Rationale for Polyclonal Intravenous Immunoglobulin Adjunctive Therapy in COVID-19 Patients: Report of a Structured Multidisciplinary Consensus
Abstract
1. Introduction
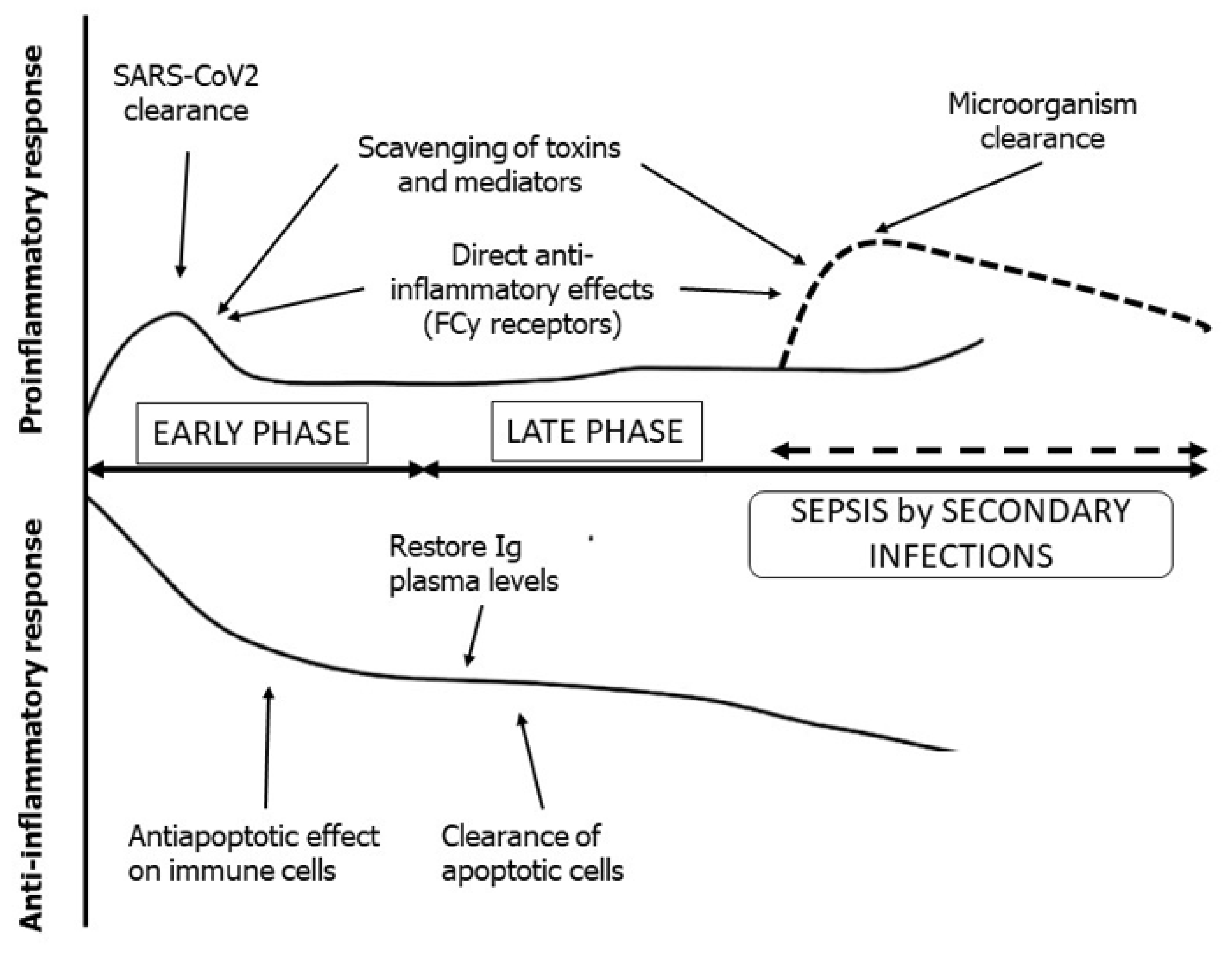
2. Adjunctive Immunoglobulin Therapy
3. Consensus Methodology
4. Consensus Results
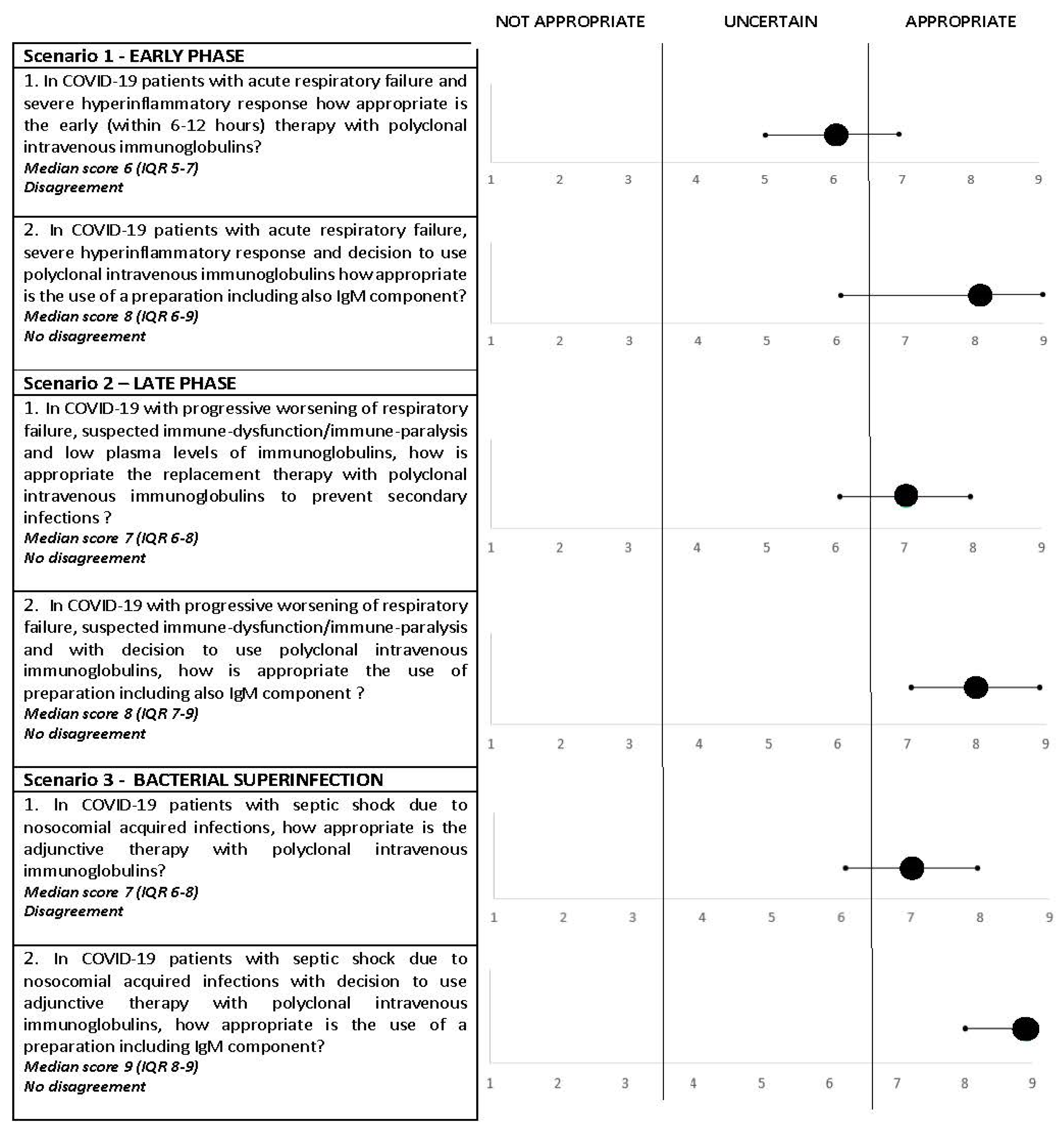
- (1)
- In COVID-19 patients with acute respiratory failure and severe hyperinflammatory response, how appropriate is the early (within 6–12 h) therapy with polyclonal intravenous immunoglobulins?Consensus rating: uncertain; median score, 6 (IQR, 5–7); disagreement: yes.
- (2)
- In COVID-19 patients with acute respiratory failure, severe hyperinflammatory response and decision to use polyclonal intravenous immunoglobulins, how appropriate is the use of preparations including also the IgM component?Consensus rating: appropriate; median score, 8 (IQR, 6–9); disagreement: no.
- (1)
- In COVID-19 with progressive worsening of respiratory failure, suspected immune dysfunction/immune paralysis and low plasma levels of immunoglobulins, how appropriate is the replacement therapy with polyclonal intravenous immunoglobulins to prevent secondary infections?Consensus rating: appropriate; median score, 7 (IQR, 6–8); disagreement: no.
- (2)
- In COVID-19 with progressive worsening of respiratory failure, suspected immune dysfunction/immune paralysis and with the decision to use polyclonal intravenous immunoglobulins, how appropriate is the use of preparations including also the IgM component?Consensus rating: appropriate; median score, 8 (IQR, 7–8); disagreement: no.
- (1)
- In COVID-19 patients with septic shock due to nosocomial acquired infections, how appropriate is the adjunctive therapy with polyclonal intravenous immunoglobulins?Consensus rating: appropriate; median score, 7 (IQR, 6–8); disagreement: yes.
- (2)
- In COVID-19 patients with septic shock due to nosocomial acquired infections with the decision to use adjunctive therapy with polyclonal intravenous immunoglobulins, how appropriate is the use of preparations including the IgM component?Consensus rating: appropriate; median score, 9 (IQR, 8–9); disagreement: no.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in critically ill patients in the Seattle region—Case series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.J.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Kox, M.; Waalders, N.J.B.; Kooistra, E.J.; Gerretsen, J.; Pickkers, P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA 2020, 324, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Nasillo, V.; Tagliafico, E.; Trenti, T.; Comoli, P.; Luppi, M. COVID-19: More than a cytokine storm. Crit. Care 2020, 24, 549. [Google Scholar] [CrossRef] [PubMed]
- Laing, A.G.; Lorenc, A.; Del Molino Del Barrio, I.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef]
- Lombardi, A.; Trombetta, E.; Cattaneo, A.; Castelli, V.; Palomba, E.; Tirone, M.; Mangioni, D.; Lamorte, G.; Manunta, M.; Prati, D.; et al. Early phases of COVID-19 are characterized by a reduction in lymphocyte populations and the presence of atypical monocytes. Front. Immunol. 2020, 11, 560330. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef]
- Riva, G.; Nasillo, V.; Tagliafico, E.; Trenti, T.; Luppi, M. COVID-19: Room for treating T cell exhaustion? Crit. Care 2020, 24, 229. [Google Scholar] [CrossRef]
- Remy, K.E.; Brakenridge, S.C.; Francois, B.; Daix, T.; Deutschman, C.S.; Monneret, G.; Jeannet, R.; La-terre, P.-F.; Hotchkiss, R.S.; Moldawer, L.L. Immunotherapies for COVID-19: Lessons learned from sepsis. Lancet Respir. Med. 2020, 8, 946–949. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Poon, L.L.M.; Ng, I.H.Y.; Luk, W.; Sia, S.-F.; Wu, M.H.S.; Chan, K.-H.; Yuen, K.-Y.; Gordon, S.; Guan, Y.; et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: Possible relevance to pathogenesis. J. Virol. 2005, 79, 7819–7826. [Google Scholar] [CrossRef] [PubMed]
- Werdan, K.; Pilz, G.; Bujdoso, O.; Fraunberger, P.; Neeser, G.; Schmieder, R.E.; Viell, B.; Marget, W.; Seewald, M.; Walger, P.; et al. Score-based immunoglobulin G therapy of patients with sepsis: The SBITS study. Crit. Care Med. 2007, 35, 2693–2701. [Google Scholar]
- Busani, S.; Damiani, E.; Cavazzuti, I.; Donati, A.; Girardis, M. Intravenous immunoglobulin in septic shock: Review of the mechanisms of action and meta-analysis of the clinical effectiveness. Minerva Anestesiol. 2016, 82, 559–572. [Google Scholar]
- Reiser, M.; Borte, M.; Huscher, D.; Baumann, U.; Pittrow, D.; Sommer, C.; Stangel, M.; Fasshauer, M.; Gold, R.; Hensel, M. Management of patients with malignancies and secondary immunodeficiencies treated with immunoglobulins in clinical practice: Long-term data of the SIGNS study. Eur. J. Haematol. 2017, 99, 169–177. [Google Scholar] [CrossRef]
- Kohler, H.; Kaveri, S. How IvIg can mitigate Covid-19 disease: A symmetrical immune network model. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 17–20. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef] [PubMed]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Use of intravenous immunoglobulins in sepsis therapy—A clinical view. Int. J. Mol. Sci. 2020, 21, 5543. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Apostolidou, E.; Lada, M.; Perdios, I.; Gatselis, N.K.; Tsangaris, I.; Georgitsi, M.; Bristianou, M.; Kanni, T.; Sereti, K.; et al. Kinetics of circulating immunoglobulin M in sepsis: Relationship with final outcome. Crit. Care 2013, 17, R247. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, X.; Zhang, F.; Xia, Y. Evaluation of the effect of intravenous immunoglobulin dosing on mortality in patients with sepsis: A network meta-analysis. Clin. Ther. 2019, 41, 1823–1838.e4. [Google Scholar] [CrossRef] [PubMed]
- Sewell, W.A.C.; Kerr, J.; Behr-Gross, M.-E.; Peter, H.-H.; Kreuth Ig Working Group. European consensus proposal for immunoglobulin therapies. Eur. J. Immunol. 2014, 44, 2207–2214. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Yaqinuddin, A.; Ambia, A.R.; Elgazzar, T.A.; AlSaud, M.B.M.; Kashir, J. Application of intravenous immunoglobulin (IVIG) to modulate inflammation in critical COVID-19—A theoretical perspective. Med. Hypotheses 2021, 151, 110592. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Feng, Y.; Zhong, L.; Xie, Q.; Lei, M.; Liu, Z.; Wang, C.; Ji, J.; Liu, H.; Gu, Z.; et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: A multicenter retrospective cohort study. Clin. Transl. Immunol. 2020, 9, e1192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-G.; Xie, S.-M.; Zhang, J.; Zheng, F.; Jiang, D.-X.; Li, K.-Y.; Zuo, Q.; Yan, Y.-S.; Liu, J.-Y.; Xie, Y.-L.; et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 Patients who have failed low-dose therapy. Preprints 2020, 2020030065. [Google Scholar] [CrossRef][Green Version]
- Cao, W.; Liu, X.; Bai, T.; Fan, H.; Hong, K.; Song, H.; Han, Y.; Lin, L.; Ruan, L.; Li, T. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease. Open Forum Infect. Dis. 2019, 7, ofaa102. Available online: https://academic.oup.com/ofid/article/7/3/ofaa102/5810740 (accessed on 10 November 2020). [CrossRef] [PubMed]
- Sakoulas, G.; Geriak, M.; Kullar, R.; Greenwood, K.; Habib, M.; Vyas, A.; Ghafourian, M.; Dintyala, V.N.K.; Haddad, F. Intravenous immunoglobulin (IVIG) significantly reduces respiratory morbidity in COVID-19 pneumonia: A prospective randomized trial. Crit. Care Explor. 2020, 2, e0280. [Google Scholar] [CrossRef]
- Xie, Y.; Cao, S.; Dong, H.; Li, Q.; Chen, E.; Zhang, W.; Yang, L.; Fu, S.; Wang, R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef]
- Mohtadi, N.; Ghaysouri, A.; Shirazi, S.; Ansari, S.; Shafiee, E.; Bastani, E.; Kokhazadeh, T.; Tavan, H. Recovery of severely ill COVID-19 patients by intravenous immunoglobulin (IVIG) treatment: A case series. Virology 2020, 548, 1–5. [Google Scholar] [CrossRef]
- Tabarsi, P.; Barati, S.; Jamaati, H.; Haseli, S.; Marjani, M.; Moniri, A.; Abtahian, Z.; Dastan, A.; Yousefian, S.; Eskandari, R.; et al. Evaluating the effects of intravenous immunoglobulin (IVIg) on the management of severe COVID-19 cases: A randomized controlled trial. Int. Immunopharmacol. 2021, 90, 107205. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.R. The new generation of liquid intravenous immunoglobulin formulations in patient care: A comparison of intravenous immunoglobulins. Postgrad. Med. 2010, 122, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Baudel, J.L.; Vigneron, C.; Pras-Landre, V.; Joffre, J.; Marjot, F.; Ait-Oufella, H.; Bigé, N.; Maury, E.; Guidet, B.; Fain, O.; et al. Transfusion-Related Acute Lung Injury (TRALI) after intravenous immuno-globulins: French multicentre study and literature review. Clin. Rheumatol. 2020, 39, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Bichuetti-Silva, D.C.; Furlan, F.P.; Nobre, F.A.; Pereira, C.T.M.; Gonçalves, T.R.T.; Gouveia-Pereira, M.; Rota, R.; Tavares, L.; Mazzucchelli, J.T.L.; Costa-Carvalho, B.T. Immediate infusion-related adverse reactions to intravenous immunoglobulin in a prospective cohort of 1765 infusions. Int. Immunopharmacol. 2014, 23, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Katz, U.; Achiron, A.; Sherer, Y.; Shoenfeld, Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun. Rev. 2007, 6, 257–259. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Kreymann, K.G.; de Heer, G.; Nierhaus, A.; Kluge, S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit. Care Med. 2007, 35, 2677–2685. [Google Scholar]
- Soares, M.O.; Welton, N.J.; Harrison, D.A.; Peura, P.; Shankar- Hari, M.; Harvey, S.E.; Madan, J.J.; Ades, A.E.; Palmer, S.J.; Rowan, K.M. An evaluation of the feasibility, cost and value of information of a multicentre randomised controlled trial of intravenous immunoglobulin for sepsis (severe sepsis and septic shock): Incorporating a systematic review, meta-analysis and value of information analysis. Health Technol. Assess. 2012, 16, 1–186. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, M.R.; Notley, C.A. The importance of natural IgM: Scavenger, protector and regulator. Nat. Rev. Immunol. 2010, 10, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Fitch, K. (Ed.) The Rand/UCLA Appropriateness Method User’s Manual; Rand: Santa Monica, CA, USA, 2001; ISBN 978-0-8330-2918-8. [Google Scholar]
- Busani, S.; Roat, E.; Tosi, M.; Biagioni, E.; Coloretti, I.; Meschiari, M.; Gelmini, R.; Brugioni, L.; De Biasi, S.; Girardis, M. Adjunctive immunotherapy with polyclonal Ig-M enriched immunoglobulins for septic shock: From bench to bedside. The rationale for a personalized treatment protocol. Front. Med. 2021, 8, 616511. [Google Scholar] [CrossRef]
- Berlot, G.; Vassallo, M.C.; Busetto, N.; Nieto Yabar, M.; Istrati, T.; Baronio, S.; Quarantotto, G.; Bixio, M.; Barbati, G.; Dattola, R.; et al. Effects of the timing of administration of IgM- and IgA-enriched intravenous polyclonal immunoglobulins on the outcome of septic shock patients. Ann. Intensive Care 2018, 8, 122. [Google Scholar] [CrossRef]
- Hung, I.F.N.; To, K.K.W.; Lee, C.-K.; Lee, K.-L.; Yan, W.-W.; Chan, K.; Chan, W.-M.; Ngai, C.-W.; Law, K.-I.; Chow, F.-L.; et al. Hyperimmune IV immunoglobulin treatment: A Multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 2013, 144, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Nierhaus, A.; Berlot, G.; Kindgen-Milles, D.; Müller, E.; Girardis, M. Best-practice IgM- and IgA-enriched immunoglobulin use in patients with sepsis. Ann. Intensive Care 2020, 10, 132. [Google Scholar] [CrossRef]
- Welte, T.; Dellinger, R.P.; Ebelt, H.; Ferrer, M.; Opal, S.M.; Singer, M.; Vincent, J.-L.; Werdan, K.; Martin-Loeches, I.; Almirall, J.; et al. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: A randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study). Intensive Care Med. 2018, 44, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-acquired infections in critically-ill COVID-19 patients. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Busani, S.; Bedini, A.; Biagioni, E.; Serio, L.; Tonelli, R.; Meschiari, M.; Franceschini, E.; Guaraldi, G.; Cossarizza, A.; Clini, E.; et al. Two fatal cases of acute liver failure due to HSV-1 infection in COVID-19 patients following immunomodulatory therapies. Clin. Infect. Dis. 2020, 73, e252–e255. [Google Scholar] [CrossRef]
- Venet, F.; Gebeile, R.; Bancel, J.; Guignant, C.; Poitevin-Later, F.; Malcus, C.; Lepape, A.; Monneret, G. Assessment of plasmatic immunoglobulin G, A and M levels in septic shock patients. Int. Immunopharmacol. 2011, 11, 2086–2090. [Google Scholar] [CrossRef]
- Husain-Syed, F.; Vadász, I.; Wilhelm, J.; Walmrath, H.-D.; Seeger, W.; Birk, H.-W.; Jennert, B.; Dietrich, H.; Herold, S.; Trauth, J.; et al. Immunoglobulin deficiency as an indicator of disease severity in patients with COVID-19. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 320, L590–L599. [Google Scholar] [CrossRef]
- Lederer, D.J.; Philip, N.; Rybak, D.; Arcasoy, S.M.; Kawut, S.M. Intravenous immunoglobulin for hypogammaglobulinemia after lung transplantation: A randomized crossover trial. PLoS ONE 2014, 9, e103908. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, E.; Diez, P.; Arraya, M.; Jaramillo, M.; Calahorra, L.; Fernandez-Yañez, J.; Palomo, J.; Sousa, I.; Hortal, J.; Barrio, J.; et al. Early intravenous immunoglobulin replacement in hypogammaglobulinemic heart transplant recipients: Results of a clinical trial. Transpl. Infect. Dis. 2016, 18, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Bourassa-Blanchette, S.; Patel, V.; Knoll, G.A.; Hutton, B.; Fergusson, N.; Bennett, A.; Tay, J.; Cameron, D.W.; Cowan, J. Clinical outcomes of polyvalent immunoglobulin use in solid organ transplant recipients: A systematic review and meta-analysis—Part II: Non-kidney transplant. Clin. Transplant. 2019, 33, e13625. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; Maria, A.D.; Dentone, C.; Biagio, A.D.; et al. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Investig. 2020, 50, e13319. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, W.; Wang, Z.; Chen, H.; Tian, L.; Liu, D. Nosocomial infection among patients with COVID-19: A retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect. Control Hosp. Epidemiol. 2020, 41, 982–983. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Busani, S.; Serafini, G.; Mantovani, E.; Venturelli, C.; Giannella, M.; Viale, P.; Mussini, C.; Cossarizza, A.; Girardis, M. Mortality in patients with septic shock by multidrug resistant bacteria: Risk factors and impact of sepsis treatments. J. Intensive Care Med. 2019, 34, 48–54. [Google Scholar] [CrossRef]
- Lye, D.C.; Earnest, A.; Ling, M.L.; Lee, T.-E.; Yong, H.-C.; Fisher, D.A.; Krishnan, P.; Hsu, L.-Y. The impact of multidrug resistance in healthcare-associated and nosocomial gram-negative bacteraemia on mortality and length of stay: Cohort study. Clin. Microbiol. Infect. 2012, 18, 502–508. [Google Scholar] [CrossRef]
- Santoro, A.; Franceschini, E.; Meschiari, M.; Menozzi, M.; Zona, S.; Venturelli, C.; Digaetano, M.; Rogati, C.; Guaraldi, G.; Paul, M.; et al. Epidemiology and risk factors associated with mortality in consecutive patients with bacterial bloodstream infection: Impact of MDR and XDR bacteria. Open Forum Infect. Dis. 2020, 7, ofaa461. [Google Scholar] [CrossRef] [PubMed]
- Alejandria, M.M.; Lansang, M.A.D.; Dans, L.F.; Mantaring, J.B., III. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst. Rev. 2013, 2013, CD001090. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coloretti, I.; Berlot, G.; Busani, S.; De Rosa, F.G.; Donati, A.; Forfori, F.; Grasselli, G.; Mirabella, L.; Tascini, C.; Viale, P.; et al. Rationale for Polyclonal Intravenous Immunoglobulin Adjunctive Therapy in COVID-19 Patients: Report of a Structured Multidisciplinary Consensus. J. Clin. Med. 2021, 10, 3500. https://doi.org/10.3390/jcm10163500
Coloretti I, Berlot G, Busani S, De Rosa FG, Donati A, Forfori F, Grasselli G, Mirabella L, Tascini C, Viale P, et al. Rationale for Polyclonal Intravenous Immunoglobulin Adjunctive Therapy in COVID-19 Patients: Report of a Structured Multidisciplinary Consensus. Journal of Clinical Medicine. 2021; 10(16):3500. https://doi.org/10.3390/jcm10163500
Chicago/Turabian StyleColoretti, Irene, Giorgio Berlot, Stefano Busani, Francesco Giuseppe De Rosa, Abele Donati, Francesco Forfori, Giacomo Grasselli, Lucia Mirabella, Carlo Tascini, Pierluigi Viale, and et al. 2021. "Rationale for Polyclonal Intravenous Immunoglobulin Adjunctive Therapy in COVID-19 Patients: Report of a Structured Multidisciplinary Consensus" Journal of Clinical Medicine 10, no. 16: 3500. https://doi.org/10.3390/jcm10163500
APA StyleColoretti, I., Berlot, G., Busani, S., De Rosa, F. G., Donati, A., Forfori, F., Grasselli, G., Mirabella, L., Tascini, C., Viale, P., & Girardis, M. (2021). Rationale for Polyclonal Intravenous Immunoglobulin Adjunctive Therapy in COVID-19 Patients: Report of a Structured Multidisciplinary Consensus. Journal of Clinical Medicine, 10(16), 3500. https://doi.org/10.3390/jcm10163500







