A Divide between the Western European and the Central and Eastern European Countries in the Peripheral Vascular Field: A Narrative Review of the Literature
Abstract
1. Introduction
2. Methods
3. East–West Health Divide
3.1. East–West Disparity across Europe in the Pattern of Vascular Risk Factors
3.2. Structure, Processes, and Outcomes of Vascular Care in CEECs and WECs
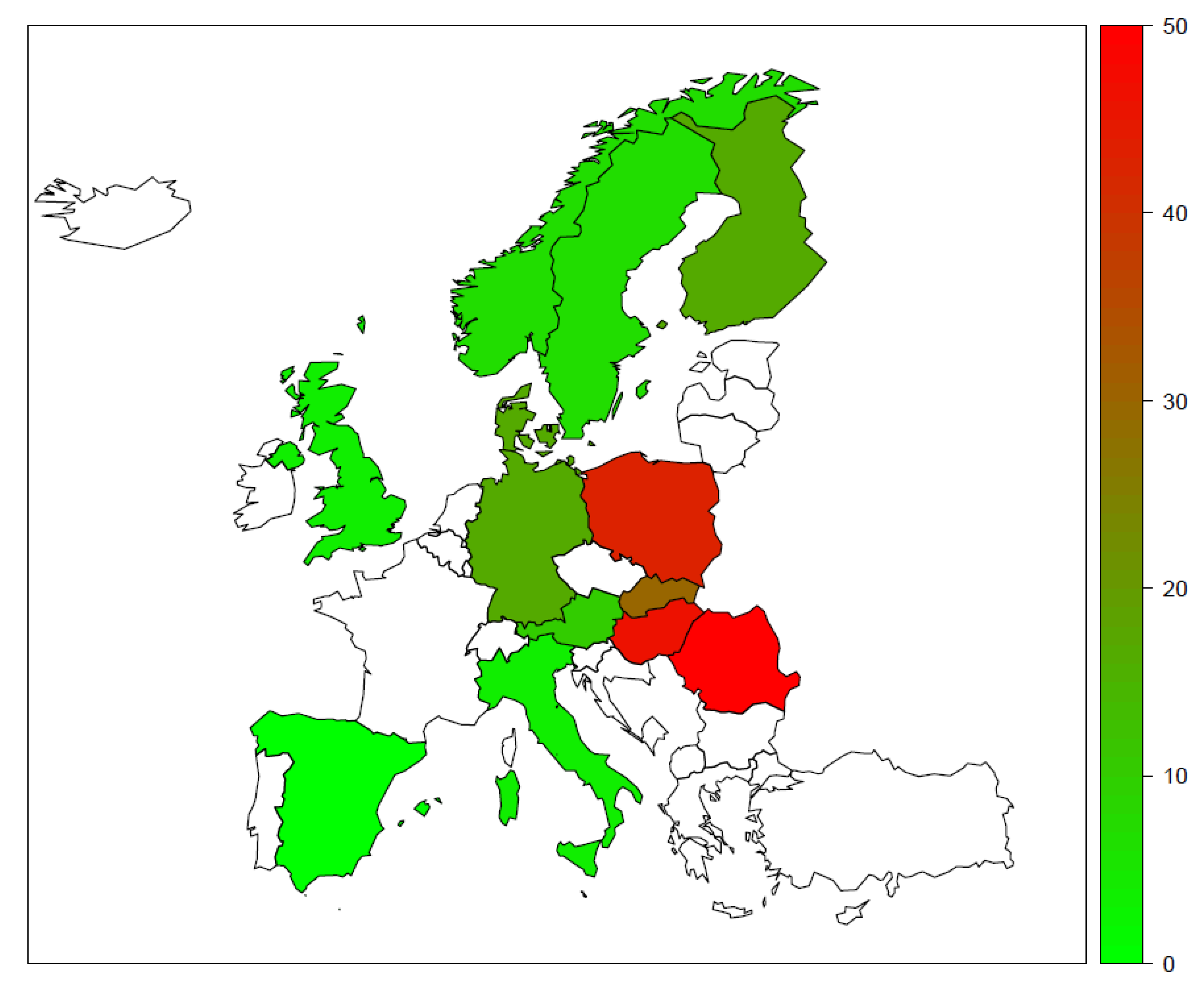
3.3. Structure Disparities
3.4. Processes
3.5. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Power, C. Health and social inequality in Europe. BMJ 1994, 308, 1153–1156. [Google Scholar] [CrossRef]
- World Health Organization. Health Status Overview for Countries of Central and Eastern Europe That Are Candidates for Accession to the European Union; WHO Regional Office for Europe: Copenhagen, Denmark, 2002; pp. 1–21. [Google Scholar]
- Mikulík, R.; Caso, V.; Bornstein, N.M.; Svobodová, V.; Pezzella, F.R.; Grecu, A.; Simsic, S.; Gdovinova, Z.; Członkowska, A.; Mishchenko, T.S.; et al. Enhancing and accelerating stroke treatment in Eastern European region: Methods and achievement of the ESO EAST program. Eur. Stroke J. 2020, 5, 204–212. [Google Scholar] [CrossRef]
- EUROASPIRE. A European Society of Cardiology survey of secondary prevention of coronary heart disease: Principal results. EUROASPIRE Study Group. European Action on Secondary Prevention through Intervention to Reduce Events. Eur. Heart J. 1997, 18, 1569–1582. [Google Scholar] [CrossRef]
- Abtan, J.; Bhatt, D.L.; Elbez, Y.; Sorbets, E.; Eagle, K.; Reid, C.M.; Baumgartner, I.; Wu, D.; Hanson, M.E.; Hannachi, H.; et al. Geographic variation and risk factors for systemic and limb ischemic events in patients with symptomatic peripheral artery disease: Insights from the REACH Registry. Clin. Cardiol. 2017, 40, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Ducrocq, G.; Bhatt, D.L.; Labreuche, J.; Corbalan, R.; Porath, A.; Gao, R.; Panchenko, E.; Liau, C.S.; Ikeda, Y.; Goto, S.; et al. Geographic differences in outcomes in outpatients with established atherothrombotic disease: Results from the REACH Registry. Eur. J. Prev. Cardiol. 2014, 21, 1509–1516. [Google Scholar] [CrossRef]
- Behrendt, C.A.; Venermo, M.; Cronenwett, J.L.; Sedrakyan, A.; Beck, A.W.; Eldrup-Jorgensen, J.; Mani, K. VASCUNET, VQI, and the International Consortium of Vascular Registries-Unique Collaborations for Quality Improvement in Vascular Surgery. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Gløersen, E.; Drăgulin, M.; Hans, S.; Kaucic, J.; Schuh, B.; Keringer, F.; Celotti, P. The Impact of Demographic Change on European Regions; European Union, Committee of the Regions: Brussels, Belgium, 2016; pp. 1–119. [Google Scholar]
- An, I.M.; Ilyina, A.; Lee, J.; Petrova, I.; Scott, A. Demographic Headwinds in Central and Eastern Europe; International Monetary Fund: Washington, DC, USA, 2019; pp. 1–101. [Google Scholar]
- OECD Library: Health at a Glance: Europe 2020: State of Health in the EU Cycle. Smoking among Adults 2020. Available online: https://www.oecd-ilibrary.org/sites/1c429c01-en/index.html?itemId=/content/component/1c429c01-en (accessed on 1 February 2021).
- Schaap, M.M.; E Kunst, A.; Leinsalu, M.; Regidor, E.; Ekholm, O.; Dzurova, D.; Helmert, U.; Klumbiene, J.; Santana, P.; Mackenbach, J.P. Effect of nationwide tobacco control policies on smoking cessation in high and low educated groups in 18 European countries. Tob. Control. 2008, 17, 248–255. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. European Tobacco Use, Trends Report; WHO Regional Office for Europe: Copenhagen, Denmark, 2019. [Google Scholar]
- Janssen, F.; El Gewily, S.; Bardoutsos, A. Smoking epidemic in Europe in the 21st century. Tob. Control. 2020. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Stefler, D.; Pikhart, H.; Kubinova, R.; Pajak, A.; Stepaniak, U.; Malyutina, S.; Simonova, G.; Peasey, A.; Marmot, M.; Bobak, M. Fruit and vegetable consumption and mortality in Eastern Europe: Longitudinal results from the Health, Alcohol and Psychosocial Factors in Eastern Europe study. Eur. J. Prev. Cardiol. 2016, 23, 493–501. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration. Repositioning of the global epicentre of non-optimal cholesterol. Nature 2020, 582, 73–77. [Google Scholar] [CrossRef]
- Chiang, C.E.; Ferrières, J.; Gotcheva, N.N.; Raal, F.J.; Shehab, A.; Sung, J.; Henriksson, K.M.; Hermans, M.P. Suboptimal Control of Lipid Levels: Results from 29 Countries Participating in the Centralized Pan-Regional Surveys on the Undertreatment of Hypercholesterolaemia (CEPHEUS). J. Atheroscler. Thromb. 2016, 23, 567–587. [Google Scholar] [CrossRef][Green Version]
- Petrov, I.; Dumitrescu, A.; Snejdrlova, M.; Zafrir, B.; Wożakowska-Kapłon, B.; Fabryova, L.; Pintarić, H.; Bridges, I.; Petkova, R. Clinical Management of High and Very High Risk Patients with Hyperlipidaemia in Central and Eastern Europe: An Observational Study. Adv Ther. 2019, 36, 608–620. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation Guideline Development G. IDF Diabetes Atlas, 9th ed.; Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf (accessed on 1 February 2021).
- Collaboration NCDRF. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- (NCD-RisC) NRFC. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Maloberti, A.; Facchetti, R.; Cuspidi, C.; Bombelli, M.; Laurent, S.; Redon, J.; Mancia, G. Within-visit BP variability, cardiovascular risk factors, and BP control in central and eastern Europe: Findings from the BP-CARE study. J. Hypertens. 2015, 33, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W.M.; Kelli, H.M.; Lisko, J.C.; Varghese, T.; Shen, J.; Sandesara, P.; Quyyumi, A.A.; Taylor, H.A.; Gulati, M.; Harold, J.G.; et al. Socioeconomic Status and Cardiovascular Outcomes: Challenges and Interventions. Circulation 2018, 137, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; DesMeules, M.; Luo, W.; Duncan, A.S.; Wielgosz, A. Socioeconomic status and cardiovascular disease: Risks and implications for care. Nat. Rev. Cardiol. 2009, 6, 712–722. [Google Scholar] [CrossRef]
- Tillmann, T.; Pikhart, H.; Peasey, A.; Kubínová, R.; Pajak, A.; Tamosiūnas, A.; Malyutina, S.; Steptoe, A.; Kivimäki, M.; Marmot, M.; et al. Psychosocial and socioeconomic determinants of cardiovascular mortality in Eastern Europe: A multicentre prospective cohort study. PLoS Med. 2017, 14, e1002459. [Google Scholar] [CrossRef] [PubMed]
- Djankov, S.; Nikolova, E.; Zilinsky, J. The happiness gap in eastern Europe. J. Comp. Econ. 2016, 44, 108–124. [Google Scholar] [CrossRef]
- Huber, A.; Höfer, S.; Saner, H.; Oldridge, N. East-West divide in health-related quality of life across Europe: Results from the HeartQoL sub-study. Eur. J. Prev. Cardiol. 2020, 27, 1112–1115. [Google Scholar] [CrossRef]
- Börsch-Supan, A.; Brandt, M.; Hunkler, C.; Kneip, T.; Korbmacher, J.; Malter, F.; Schaan, B.; Stuck, S.; Zuber, S. Data Resource Profile: The Survey of Health, Ageing and Retirement in Europe (SHARE). Int. J. Epidemiol. 2013, 42, 992–1001. [Google Scholar] [CrossRef]
- Bíró, A.; Branyiczki, R. Transition shocks during adulthood and health a few decades later in post-socialist Central and Eastern Europe. BMC Public Health 2020, 20, 698. [Google Scholar] [CrossRef] [PubMed]
- Romaniuk, P.; Szromek, A.R. The evolution of the health system outcomes in Central and Eastern Europe and their association with social, economic and political factors: An analysis of 25 years of transition. BMC Health Serv. Res. 2016, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Donabedian, A. The quality of care. How can it be assessed? JAMA 1988, 260, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Baeten, R.; Spasova, S.; Vanhercke, B.; Coster, S. Inequalities in Access to Healthcare, a Study of National Policies 2018; European Commission: Brussels, Belgium, 2018; pp. 5–62. [Google Scholar]
- Jakovljevic, M.; Fernandes, P.O.; Teixeira, J.P.; Rancic, N.; Timofeyev, Y.; Reshetnikov, V. Underlying Differences in Health Spending Within the World Health Organisation Europe Region-Comparing EU15, EU Post-2004, CIS, EU Candidate, and CARINFONET Countries. Int. J. Environ. Res. Public Health 2019, 16, 3043. [Google Scholar] [CrossRef]
- Eurostat. Healthcare Expenditure Statistics; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Tambor, M.; Klich, J.; Domagała, A. Financing Healthcare in Central and Eastern European Countries: How Far Are We from Universal Health Coverage? Int. J. Environ. Res. Public Health 2021, 18, 1382. [Google Scholar] [CrossRef]
- O’Connor, A.; Čihula, T. Central and Eastern Europe: Exploring Emerging Healthcare Investment Opportunities; Kinstellar: Kyiv, Ukraine, 2018. [Google Scholar]
- Brownlee, S.; Chalkidou, K.; Doust, J.; Elshaug, A.G.; Glasziou, P.; Heath, I.; Nagpal, S.; Saini, V.; Srivastava, D.; Chalmers, K.; et al. Evidence for overuse of medical services around the world. Lancet 2017, 390, 156–168. [Google Scholar] [CrossRef]
- Harkin, D.W.; Beard, J.D.; Shearman, C.P.; Wyatt, M.G. Predicted shortage of vascular surgeons in the United Kingdom: A matter for debate? Surgeon 2016, 14, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Go, M.R.; Oslock, W.M.; Way, D.P.; Baselice, H.E.; Tamer, R.M.; Kent, K.C.; Williams, T.E.; Satiani, B. An Updated Physician Workforce Model Predicts a Shortage of Vascular Surgeons for the Next 20 Years. Ann. Vasc. Surg. 2020, 66, 282–288. [Google Scholar] [CrossRef]
- Glinos, I.A. Health professional mobility in the European Union: Exploring the equity and efficiency of free movement. Health policy (Amsterdam, Netherlands). Health Policy 2015, 119, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Glinos, I.; Wismar, M.; Buchan, J.; Rakovac, I. How can countries address the efficiency and equity implications of health professional mobility in Europe? In Adapting Policies in the Context of the WHO Code of Practice and EU Freedom of Movement. Copenhagen (Denmark): European Observatory on Health Systems and Policies. World Health Organization 2015 (Acting as the Host Organization for, and Secretariat of, the European Observatory on Health Systems and Policies); World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Lilford, R.J.; Brown, C.A.; Nicholl, J. Use of process measures to monitor the quality of clinical practice. BMJ 2007, 335, 648–650. [Google Scholar] [CrossRef]
- Kolossváry, E.; Ferenci, T.; Kováts, T.; Kovács, L.; Szeberin, Z.; Sótonyi, P.; Dósa, E.; Járai, Z.; Farkas, K. Lower Limb Amputations and Revascularisation Procedures in the Hungarian Population: A 14 Year Retrospective Cohort Study. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, C.A.; Sigvant, B.; Kuchenbecker, J.; Grima, M.J.; Schermerhorn, M.; Thomson, I.A.; Altreuther, M.; Setacci, C.; Svetlikov, A.; Laxdal, E.H.; et al. International Variations and Sex Disparities in the Treatment of Peripheral Arterial Occlusive Disease: A Report from VASCUNET and the International Consortium of Vascular Registries. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Grip, O.; Mani, K.; Altreuther, M.; Gonçalves, F.B.; Beiles, B.; Cassar, K.; Davidovic, L.; Eldrup, N.; Lattmann, T.; Laxdal, E.; et al. Contemporary Treatment of Popliteal Artery Aneurysms in 14 Countries: A Vascunet Report. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 721–729. [Google Scholar] [CrossRef]
- Mani, K.; Lees, T.; Beiles, B.; Jensen, L.; Venermo, M.; Simo, G.; Palombo, D.; Halbakken, E.; Troëng, T.; Wigger, P.; et al. Treatment of abdominal aortic aneurysm in nine countries 2005–2009: A vascunet report. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 598–607. [Google Scholar] [CrossRef]
- Movsisyan, N.K.; Vinciguerra, M.; Medina-Inojosa, J.R.; Lopez-Jimenez, F. Cardiovascular Diseases in Central and Eastern Europe: A Call for More Surveillance and Evidence-Based Health Promotion. Ann. Glob. Health 2020, 86, 21. [Google Scholar] [CrossRef]
- Cífková, R.; Bruthans, J.; Wohlfahrt, P.; Krajčoviechová, A.; Šulc, P.; Jozífová, M.; Eremiášová, L.; Pudil, J.; Linhart, A.; Widimský, J.; et al. 30-year trends in major cardiovascular risk factors in the Czech population, Czech MONICA and Czech post-MONICA, 1985–2016/17. PLoS ONE 2020, 15, e0232845. [Google Scholar] [CrossRef]
- Goodall, R.; Salciccioli, J.D.; Davies, A.H.; Marshall, D.; Shalhoub, J. Trends in peripheral arterial disease incidence and mortality in EU15+ countries 1990–2017. Eur. J. Prev. Cardiol. 2020. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.K.; Day, J.; Horrocks, M. Outcomes for Angioplasty and Bypass Lower Limb Revascularisation Procedures for Limb Salvage in England: Findings From the Getting It Right First Time Programme. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Al-Balah, A.; Goodall, R.; Salciccioli, J.D.; Marshall, D.C.; Shalhoub, J. Mortality from abdominal aortic aneurysm: Trends in European Union 15+ countries from 1990 to 2017. Br. J. Surg. 2020, 107, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, A.; Kakkos, S.; Abbott, A.; Naylor, A.R.; Richards, T.; Mikhailidis, D.P.; Geroulakos, G.; Nicolaides, A.N. Long-term Mortality in Patients with Asymptomatic Carotid Stenosis: Implications for Statin Therapy. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 573–582. [Google Scholar] [CrossRef]
- Hidi, L.; Menyhei, G.; Kováts, T.; Dobai, A.; Szeberin, Z. Report of the Hungarian Vascular Registry’s data of infrarenal aortic aneurysms (2010–2014). Orv. Hetil. 2015, 156, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Ruzsa, Z.; Januszek, R.; Óriás, V.; Chyrchel, M.; Wojtasik-Bakalarz, J.; Bartuś, J.; Arif, S.; Pawel, K.; Tokarek, T.; Nyerges, A.; et al. Mortality and chronic obstructive pulmonary disease in patients treated with endovascular revascularization of the infra-inguinal lower limb arteries from retrograde access. Ann. Transl. Med. 2020, 8, 206. [Google Scholar] [CrossRef]
- Behrendt, C.-A.; Sigvant, B.; Szeberin, Z.; Beiles, B.; Eldrup, N.; Thomson, I.A.; Venermo, M.; Altreuther, M.; Menyhei, G.; Nordanstig, J.; et al. International Variations in Amputation Practice: A VASCUNET Report. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 391–399. [Google Scholar] [CrossRef]
- Kolossváry, E.; Ferenci, T.; Kováts, T.; Kovács, L.; Járai, Z.; Menyhei, G.; Farkas, K. Trends in Major Lower Limb Amputation Related to Peripheral Arterial Disease in Hungary: A Nationwide Study (2004–2012). Eur. J. Vasc. Endovasc. Surg. 2015, 50, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wierzba, W.; Krasnodębski, P.; Śliwczyński, A.; Karnafel, W. Geographic variability of major non-traumatic lower limb amputations in diabetic and non-diabetic patients in Poland. Ann. Agric. Environ. Med. 2020, 27, 76–79. [Google Scholar] [CrossRef]
- Veresiu, I.A.; Iancu, S.S.; Bondor, C. Trends in diabetes-related lower extremities amputations in Romania—A five year nationwide evaluation. Diabetes Res. Clin. Pr. 2015, 109, 293–298. [Google Scholar] [CrossRef]
- Aziz, F.; Reichardt, B.; Sourij, C.; Dimai, H.-P.; Reichart, D.; Köhler, G.; Brodmann, M.; Sourij, H. Epidemiology of major lower extremity amputations in individuals with diabetes in Austria, 2014–2017: A retrospective analysis of health insurance database. Diabetes Res. Clin. Pr. 2020, 170, 108477. [Google Scholar] [CrossRef] [PubMed]
- Heyer, K.; Debus, E.S.; Mayerhoff, L.; Augustin, M. Prevalence and Regional Distribution of Lower Limb Amputations from 2006 to 2012 in Germany: A Population based Study. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Kolossváry, E.; Björck, M.; Behrendt, C.A. Lower Limb Major Amputation Data as a Signal of an East/West Health Divide Across Europe. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Płoszaj, A.; Olechnicka, A. Running Faster or Measuring Better? How Is the R&D Sector in Central and Eastern Europe Catching up with Western Europe? Centre for European Regional and Local Studies (EUROREG), University of Warsaw: Warszawa, Poland, 2015; pp. 2–21. [Google Scholar]
- Motovska, Z.; Ionita, O. Perspectives of cardiovascular research in Central and Eastern Europe (letter). Eur. Heart J. Suppl. 2020, 22, F51–F53. [Google Scholar] [CrossRef]
- Garrido, M.V.; Kristensen, F.B.; Nielsen, C.P.; Busse, R. Health Technology Assessment and Health Policy-Making in Europe; The European Observatory on Health Systems and Policies: London, UK, 2009. [Google Scholar]
- Gulácsi, L.; Rotar, A.M.; Niewada, M.; Löblová, O.; Rencz, F.; Petrova, G.; Boncz, I.; Klazinga, N.S. Health technology assessment in Poland, the Czech Republic, Hungary, Romania and Bulgaria. Eur. J. Health Econ. 2014, 15 (Suppl. 1), S13–S25. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, C.A. Routinely collected data from health insurance claims and electronic health records in vascular research-a success story and way to go. VASA Z. Gefasskrankh. 2020, 49, 85–86. [Google Scholar] [CrossRef]
- Sutzko, D.C.; Mani, K.; Behrendt, C.A.; Wanhainen, A.; Beck, A.W. Big data in vascular surgery: Registries, international collaboration and future directions. J. Intern. Med. 2020, 288, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Lomba, N. The Benefit of EU Action in Health Policy: The Record to Date; European Added Value in Action: European Added Value Unit, European Parliamentary Research Service; European Union: Brussels, Belgium, 2019; pp. 1–44. [Google Scholar]
- Scholz, N. Addressing Health Inequalities in the European Union. Concepts, Action, State of Play: European Parliamentary Research Service; European Parliament Think Tank: Brussels, Belgium, 2020; pp. 1–35. [Google Scholar]
| Domain of Differences | Examples |
|---|---|
| Vascular risk factors | Demography, tobacco use, hyperlipidaemia, diabetes, socioeconomic status, environment |
| Structure of vascular care | Health expenditure, availability of vascular specialists |
| Processes of vascular care | Volume of vascular procedures, availability of new technologies |
| Outcomes of vascular care | Mortality, lower limb amputations |
| Availability of scientific data | Backwardness in research and development, organisation of health technology assessment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolossváry, E.; Björck, M.; Behrendt, C.-A. A Divide between the Western European and the Central and Eastern European Countries in the Peripheral Vascular Field: A Narrative Review of the Literature. J. Clin. Med. 2021, 10, 3553. https://doi.org/10.3390/jcm10163553
Kolossváry E, Björck M, Behrendt C-A. A Divide between the Western European and the Central and Eastern European Countries in the Peripheral Vascular Field: A Narrative Review of the Literature. Journal of Clinical Medicine. 2021; 10(16):3553. https://doi.org/10.3390/jcm10163553
Chicago/Turabian StyleKolossváry, Endre, Martin Björck, and Christian-Alexander Behrendt. 2021. "A Divide between the Western European and the Central and Eastern European Countries in the Peripheral Vascular Field: A Narrative Review of the Literature" Journal of Clinical Medicine 10, no. 16: 3553. https://doi.org/10.3390/jcm10163553
APA StyleKolossváry, E., Björck, M., & Behrendt, C.-A. (2021). A Divide between the Western European and the Central and Eastern European Countries in the Peripheral Vascular Field: A Narrative Review of the Literature. Journal of Clinical Medicine, 10(16), 3553. https://doi.org/10.3390/jcm10163553







