Longitudinal Changes in Body Composition of Long-Term Survivors of Pancreatic Head Cancer and Factors Affecting the Changes
Abstract
1. Introduction
2. Materials and Methods
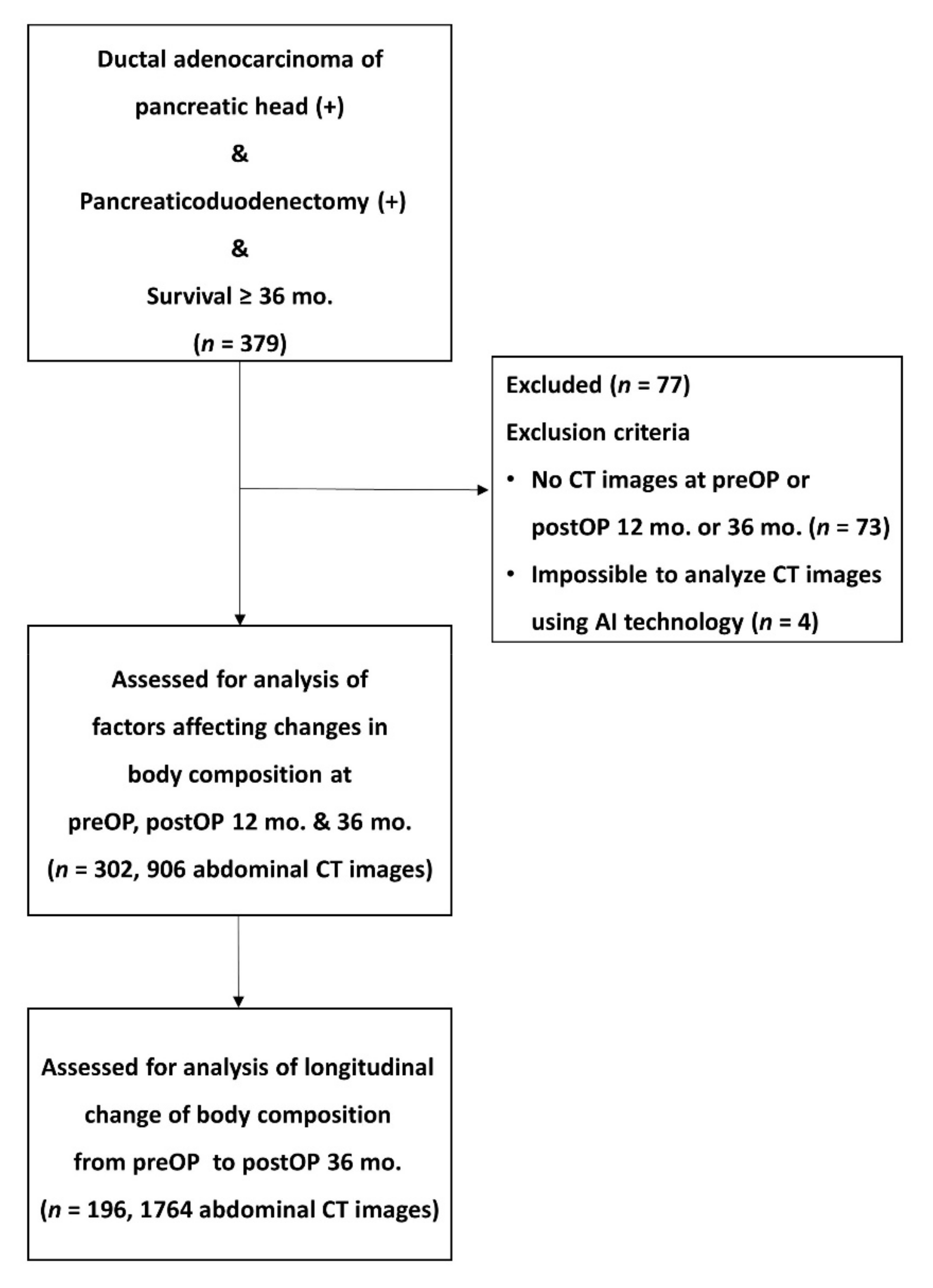
2.1. Patients Selection and Study Design
2.2. Surgical Procedure and Adjuvant Treatments
2.3. Anthropometrics and Laboratory Data
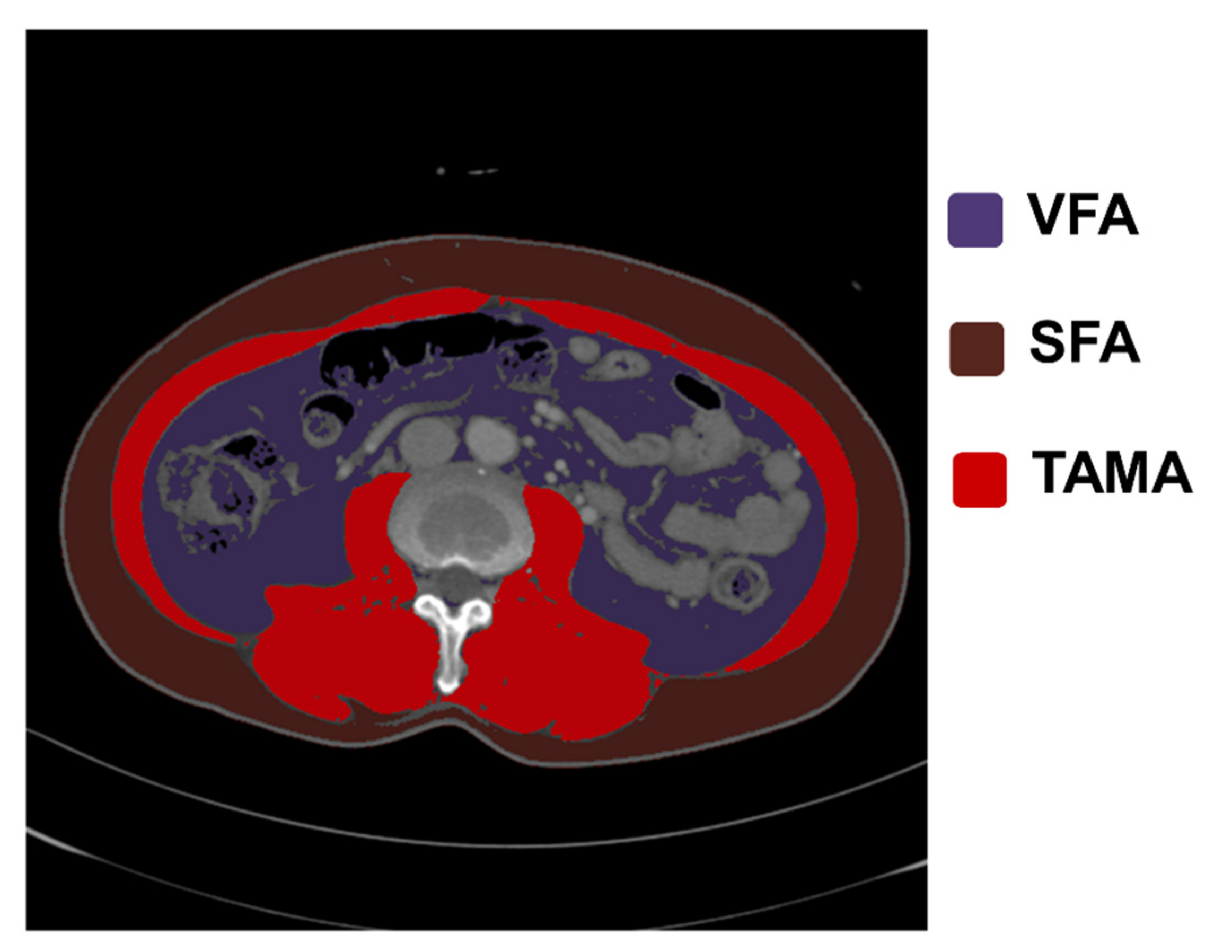
2.4. Analysis of CT Images and Body Composition
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Changes of Body Composition during the Survival Period
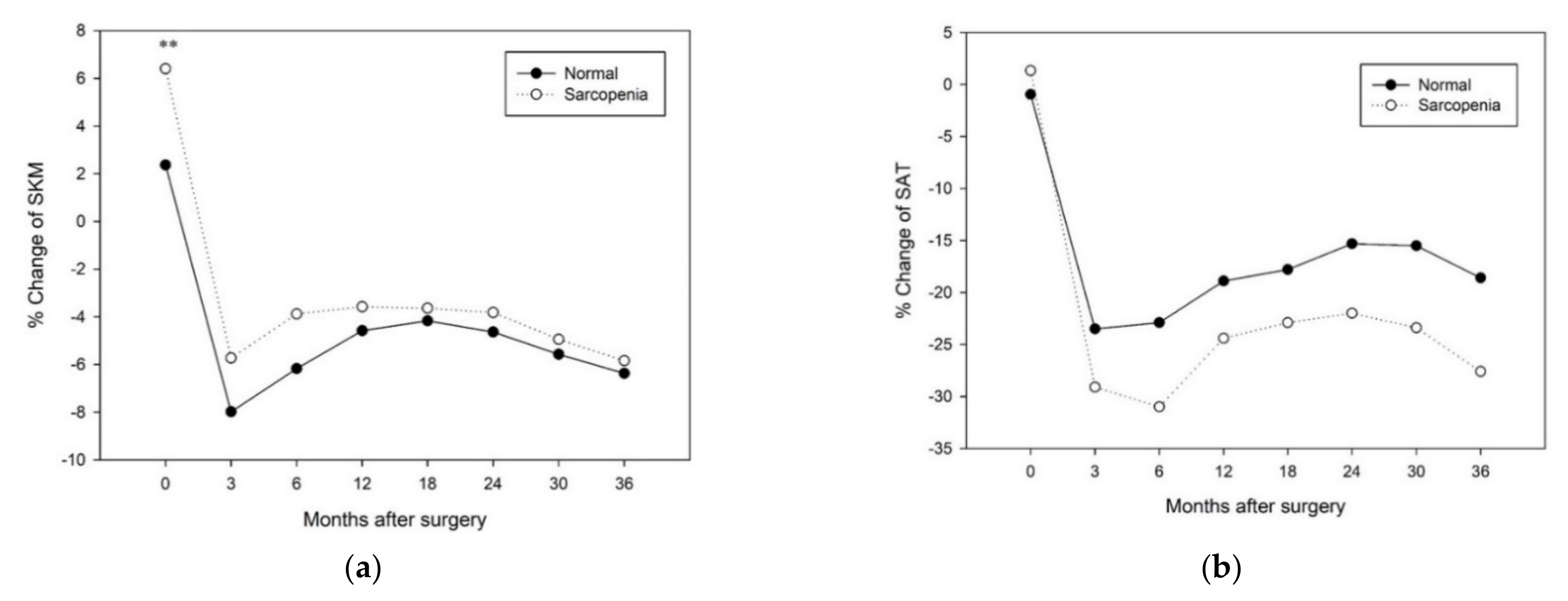
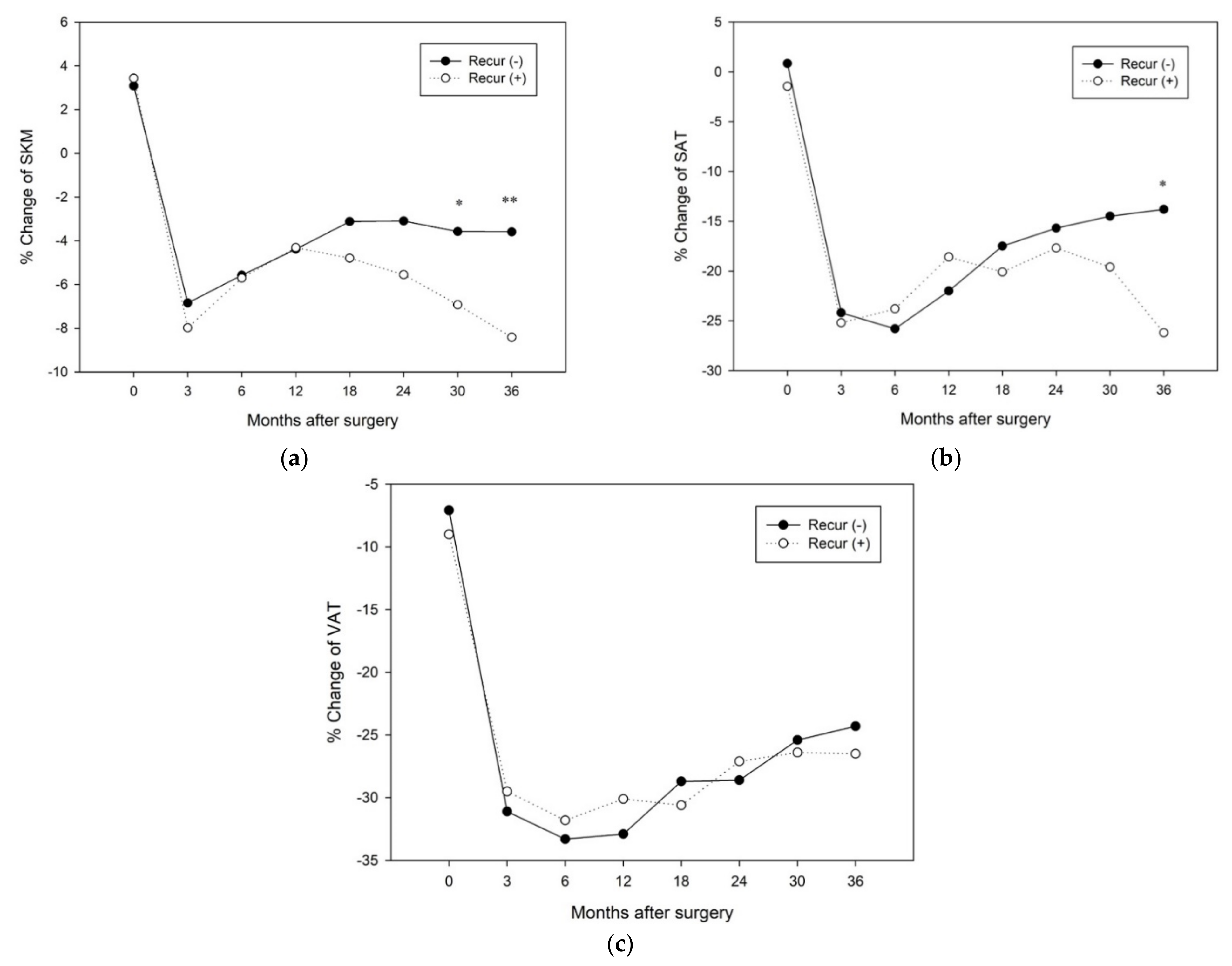
3.3. Factors Affecting Changes in Body Composition after Surgery
3.4. Changes in Longitudinal Body Composition According to Each Factor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Serrano, P.E.; Cleary, S.P.; Dhani, N.; Kim, P.T.; Greig, P.D.; Leung, K.; Moulton, C.A.; Gallinger, S.; Wei, A.C. Improved long-term outcomes after resection of pancreatic adenocarcinoma: A comparison between two time periods. Ann. Surg. Oncol. 2015, 22, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.L.; Ryan, A.M.; Reynolds, J.V. Cancer cachexia: Mechanisms and clinical implications. Gastroenterol. Res. Pract. 2011, 2011, 601434. [Google Scholar] [CrossRef] [PubMed]
- Vujasinovic, M.; Valente, R.; Del Chiaro, M.; Permert, J.; Lohr, J.M. Pancreatic Exocrine Insufficiency in Pancreatic Cancer. Nutrients 2017, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Barreto, R.; Couch, M.E.; Bonetto, A.; O’Connell, T.M. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J. Cachexia Sarcopenia Muscle 2019, 10, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Di Sebastiano, K.M.; Yang, L.; Zbuk, K.; Wong, R.K.; Chow, T.; Koff, D.; Moran, G.R.; Mourtzakis, M. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: The relationship with diabetes and anaemia. Br. J. Nutr. 2013, 109, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Dalal, S.; Hui, D.; Bidaut, L.; Lem, K.; Del Fabbro, E.; Crane, C.; Reyes-Gibby, C.C.; Bedi, D.; Bruera, E. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: A pilot study. J. Pain Symptom Manag. 2012, 44, 181–191. [Google Scholar] [CrossRef]
- Richter, E.; Denecke, A.; Klapdor, S.; Klapdor, R. Parenteral nutrition support for patients with pancreatic cancer--improvement of the nutritional status and the therapeutic outcome. Anticancer Res. 2012, 32, 2111–2118. [Google Scholar]
- Florez Bedoya, C.A.; Cardoso, A.C.F.; Parker, N.; Ngo-Huang, A.; Petzel, M.Q.; Kim, M.P.; Fogelman, D.; Romero, S.G.; Wang, H.; Park, M.; et al. Exercise during preoperative therapy increases tumor vascularity in pancreatic tumor patients. Sci. Rep. 2019, 9, 13966. [Google Scholar] [CrossRef]
- Beger, H.G.; Poch, B.; Mayer, B.; Siech, M. New Onset of Diabetes and Pancreatic Exocrine Insufficiency After Pancreaticoduodenectomy for Benign and Malignant Tumors: A Systematic Review and Meta-analysis of Long-term Results. Ann. Surg. 2018, 267, 259–270. [Google Scholar] [CrossRef]
- Hallac, A.; Aleassa, E.M.; Rogers, M.; Falk, G.A.; Morris-Stiff, G. Exocrine pancreatic insufficiency in distal pancreatectomy: Incidence and risk factors. HPB 2020, 22, 275–281. [Google Scholar] [CrossRef]
- Armstrong, T.; Strommer, L.; Ruiz-Jasbon, F.; Shek, F.W.; Harris, S.F.; Permert, J.; Johnson, C.D. Pancreaticoduodenectomy for peri-ampullary neoplasia leads to specific micronutrient deficiencies. Pancreatology 2007, 7, 37–44. [Google Scholar] [CrossRef]
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia-What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef]
- Lee, K.; Shin, Y.; Huh, J.; Sung, Y.S.; Lee, I.S.; Yoon, K.H.; Kim, K.W. Recent Issues on Body Composition Imaging for Sarcopenia Evaluation. Korean J. Radiol. 2019, 20, 205–217. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, C.J.; Yum, K.S. Cut-off values of visceral fat area and waist circumference: Diagnostic criteria for abdominal obesity in a Korean population. J. Korean Med. Sci. 2006, 21, 1048–1053. [Google Scholar] [CrossRef]
- Gross, A.L.; May, B.J.; Axilbund, J.E.; Armstrong, D.K.; Roden, R.B.; Visvanathan, K. Weight change in breast cancer survivors compared to cancer-free women: A prospective study in women at familial risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1262–1269. [Google Scholar] [CrossRef]
- Xiao, D.Y.; Luo, S.; O’Brian, K.; Sanfilippo, K.M.; Ganti, A.; Riedell, P.; Lynch, R.C.; Liu, W.; Kahl, B.S.; Cashen, A.F.; et al. Longitudinal Body Composition Changes in Diffuse Large B-cell Lymphoma Survivors: A Retrospective Cohort Study of United States Veterans. J. Natl. Cancer Inst. 2016, 108, djw145. [Google Scholar] [CrossRef]
- Vaughan, V.C.; Martin, P.; Lewandowski, P.A. Cancer cachexia: Impact, mechanisms and emerging treatments. J. Cachexia Sarcopenia Muscle 2013, 4, 95–109. [Google Scholar] [CrossRef]
- Shimokata, H.; Ando, F.; Yuki, A.; Otsuka, R. Age-related changes in skeletal muscle mass among community-dwelling Japanese: A 12-year longitudinal study. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 85–92. [Google Scholar] [CrossRef]
- Waters, D.L.; Yau, C.L.; Montoya, G.D.; Baumgartner, R.N. Serum Sex Hormones, IGF-1, and IGFBP3 Exert a Sexually Dimorphic Effect on Lean Body Mass in Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, 648–652. [Google Scholar] [CrossRef][Green Version]
- Bhasin, S.; Storer, T.W.; Berman, N.; Callegari, C.; Clevenger, B.; Phillips, J.; Bunnell, T.J.; Tricker, R.; Shirazi, A.; Casaburi, R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N. Engl. J. Med. 1996, 335, 1–7. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Waters, D.L.; Gallagher, D.; Morley, J.E.; Garry, P.J. Predictors of skeletal muscle mass in elderly men and women. Mech. Ageing Dev. 1999, 107, 123–136. [Google Scholar] [CrossRef]
- Kong, H.H.; Won, C.W.; Kim, W. Effect of sarcopenic obesity on deterioration of physical function in the elderly. Arch. Gerontol. Geriatr. 2020, 89, 104065. [Google Scholar] [CrossRef]
- Schaap, L.A.; van Schoor, N.M.; Lips, P.; Visser, M. Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1199–1204. [Google Scholar] [CrossRef]
- Beaudart, C.; Biver, E.; Reginster, J.Y.; Rizzoli, R.; Rolland, Y.; Bautmans, I.; Petermans, J.; Gillain, S.; Buckinx, F.; Dardenne, N.; et al. Validation of the SarQoL(R), a specific health-related quality of life questionnaire for Sarcopenia. J. Cachexia Sarcopenia Muscle 2017, 8, 238–244. [Google Scholar] [CrossRef]
- Rohm, M.; Zeigerer, A.; Machado, J.; Herzig, S. Energy metabolism in cachexia. EMBO Rep. 2019, 20, e47258. [Google Scholar] [CrossRef]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Romero, L.; Garry, P.J. Serum albumin is associated with skeletal muscle in elderly men and women. Am. J. Clin. Nutr. 1996, 64, 552–558. [Google Scholar] [CrossRef]
- Todorov, P.; Cariuk, P.; McDevitt, T.; Coles, B.; Fearon, K.; Tisdale, M. Characterization of a cancer cachectic factor. Nature 1996, 379, 739–742. [Google Scholar] [CrossRef]
- Hirai, K.; Hussey, H.J.; Barber, M.D.; Price, S.A.; Tisdale, M.J. Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Res. 1998, 58, 2359–2365. [Google Scholar] [PubMed]
- Daly, L.E.; Éb, N.B.; Power, D.G.; Cushen, S.J.; James, K.; Ryan, A.M. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hayashidani, Y.; Hashimoto, Y.; Nakashima, A.; Yuasa, Y.; Kondo, N.; Ohge, H.; Sueda, T. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J. Am. Coll. Surg. 2010, 211, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.Y.; Lee, S.G.; Park, M.; Lee, J.; Lee, S.H.; Hwang, H.K.; Lee, M.J.; Paik, Y.K.; Lee, W.J.; Kang, C.M. Developing a preoperative serum metabolome-based recurrence-predicting nomogram for patients with resected pancreatic ductal adenocarcinoma. Sci. Rep. 2019, 9, 18634. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.L., Jr.; Olivan, M.; Alcantara, P.S.; Sandoval, R.; Peres, S.B.; Neves, R.X.; Silverio, R.; Maximiano, L.F.; Otoch, J.P.; Seelaender, M. Adipose tissue-derived factors as potential biomarkers in cachectic cancer patients. Cytokine 2013, 61, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.L., Jr.; Henriques, F.S.; Neves, R.X.; Olivan, M.R.; Matos-Neto, E.M.; Alcantara, P.S.; Maximiano, L.F.; Otoch, J.P.; Alves, M.J.; Seelaender, M. Cachexia-associated adipose tissue morphological rearrangement in gastrointestinal cancer patients. J. Cachexia Sarcopenia Muscle 2016, 7, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.E.; Tewari, N.; Ackner, A.; Awwad, A.; Madhusudan, S.; Macdonald, I.A.; Fearon, K.C.; Lobo, D.N. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin. Nutr. 2016, 35, 1103–1109. [Google Scholar] [CrossRef]

| Variables | Male (n = 181) | Female (n = 121) | p Value |
|---|---|---|---|
| Clinical information | |||
| Age (year), mean (SD) | 58.9 (9.5) | 59.4 (8.9) | 0.68 |
| BMI (kg/m2), mean (SD) | 23.2 (2.3) | 23.1 (2.5) | 0.57 |
| Cancer stage, n (%) I/II/III/IV | 87/77/13/4 (48.1/42.5/7.2/2.2) | 65/43/8/5 (53.7/35.5/6.6/4.1) | 0.79 |
| Recurrence, n (%) | |||
| (+) | 100 (55.2) | 55 (45.5) | 0.10 |
| (−) | 81 (44.8) | 66 (54.5) | |
| Duration of recurrence after surgery (day), mean (SD) | 938.5 (761.2) | 1007.9 (784.8) | 0.59 |
| Adjuvant Tx, n (%) | |||
| (−) | 38 (21.0) | 31 (25.6) | 0.58 |
| CTx only | 111 (61.3) | 68 (56.2) | |
| CTx & RTx | 32 (17.7) | 22 (18.2) | |
| Laboratory findings | |||
| Albumin (g/dL), mean (SD) | 3.8 (0.4) | 3.6 (0.4) | <0.05 |
| NLR, mean (SD) | 2.1 (1.2) | 2.2 (2.1) | 0.51 |
| CRP (mL/dL), mean (SD) | 0.93 (1.7) | 1.0 (2.3) | 0.72 |
| CA 19-9 (U/mL), mean (SD) | 242.9 (795.1) | 260.6 (501.8) | 0.83 |
| CEA (mg/mL), mean (SD) | 2.7 (2.4) | 3.3 (6.9) | 0.34 |
| Body composition | |||
| SMI (cm2/m2), mean (SD) | 55.1 (7.9) | 44.2 (5.2) | <0.001 |
| SFI (cm2/m2), mean (SD) | 37.4 (15.2) | 66.0 (21.4) | <0.001 |
| VFI (cm2/m2), mean (SD) | 39.7 (18.4) | 35.4 (17.3) | <0.05 |
| Obesity, n (%) | 91 (50.3) | 39 (32.2) | <0.01 |
| Sarcopenia, n (%) | 44 (24.3) | 29 (24.0) | 0.95 |
| Male (n = 181) | Female (n = 121) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-OP | Post-OP 36 mo | p Value | % Change (%) | Pre-OP | Post-OP 36 mo | p Value | % Change (%) | |
| SMI (cm2/m2), mean (SD) | 55.1 (7.9) | 51.1 (8.6) | <0.001 | −7.0 (10.2) | 44.2 (5.2) | 42.5 (5.9) | <0.001 | −3.6 (9.2) ** |
| SFI (cm2/m2), mean (SD) | 37.4 (15.2) | 29.9 (15.4) | <0.001 | −6.6 (38.1) | 66.0 (21.4) | 51.5 (27.8) | <0.001 | −22.7 (32.5) |
| VFI (cm2/m2), mean (SD) | 39.7 (18.4) | 26.9 (16.4) | <0.001 | −27.5 (39.1) | 35.4 (17.3) | 25.9 (16.1) | <0.001 | −19.9 (55.3) |
| Post OP 1 Year | Post OP 3 Years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| Variables | OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value |
| skeletal muscle | ||||||||||||
| Age > 65 years | 0.67 | 0.37–1.22 | 0.19 | 0.61 | 0.33–1.12 | 0.11 | ||||||
| Sex | 0.97 | 0.57–1.66 | 0.92 | 1.67 | 0.96–2.92 | 0.07 | 1.55 | 0.87–2.74 | 0.13 | |||
| Stage (I vs. others) | 1.02 | 0.60–1.72 | 0.95 | 1.26 | 0.75–2.14 | 0.39 | ||||||
| Adjuvant treatment | 1.95 | 0.96–3.96 | 0.06 | 1.67 | 0.81–3.44 | 0.17 | 1.72 | 0.87–3.42 | 0.12 | |||
| Recurrence | 0.75 | 0.44–1.27 | 0.29 | 2.87 | 1.63–5.04 | <0.001 | 2.78 | 1.58–4.89 | <0.001 | |||
| Body composition | ||||||||||||
| Normal vs. Sarcopenia | 0.41 | 0.20–0.85 | <0.05 | 0.43 | 0.21–0.91 | <0.05 | 0.75 | 0.39–1.41 | 0.37 | |||
| Normal vs. Obesity | 1.09 | 0.64–1.84 | 0.76 | 1.45 | 0.86–2.46 | 0.16 | ||||||
| Laboratory findings | ||||||||||||
| Albumin < 3.5 (g/dL) | 0.39 | 0.18–0.83 | <0.05 | 0.41 | 0.19–0.87 | <0.05 | 0.66 | 0.34–1.28 | 0.21 | |||
| NLR > 4.0 | 0.94 | 0.30–2.98 | 1.00 | 0.94 | 0.30–2.98 | 1.00 | ||||||
| CRP > 1.0 (mL/dL) | 0.98 | 0.47–2.06 | 0.97 | 1.22 | 0.58–2.58 | 0.60 | ||||||
| CA19-9 > 200 (U/mL) | 0.60 | 0.30–1.19 | 0.14 | 0.71 | 0.36–1.39 | 0.31 | ||||||
| CEA > 5.0 (mg/mL) | 0.71 | 0.29–1.71 | 0.44 | 0.64 | 0.25–1.62 | 0.34 | ||||||
| subcutaneous adipose tissue | ||||||||||||
| Age > 65 years | 0.58 | 0.32–1.06 | 0.08 | 0.53 | 0.28–0.98 | <0.05 | 0.67 | 0.37–1.22 | 0.19 | |||
| Sex | 1.03 | 0.61–1.76 | 0.90 | 1.05 | 0.61–1.80 | 0.86 | ||||||
| Stage (I vs. others) | 0.88 | 0.53–1.49 | 0.64 | 1.36 | 0.80–2.30 | 0.26 | ||||||
| Adjuvant treatment | 1.42 | 0.74–2.74 | 0.29 | 0.90 | 0.48–1.66 | 0.73 | ||||||
| Recurrence | 1.53 | 0.90–2.59 | 0.11 | 3.40 | 1.91–6.04 | <0.001 | 3.76 | 2.07–6.82 | <0.001 | |||
| Body composition | ||||||||||||
| Normal vs. Sarcopenia | 2.16 | 1.22–3.82 | <0.01 | 2.33 | 1.30–4.17 | <0.01 | 2.48 | 1.40–4.39 | <0.01 | 2.78 | 1.51–5.11 | <0.01 |
| Normal vs. Obesity | 0.71 | 0.42–1.21 | 0.21 | 0.87 | 0.51–1.49 | 0.62 | ||||||
| Laboratory findings | ||||||||||||
| Albumin < 3.5 (g/dL) | 0.78 | 0.41–1.49 | 0.46 | 0.66 | 0.34–1.28 | 0.21 | ||||||
| NLR > 4.0 | 1.67 | 0.60–4.67 | 0.39 | 0.18 | 0.02–1.39 | 0.08 | 0.16 | 0.02–1.26 | 0.08 | |||
| CRP > 1.0 (mL/dL) | 0.93 | 0.45–1.94 | 0.84 | 1.73 | 0.86–3.46 | 0.12 | ||||||
| CA19-9 > 200 (U/mL) | 0.81 | 0.43–1.55 | 0.52 | 1.10 | 0.59–2.06 | 0.77 | ||||||
| CEA > 5.0 (mg/mL) | 0.74 | 0.31–1.79 | 0.50 | 0.49 | 0.18–1.33 | 0.15 | ||||||
| visceral adipose tissue | ||||||||||||
| Age > 65 years | 0.93 | 0.53–1.65 | 0.81 | 0.85 | 0.48–1.52 | 0.59 | ||||||
| Sex | 1.72 | 0.98–3.00 | 0.06 | 1.73 | 0.99–3.04 | 0.06 | 1.08 | 0.63–1.85 | 0.78 | |||
| Stage (I vs. others) | 0.85 | 0.51–1.44 | 0.55 | 0.98 | 0.58–1.66 | 0.95 | ||||||
| Adjuvant treatment | 1.56 | 0.80–3.05 | 0.19 | 1.39 | 0.72–2.68 | 0.32 | ||||||
| Recurrence | 1.38 | 0.82–2.34 | 0.23 | 2.32 | 1.35–4.01 | <0.01 | 2.34 | 1.35–4.06 | <0.01 | |||
| Body composition | ||||||||||||
| Normal vs. Sarcopenia | 2.03 | 1.15–3.61 | <0.05 | 2.05 | 1.15–3.65 | <0.05 | 1.19 | 0.66–2.17 | 0.56 | |||
| Normal vs. Obesity | 1.40 | 0.83–2.37 | 0.21 | 1.62 | 0.96–2.74 | 0.07 | 1.64 | 0.96–2.80 | 0.07 | |||
| Laboratory findings | ||||||||||||
| Albumin < 3.5 (g/dL) | 0.72 | 0.37–1.38 | 0.32 | 0.89 | 0.47–1.68 | 0.72 | ||||||
| NLR > 4.0 | 1.70 | 0.61–4.77 | 0.39 | 0.63 | 0.18–2.26 | 0.58 | ||||||
| CRP > 1.0 (mL/dL) | 0.85 | 0.40–1.81 | 0.68 | 1.39 | 0.69–2.79 | 0.36 | ||||||
| CA19-9 > 200 (U/mL) | 0.94 | 0.50–1.78 | 0.86 | 0.87 | 0.45–1.66 | 0.67 | ||||||
| CEA > 5.0 (mg/mL) | 0.94 | 0.40–2.19 | 0.88 | 1.00 | 0.43–2.35 | 0.99 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, H.-H.; Kim, K.-W.; Ko, Y.-S.; Kim, S.-C.; Lee, J.-H.; Song, K.-B.; Hwang, D.-W.; Kim, W. Longitudinal Changes in Body Composition of Long-Term Survivors of Pancreatic Head Cancer and Factors Affecting the Changes. J. Clin. Med. 2021, 10, 3436. https://doi.org/10.3390/jcm10153436
Kong H-H, Kim K-W, Ko Y-S, Kim S-C, Lee J-H, Song K-B, Hwang D-W, Kim W. Longitudinal Changes in Body Composition of Long-Term Survivors of Pancreatic Head Cancer and Factors Affecting the Changes. Journal of Clinical Medicine. 2021; 10(15):3436. https://doi.org/10.3390/jcm10153436
Chicago/Turabian StyleKong, Hyun-Ho, Kyung-Won Kim, You-Sun Ko, Song-Cheol Kim, Jae-Hoon Lee, Ki-Byung Song, Dae-Wook Hwang, and Won Kim. 2021. "Longitudinal Changes in Body Composition of Long-Term Survivors of Pancreatic Head Cancer and Factors Affecting the Changes" Journal of Clinical Medicine 10, no. 15: 3436. https://doi.org/10.3390/jcm10153436
APA StyleKong, H.-H., Kim, K.-W., Ko, Y.-S., Kim, S.-C., Lee, J.-H., Song, K.-B., Hwang, D.-W., & Kim, W. (2021). Longitudinal Changes in Body Composition of Long-Term Survivors of Pancreatic Head Cancer and Factors Affecting the Changes. Journal of Clinical Medicine, 10(15), 3436. https://doi.org/10.3390/jcm10153436








