmune endocrine diseases. Autoimmun. Rev. 2020, 19, 102496. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, M.; Baron, B. The Role of Toll-Like Receptors in Autoimmune Diseases through Failure of the Self-Recognition Mechanism. Int. J. Inflamm. 2017, 2017, 8391230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunc, M.; Gabrych, A.; Witkowski, J.M. Microbiome impact on metabolism and function of sex, thyroid, growth and parathyroid hormones. Acta Biochim. Pol. 2016, 63, 189–201. [Google Scholar] [CrossRef]
- Su, X.; Zhao, Y.; Li, Y.; Ma, S.; Wang, Z. Gut dysbiosis is associated with primary hypothyroidism with interaction on gut-thyroid axis. Clin. Sci. 2020, 134, 1521–1535. [Google Scholar] [CrossRef]
- Dolan, K.; Finley, H.; Gasta, M.; Houseman, S. Managing Hashimoto’s Thyroiditis Through Personalized Care: A Case Report. Altern. Ther. Health Med. 2018, 24, 56–61. [Google Scholar]
- Cornejo-Pareja, I.; Ruiz-Limón, P.; Gómez-Pérez, A.M.; Molina-Vega, M.; Moreno-Indias, I.; Tinahones, F.J. Differential Microbial Pattern Description in Subjects with Autoimmune-Based Thyroid Diseases: A Pilot Study. J. Pers. Med. 2020, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Kuczyńska, B.; Wasilewska, A.; Biczysko, M.; Banasiewicz, T.; Drews, M. Krótkołańcuchowe kwasy Tłuszczowe–Mechanizmy działania, potencjalne zastosowania kliniczne oraz zalecenia dietetyczne. Now. Lek. 2011, 80, 299–304. [Google Scholar]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef]
- Memba, R.; Duggan, S.N.; Chonchubhair, H.M.N.; Griffin, O.M.; Bashir, Y.; O’Connor, D.B.; Murphy, A.; McMahon, J.; Volcov, Y.; Ryan, B.M.; et al. The potential role of gut microbiota in pancreatic disease: A systematic review. Pancreatology 2017, 17, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. Short-chain fatty acids and thyroid hormone interact in regulating enterocyte gene transcription. Surgery 1999, 126, 293–298. [Google Scholar] [CrossRef]
- Cayres, L.C.d.F.; de Salis, L.V.V.; Rodrigues, G.S.P.; Lengert, A.V.H.; Biondi, A.P.C.; Sargentini, L.D.B.; Brisotti, J.L.; Gomes, E.; de Oliveira, G.L.V. Detection of Alterations in the Gut Microbiota and Intestinal Permeability in Patients With Hashimoto Thyroiditis. Front. Immunol. 2021, 12, 579140. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Czajkowska, A.; Szponar, B. Short Chain Fatty Acids (SCFA), the Products of Gut Bacteria Metabolism and Their Role in the Host. Available online: https://phmd.pl/resources/html/article/details?id=168203&language=en (accessed on 9 May 2021).
- Köhling, H.L.; Plummer, S.F.; Marchesi, J.R.; Davidge, K.S.; Ludgate, M. The microbiota and autoimmunity: Their role in thyroid autoimmune diseases. Clin. Immunol. 2017, 183, 63–74. [Google Scholar] [CrossRef]
- Sepahi, A.; Liu, Q.; Friesen, L.; Kim, C.H. Dietary fiber metabolites regulate innate lymphoid cell responses. Mucosal Immunol. 2021, 14, 317–330. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Zagrodzki, P.; Prochownik, E.; Jarkiewicz, A.; Paśko, P. Influence of brassica sprouts on short chain fatty acids concentration in stools of rats with thyroid dysfunction. Acta Pol. Pharm. Drug Res. 2019, 76, 1005–1014. [Google Scholar] [CrossRef]
- Liu, S.; An, Y.; Cao, B.; Sun, R.; Ke, J.; Zhao, D. The Composition of Gut Microbiota in Patients Bearing Hashimoto’s Thyroiditis with Euthyroidism and Hypothyroidism. Int. J. Endocrinol. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Jakubczyk, K.; Maciejewska-Markiewicz, D.; Janda, K.; Kaźmierczak-Siedlecka, K.; Kaczmarczyk, M.; Łoniewski, I.; Marlicz, W. Gut Biofactory—Neurocompetent Metabolites within the Gastrointestinal Tract. A Scoping Review. Nutrients 2020, 12, 3369. [Google Scholar] [CrossRef]
- Kim, B.-T.; Kim, K.-M.; Kim, K.-N. The Effect of Ursodeoxycholic Acid on Small Intestinal Bacterial Overgrowth in Patients with Functional Dyspepsia: A Pilot Randomized Controlled Trial. Nutrients 2020, 12, 1410. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Regulation of bile acid synthesis: Pathways, nuclear receptors, and mechanisms. J. Hepatol. 2004, 40, 539–551. [Google Scholar] [CrossRef]
- Majewska, K.; Szulińska, M.; Michałowska, J.; Markuszewski, L.; Bogdański, P. Flora bakteryjna przewodu pokarmowego a choroby układu sercowo-naczyniowego. Forum Zaburzeń Metab. 2017, 8, 1–6. [Google Scholar]
- Pols, T.W.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor TGR5: A valuable metabolic target. Dig. Dis. 2011, 29, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.; Auwerx, J.; Schoonjans, K. Bile acids and the membrane bile acid receptor TGR5—Connecting nutrition and metabolism. Thyroid 2008, 18, 167–174. [Google Scholar] [CrossRef]
- Sonne, D.P. MECHANISMS IN ENDOCRINOLOGY: FXR signalling: A novel target in metabolic diseases. Eur. J. Endocrinol. 2021, 184, R193–R205. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, M.; Zhang, H.; Zhang, X.; Zhao, J.; Xu, J.; Gao, L. Thyroid-Stimulating hormone levels are inversely associated with serum total bile acid levels: A Cross-Sectional study. Endocr. Pract. 2016, 22, 420–426. [Google Scholar] [CrossRef]
- Rizos, C.V.; Elisaf, M.S.; Liberopoulos, E.N. Effects of Thyroid Dysfunction on Lipid Profile. Open Cardiovasc. Med. J. 2011, 5, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Fu, J.; Jia, Y.; Yang, N.; Li, J.; Wang, G. Serum metabolomic patterns in patients with autoimmune thyroid disease. Endocr. Pract. 2020, 26, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Bonde, Y.; Breuer, O.; Lütjohann, D.; Sjöberg, S.; Angelin, B.; Rudling, M. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J. Lipid Res. 2014, 55, 2408–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gérard, P. Metabolism of Cholesterol and Bile Acids by the Gut Microbiota. Pathogens 2013, 3, 14–24. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bargiel, P.; Szczuko, M.; Stachowska, L.; Prowans, P.; Czapla, N.; Markowska, M.; Petriczko, J.; Kledzik, J.; Jędrzejczyk-Kledzik, A.; Palma, J.; et al. Microbiome Metabolites and Thyroid Dysfunction. J. Clin. Med. 2021, 10, 3609. https://doi.org/10.3390/jcm10163609
Bargiel P, Szczuko M, Stachowska L, Prowans P, Czapla N, Markowska M, Petriczko J, Kledzik J, Jędrzejczyk-Kledzik A, Palma J, et al. Microbiome Metabolites and Thyroid Dysfunction. Journal of Clinical Medicine. 2021; 10(16):3609. https://doi.org/10.3390/jcm10163609
Chicago/Turabian StyleBargiel, Piotr, Małgorzata Szczuko, Laura Stachowska, Piotr Prowans, Norbert Czapla, Marta Markowska, Jan Petriczko, Jakub Kledzik, Alicja Jędrzejczyk-Kledzik, Joanna Palma, and et al. 2021. "Microbiome Metabolites and Thyroid Dysfunction" Journal of Clinical Medicine 10, no. 16: 3609. https://doi.org/10.3390/jcm10163609
APA StyleBargiel, P., Szczuko, M., Stachowska, L., Prowans, P., Czapla, N., Markowska, M., Petriczko, J., Kledzik, J., Jędrzejczyk-Kledzik, A., Palma, J., Zabielska, P., & Maciejewska-Markiewicz, D. (2021). Microbiome Metabolites and Thyroid Dysfunction. Journal of Clinical Medicine, 10(16), 3609. https://doi.org/10.3390/jcm10163609







