Association of Body Composition and Sarcopenia with NASH in Obese Patients
Abstract
:1. Introduction
2. Results
2.1. Clinical and Histological Characteristics
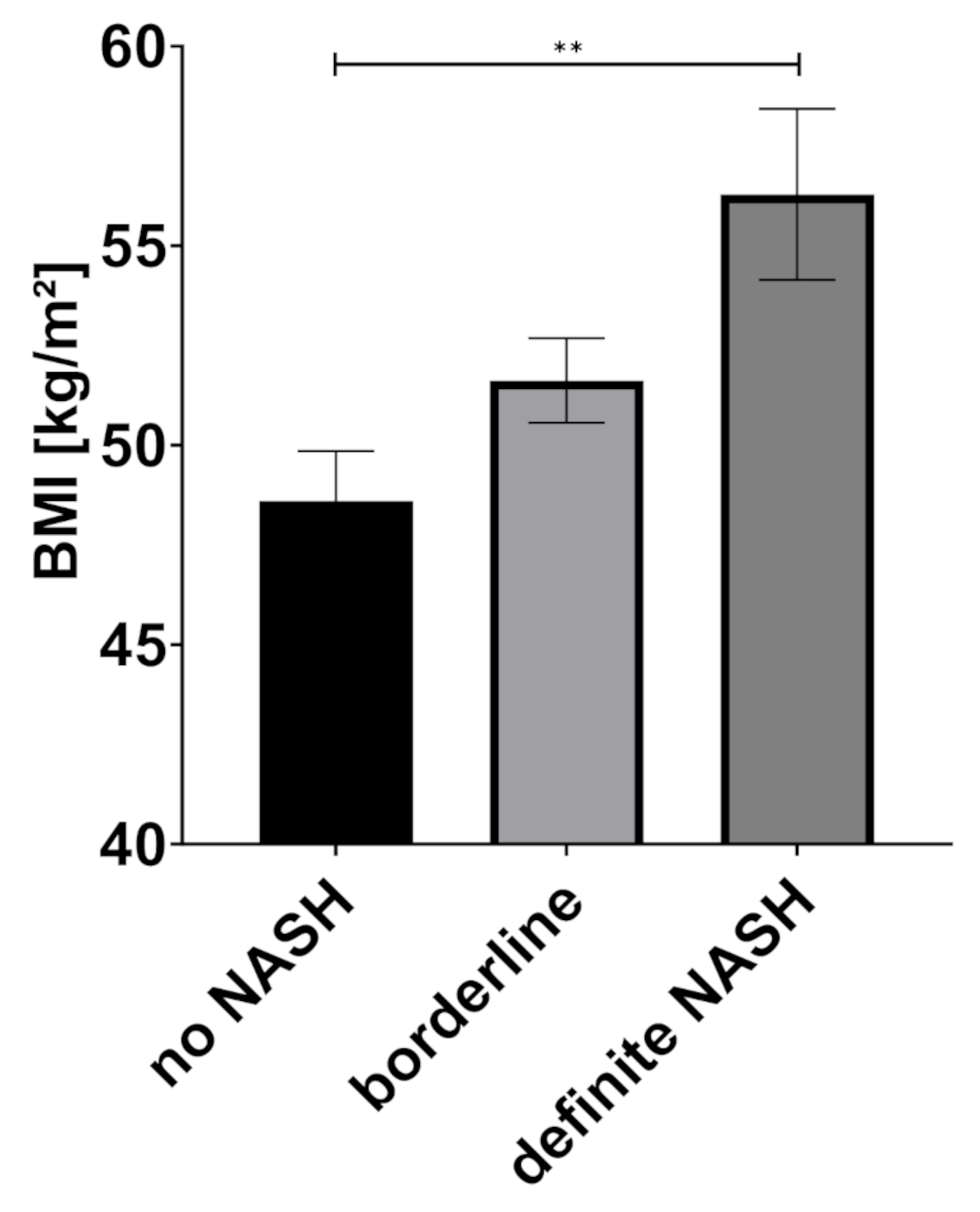
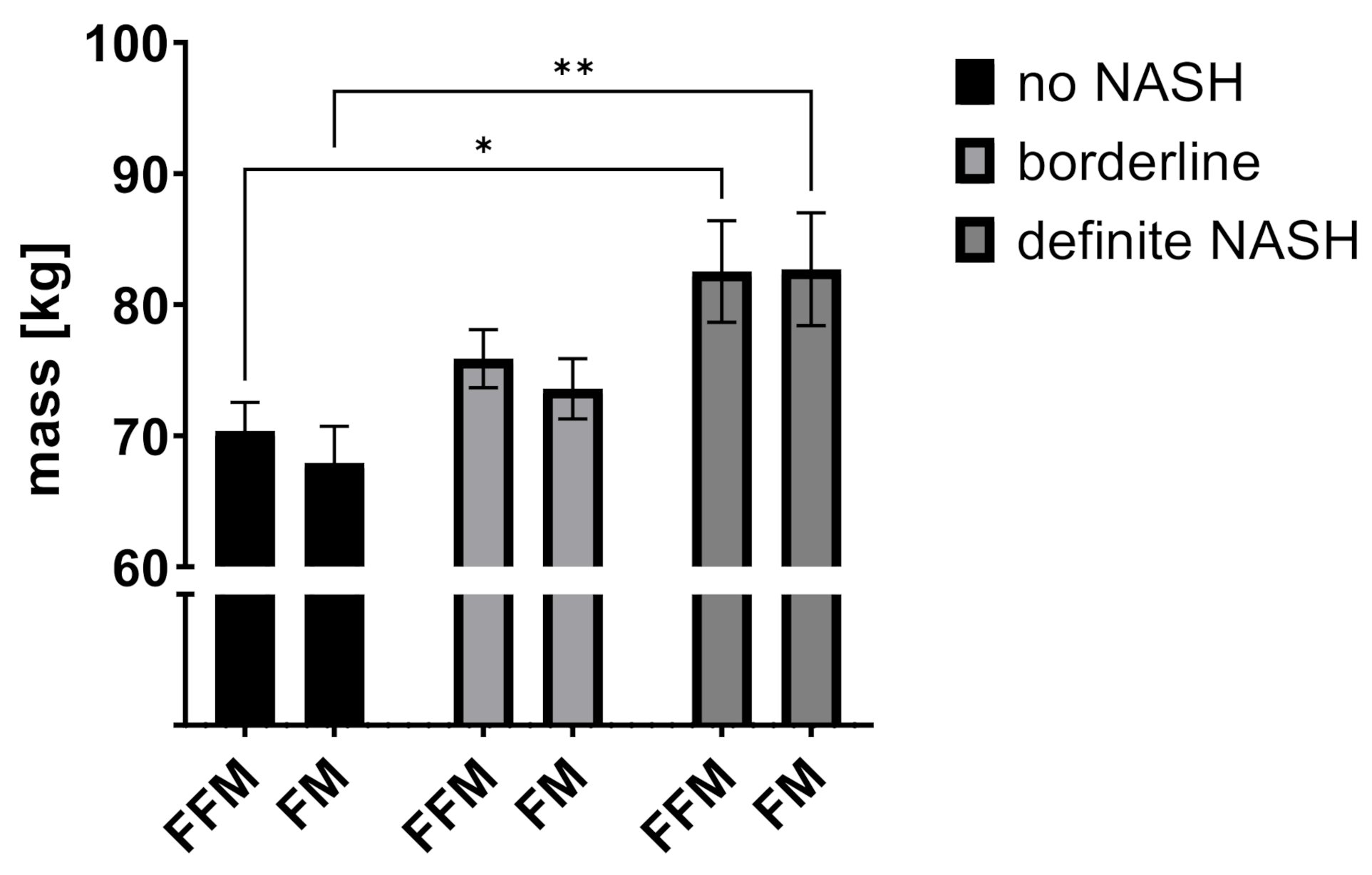
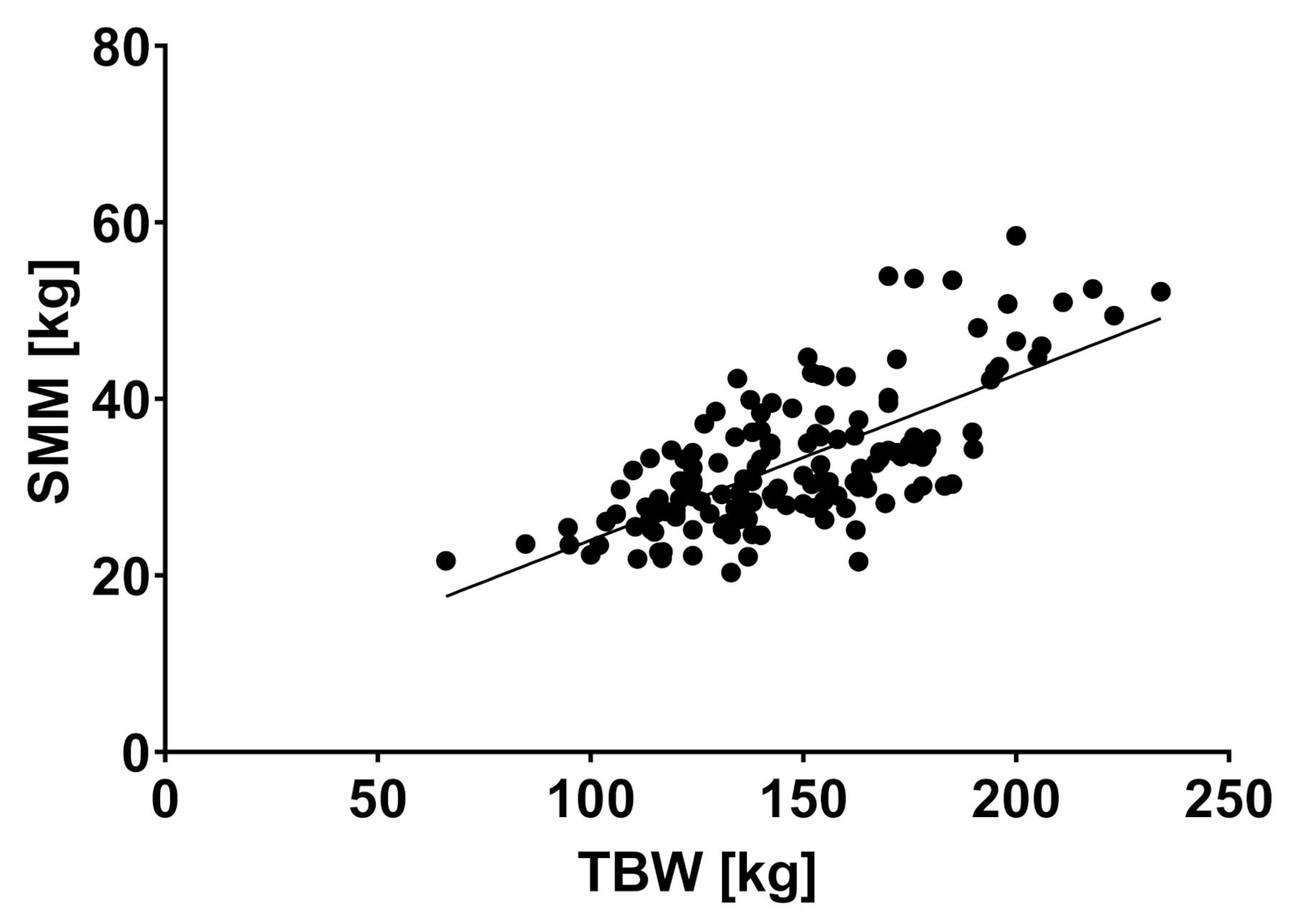
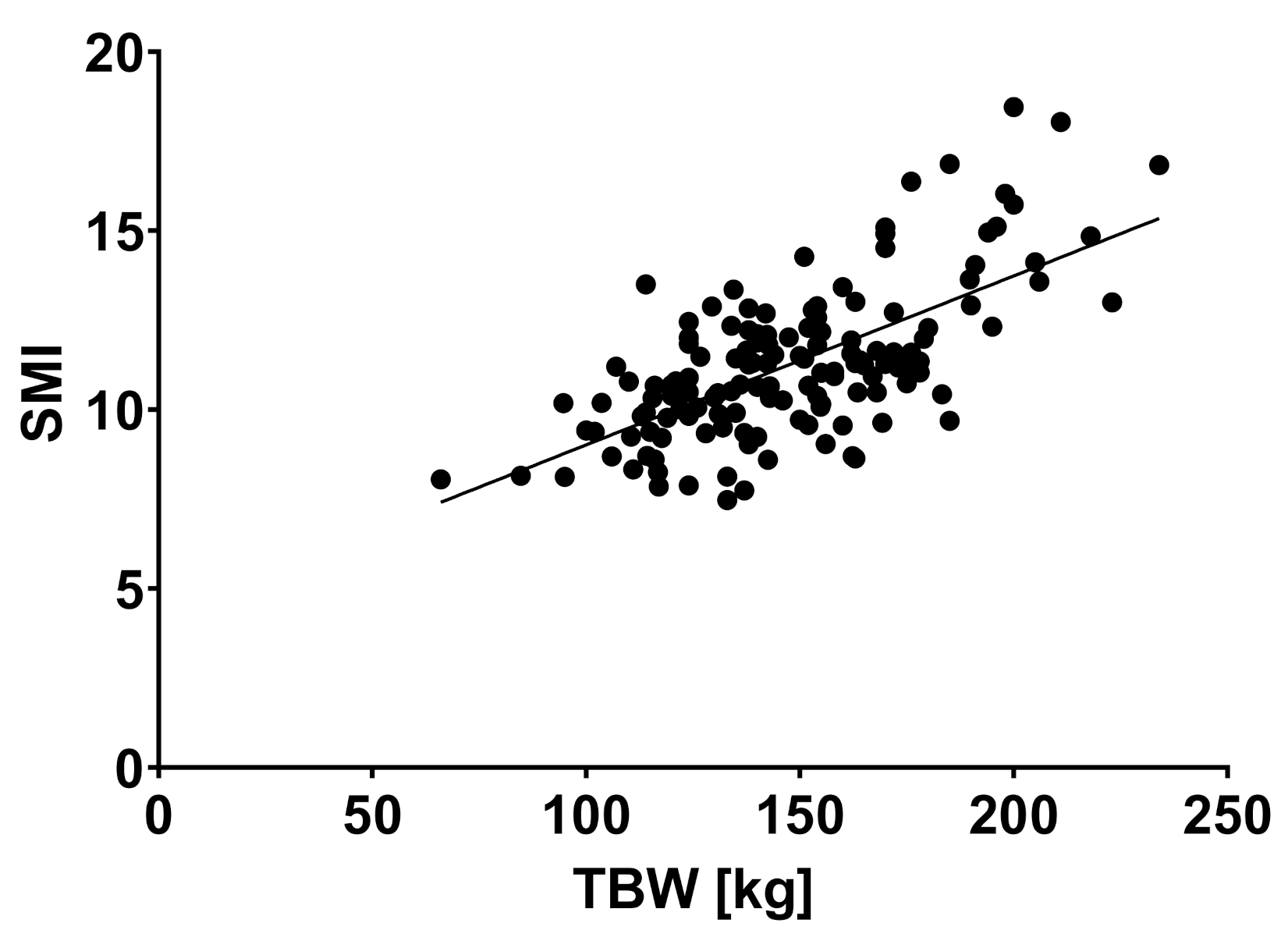
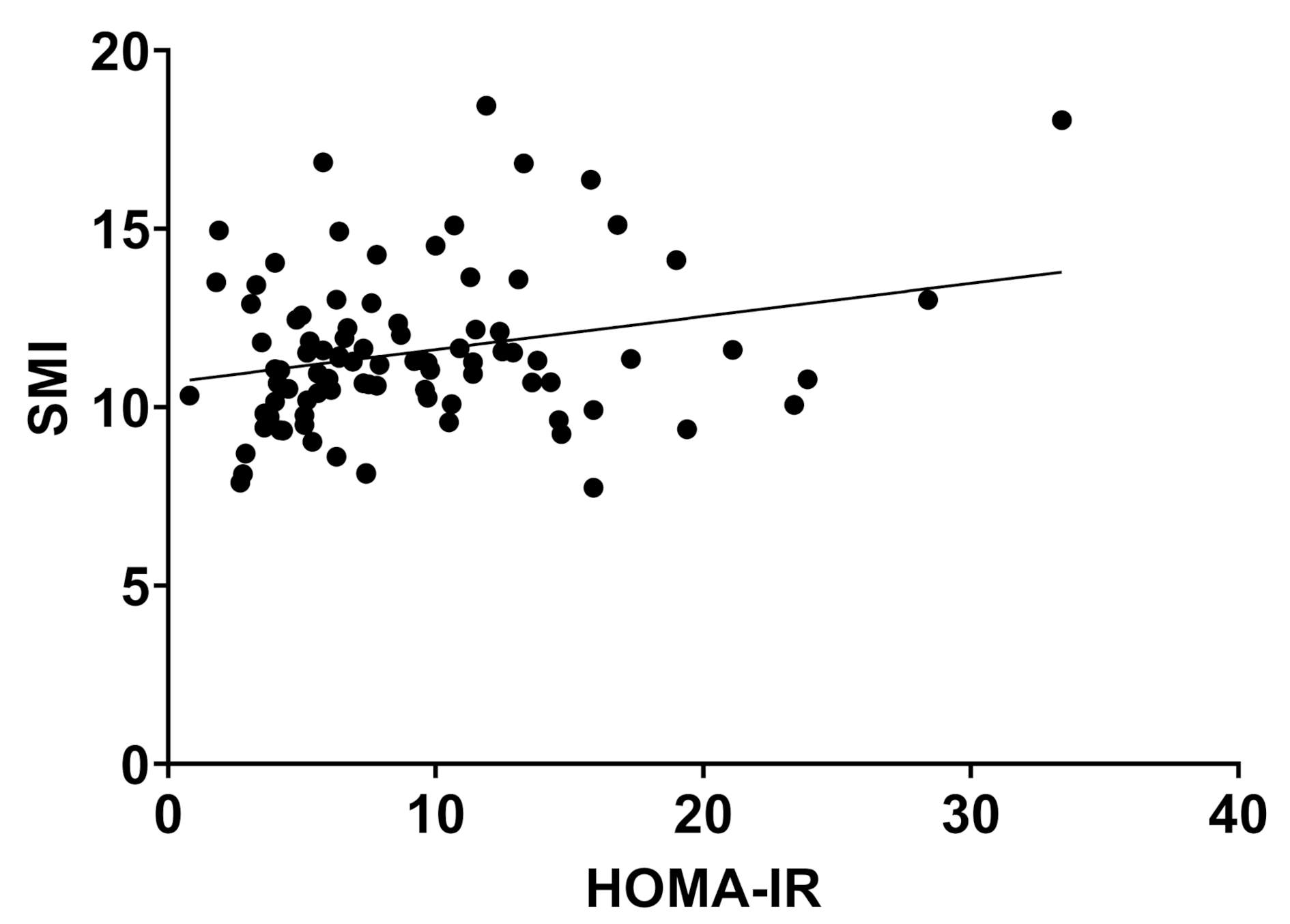
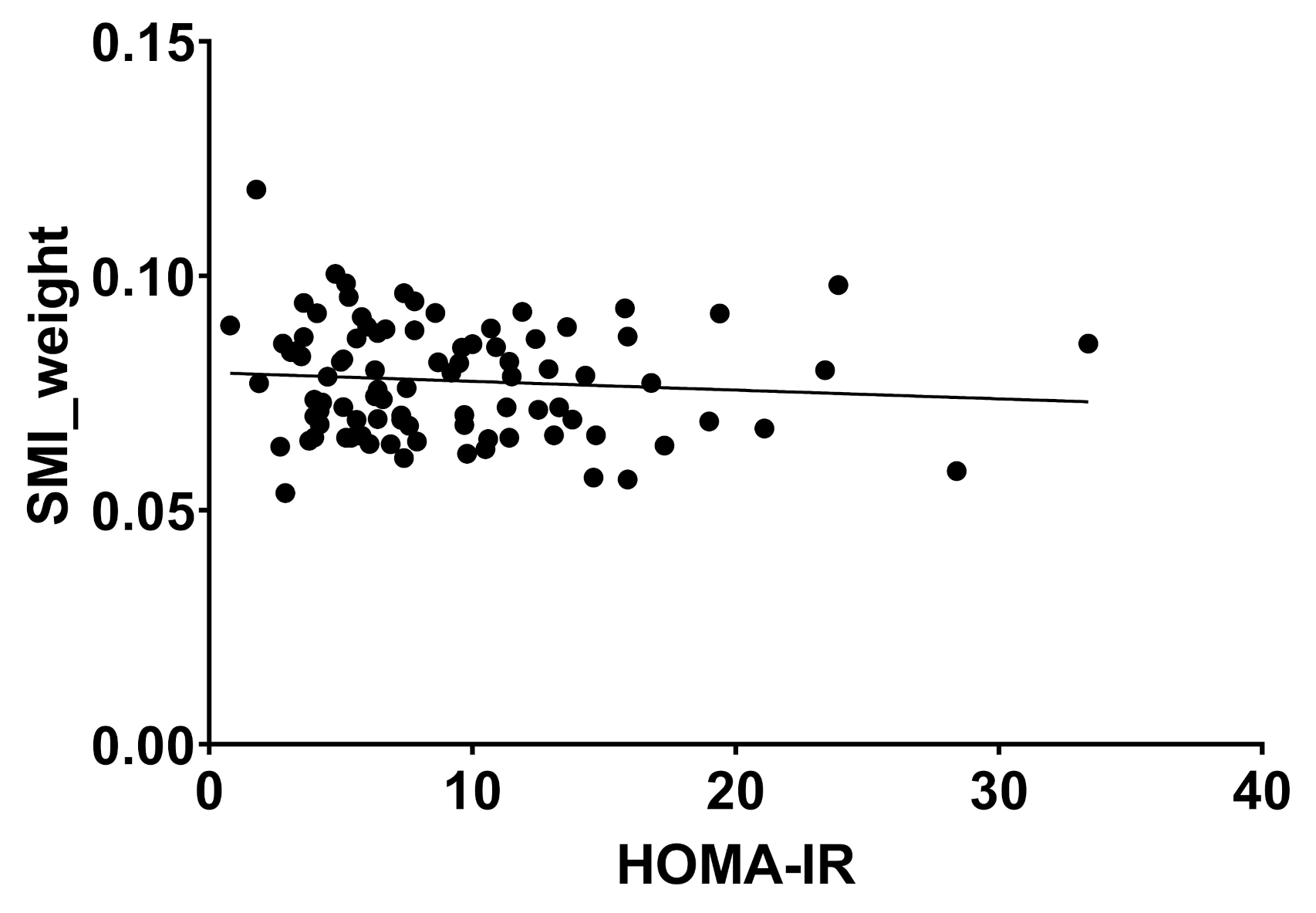
2.2. Body Composition and Liver Disease
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, H.S.; Park, Y.H.; Suh, K.; Yoo, M.H.; Park, H.K.; Kim, H.J.; Lee, J.H.; Byun, D.W. Association between Sarcopenia, Sarcopenic Obesity, and Chronic Disease in Korean Elderly. J. Bone Metab. 2018, 25, 187–193. [Google Scholar] [CrossRef]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, K.M. Sarcopenia and fatty liver disease. Hepatol. Int. 2019, 13, 674–687. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, C.W.; Park, C.H.; Choi, J.Y.; Han, K.; Merchant, A.T.; Park, Y.M. Low skeletal muscle mass is associated with non-alcoholic fatty liver disease in Korean adults: The Fifth Korea National Health and Nutrition Examination Survey. Hepatobiliary Pancreat Dis. Int. 2016, 15, 39–47. [Google Scholar] [CrossRef]
- Wang, Y.M.; Zhu, K.F.; Zhou, W.J.; Zhang, Q.; Deng, D.F.; Yang, Y.C.; Lu, W.W.; Xu, J.; Yang, Y.M. Sarcopenia is associated with the presence of nonalcoholic fatty liver disease in Zhejiang Province, China: A cross-sectional observational study. BMC Geriatr. 2021, 21, 55. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.E.; Lee, Y.B.; Jun, J.E.; Ahn, J.; Bae, J.C.; Jin, S.M.; Hur, K.Y.; Jee, J.H.; Lee, M.K.; et al. Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology 2018, 68, 1755–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, G.E.; Kim, M.J.; Yim, J.Y.; Kim, J.S.; Yoon, J.W. Sarcopenia Is Significantly Associated with Presence and Severity of Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2019, 28, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.S.; Yoon, J.S.; Won, K.C.; Lee, H.W. The role of skeletal muscle in development of nonalcoholic Fatty liver disease. Diabetes Metab. J. 2013, 37, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Golabi, P.; Gerber, L.; Paik, J.M.; Deshpande, R.; de Avila, L.; Younossi, Z.M. Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100171. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Marchesini, G.; Pinto-Cortez, H.; Petta, S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation 2019, 103, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; El-Serag, H.B.; Nguyen, T.; Hilal, J.; Kanwal, F.; Thrift, A.P. Obesity and Risk of Nonalcoholic Fatty Liver Disease: A Comparison of Bioelectrical Impedance Analysis and Conventionally-Derived Anthropometric Measures. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2017, 15, 1965–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.H.; Wong, T.C.; Hsu, Y.Y.; Kuo, K.L.; Yang, S.H. The Correlation Between Body Fat, Visceral Fat, and Nonalcoholic Fatty Liver Disease. Metab. Syndr. Relat Disord. 2017, 15, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.Y.; Baik, S.J.; Lee, H.A.; Lee, B.K.; Lee, H.S.; Kim, T.H.; Yoo, K. Relative fat mass at baseline and its early change may be a predictor of incident nonalcoholic fatty liver disease. Sci. Rep. 2020, 10, 17491. [Google Scholar] [CrossRef]
- Cai, C.; Song, X.; Chen, Y.; Chen, X.; Yu, C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Hepatol. Int. 2020, 14, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.C.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean Sarcopenic Obesity Study. Hepatology 2014, 59, 1772–1778. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Atkins, J.L. Muscle loss and obesity: The health implications of sarcopenia and sarcopenic obesity. Proc. Nutr. Soc. 2015, 74, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Siervo, M.; Mire, E.; Heymsfield, S.B.; Stephan, B.C.; Broyles, S.; Smith, S.R.; Wells, J.C.; Katzmarzyk, P.T. A population-based approach to define body-composition phenotypes. Am. J. Clin. Nutr. 2014, 99, 1369–1377. [Google Scholar] [CrossRef] [Green Version]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Xu, Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatr. Gerontol. Int. 2016, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Ofenheimer, A.; Breyer-Kohansal, R.; Hartl, S.; Burghuber, O.C.; Krach, F.; Schrott, A.; Wouters, E.F.M.; Franssen, F.M.E.; Breyer, M.K. Reference values of body composition parameters and visceral adipose tissue (VAT) by DXA in adults aged 18–81 years-results from the LEAD cohort. Eur. J. Clin. Nutr. 2020, 74, 1181–1191. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Lim, K.I.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Choi, H.; Baik, S.H.; et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: The Korean Sarcopenic Obesity Study. Clin. Endocrinol. 2013, 78, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, A.Y.; Meskers, C.G.; van Heemst, D.; Westendorp, R.G.; de Craen, A.J.; Maier, A.B. Diagnostic criteria for sarcopenia relate differently to insulin resistance. Age 2013, 35, 2367–2375. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K. Body composition measurement in severe obesity. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 602–606. [Google Scholar] [CrossRef] [Green Version]
- Beato, G.C.; Ravelli, M.N.; Crisp, A.H.; de Oliveira, M.R.M. Agreement Between Body Composition Assessed by Bioelectrical Impedance Analysis and Doubly Labeled Water in Obese Women Submitted to Bariatric Surgery: Body Composition, BIA, and DLW. Obes. Surg. 2019, 29, 183–189. [Google Scholar] [CrossRef]
- Kyle, U.G.; Genton, L.; Hans, D.; Pichard, C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin. Nutr. 2003, 22, 537–543. [Google Scholar] [CrossRef]
- Bera, T.K. Bioelectrical Impedance Methods for Noninvasive Health Monitoring: A Review. J. Med. Eng. 2014, 2014, 381251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.K.; Roberts, S.B.; Kehayias, J.J.; Wang, J.; Hsu, L.K.; Shikora, S.A.; Saltzman, E.; McCrory, M.A. Body composition assessment in extreme obesity and after massive weight loss induced by gastric bypass surgery. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E1080–E1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lv, Y.; Ni, W.; Shi, Q.; Xiang, X.; Li, S.; Song, C.; Xiao, M.; Jin, S. Associations between Phase Angle Values Obtained by Bioelectrical Impedance Analysis and Nonalcoholic Fatty Liver Disease in an Overweight Population. Can. J. Gastroenterol. Hepatol. 2020, 2020, 8888405. [Google Scholar] [CrossRef]
- Kang, M.K.; Park, J.G.; Lee, H.J.; Kim, M.C. Association of low skeletal muscle mass with advanced liver fibrosis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2019, 34, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Jeong, S.J. Relative Skeletal Muscle Mass Is an Important Factor in Non-Alcoholic Fatty Liver Disease in Non-Obese Children and Adolescents. J. Clin. Med. 2020, 9, 3355. [Google Scholar] [CrossRef]
- Noh, J. Sarcopenia as a Novel Risk Factor for Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2020, 29, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Zhai, Y.; Xiao, Q. The Common Mechanisms of Sarcopenia and NAFLD. BioMed. Res. Int. 2017, 2017, 6297651. [Google Scholar] [CrossRef] [Green Version]
- Pagano, A.P.; Sicchieri, J.M.F.; Schiavoni, I.L.; Barbeiro, D.; Manca, C.S.; da Silva, B.R.; Bezerra, A.E.; Pinto, L.C.M.; Araujo, R.C.; Teixeira, A.C.; et al. Phase angle as a severity indicator for liver diseases. Nutrition 2020, 70, 110607. [Google Scholar] [CrossRef]
- Peres, W.A.; Lento, D.F.; Baluz, K.; Ramalho, A. Phase angle as a nutritional evaluation tool in all stages of chronic liver disease. Nutr. Hosp. 2012, 27, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Coppini, L.Z.; Waitzberg, D.L.; Campos, A.C. Limitations and validation of bioelectrical impedance analysis in morbidly obese patients. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Tan, C.S.; Tan, K.W.; Lim, S.P.Y.; So, J.B.Y.; Shabbir, A. Sleeve Gastrectomy and Roux-En-Y Gastric Bypass Lead to Comparable Changes in Body Composition in a Multiethnic Asian Population. J. Gastrointest. Surg. 2019, 23, 445–450. [Google Scholar] [CrossRef]
- Widen, E.M.; Strain, G.; King, W.C.; Yu, W.; Lin, S.; Goodpaster, B.; Thornton, J.; Courcoulas, A.; Pomp, A.; Gallagher, D. Validity of bioelectrical impedance analysis for measuring changes in body water and percent fat after bariatric surgery. Obes. Surg. 2014, 24, 847–854. [Google Scholar] [CrossRef] [Green Version]
- de Paris, F.G.C.; Padoin, A.V.; Mottin, C.C.; de Paris, M.F. Assessment of Changes in Body Composition During the First Postoperative Year After Bariatric Surgery. Obes. Surg. 2019, 29, 3054–3061. [Google Scholar] [CrossRef] [PubMed]

| No NASH | Borderline NASH | Definite NASH | |||||
|---|---|---|---|---|---|---|---|
| Count | n % | Count | n % | Count | n % | ||
| Steatosis | 0: <5% | 22 | 44.0 | 1 | 1.5 | 0 | 0.0 |
| 1: 5–33% | 26 | 52.0 | 28 | 43.1 | 2 | 8.7 | |
| 2: 34–4: 66% | 2 | 4.0 | 29 | 44.6 | 8 | 34.8 | |
| 3: >66% | 0 | 0.0 | 7 | 10.8 | 13 | 56.5 | |
| Ballooning | 0: none | 43 | 86.0 | 17 | 26.2 | 1 | 4.3 |
| 1: few ballooned | 7 | 14.0 | 44 | 67.7 | 16 | 69.6 | |
| 2: many ballooned | 0 | 0.0 | 4 | 6.2 | 6 | 26.1 | |
| Inflammation | 0: none | 27 | 54.0 | 7 | 10.8 | 0 | 0.0 |
| 1: <2 foci | 23 | 46.0 | 49 | 75.4 | 7 | 30.4 | |
| 2: 2–4 foci | 0 | 0.0 | 9 | 13.8 | 12 | 52.2 | |
| 3: >4 foci | 0 | 0.0 | 0 | 0.0 | 4 | 17.4 | |
| No NASH (n = 50) | Borderline NASH (n = 65) | Definite NASH (n = 23) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value | |
| Age (years) | 41.8 | 11.6 | 45.9 | 11.2 | 42.4 | 10-5 | 0.124 |
| BMI (kg/m2) | 48.6 | 8.8 | 51.6 | 8.5 | 56.3 | 10.3 | 0.004 * |
| Weight (kg) | 138.0 | 29.3 | 149.5 | 27.9 | 165.2 | 31.2 | 0.001 * |
| WC (cm) | 141.2 | 20.3 | 149.1 | 12.9 | 155.6 | 22.5 | 0.068 |
| AST | 22.7 | 6.0 | 28.9 | 17.2 | 33.4 | 11.1 | 0.003 * |
| ALT | 24.5 | 7.9 | 38 | 30.5 | 44.3 | 19.8 | 0.001 * |
| APRI | 0.08 | 0.03 | 0.11 | 0.1 | 0.12 | 0.05 | 0.030 * |
| Trigylcerides | 134.9 | 75.0 | 175.1 | 86.9 | 187.6 | 83.6 | 0.016 * |
| Cholesterol | 194.9 | 31.9 | 191.0 | 35.4 | 180.2 | 36.8 | 0.252 |
| HbA1c (%) | 5.8 | 1.3 | 6,4 | 1.6 | 6.6 | 1.7 | 0.060 |
| HOMA-IR | 6.9 | 4.6 | 9.8 | 5.7 | 21.0 | 25.3 | 0.000 * |
| PhA (°) | 5.3 | 0.9 | 5.3 | 0.9 | 5.3 | 0.8 | 0.959 |
| TBW (liter) | 51.5 | 11.3 | 55.6 | 13.1 | 60.4 | 13.6 | 0.018 * |
| FFM (kg) | 70.4 | 15.4 | 75.9 | 17.9 | 82.5 | 18.6 | 0.018 * |
| FM (kg) | 67.9 | 19.8 | 73.6 | 18.5 | 82.7 | 20.6 | 0.011 * |
| FMI (kg/cm2) | 23.8 | 7.3 | 25.6 | 6.8 | 28.0 | 7.9 | 0.067 |
| FFMI (kg/cm2) | 24.4 | 4.2 | 26.0 | 4.9 | 27.4 | 4.2 | 0.026 * |
| SMM (kg) | 31.0 | 6.9 | 33.4 | 8.1 | 37.5 | 8.2 | 0.005 * |
| SMI (kg/cm2) | 10.8 | 1.9 | 11.4 | 2.2 | 12.5 | 1.9 | 0.006 * |
| SMI_weight | 0.08 | 0.0 | 0.08 | 0.0 | 0.08 | 0-0 | 0.661 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitz, S.M.-T.; Schooren, L.; Kroh, A.; Koch, A.; Stier, C.; Neumann, U.P.; Ulmer, T.F.; Alizai, P.H. Association of Body Composition and Sarcopenia with NASH in Obese Patients. J. Clin. Med. 2021, 10, 3445. https://doi.org/10.3390/jcm10153445
Schmitz SM-T, Schooren L, Kroh A, Koch A, Stier C, Neumann UP, Ulmer TF, Alizai PH. Association of Body Composition and Sarcopenia with NASH in Obese Patients. Journal of Clinical Medicine. 2021; 10(15):3445. https://doi.org/10.3390/jcm10153445
Chicago/Turabian StyleSchmitz, Sophia Marie-Therese, Lena Schooren, Andreas Kroh, Alexander Koch, Christine Stier, Ulf Peter Neumann, Tom Florian Ulmer, and Patrick Hamid Alizai. 2021. "Association of Body Composition and Sarcopenia with NASH in Obese Patients" Journal of Clinical Medicine 10, no. 15: 3445. https://doi.org/10.3390/jcm10153445
APA StyleSchmitz, S. M.-T., Schooren, L., Kroh, A., Koch, A., Stier, C., Neumann, U. P., Ulmer, T. F., & Alizai, P. H. (2021). Association of Body Composition and Sarcopenia with NASH in Obese Patients. Journal of Clinical Medicine, 10(15), 3445. https://doi.org/10.3390/jcm10153445







