Systemic Vulnerability, as Expressed by I-CAM and MMP-9 at Presentation, Predicts One Year Outcomes in Patients with Acute Myocardial Infarction—Insights from the VIP Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Study Protocol
2.2.1. Laboratory Testing for Inflammatory Biomarkers
2.2.2. Evaluation of Imaging Markers
2.2.3. Study End-Points and Follow-Up
- -
- hemodynamic instability (cardiogenic shock, need for inotropic medication);
- -
- new onset atrial fibrillation;
- -
- ventricular arrhythmias (non-sustained or sustained VT not requiring electrical DC, polymorphic ventricular premature contractions);
- -
- resuscitated cardiac arrest (out-of-hospital and in-hospital cardiac arrest);
- -
- high-degree AV conduction abnormalities requiring temporary pacing;
- -
- mechanical complications (rupture of free ventricular wall, interventricular septum, papillary muscle).
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population and End-Points
3.2. Accuracy of Serum and Imaging Markers in Predicting 1-Year MACE Rates
3.3. Uni- and Multivariable Analysis for Predictors of MACE during the 1 Year Follow-Up
4. Discussions
4.1. Imaging Predictors for MACE in the Context of an Enhanced Systemic Inflammation
4.2. Clinical Applications
4.3. Study Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 12 December 2020).
- Cabrera-Fuentes, H.A.; Alba-Alba, C.; Aragones, J.; Bernhagen, J.; Boisvert, W.A.; Bøtker, H.E.; Cesarman-Maus, G.; Fleming, I.; Garcia-Dorado, D.; Lecour, S.; et al. Meeting report from the 2nd International Symposium on New Frontiers in Cardiovascular Research. Protecting the cardiovascular system from ischemia: Between bench and bedside. Basic Res. Cardiol. 2016, 111, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Li, N.; Li, Y.; Zeng, X.T.; Liu, M.Y. Cardiac Biomarkers Predicting MACE in Patients Undergoing Noncardiac Surgery: A Meta-Analysis. Front. Physiol. 2019, 9, 1923. [Google Scholar] [CrossRef] [PubMed]
- Abu-Assi, E.; García-Acuña, J.M.; Peña-Gil, C.; González-Juanatey, J.R. Validation of the GRACE risk score for predicting death within 6 months of follow-up in a contemporary cohort of patients with acute coronary syndrome. Rev. Esp. Cardiol. 2010, 63, 640–648. [Google Scholar] [CrossRef]
- Correia, L.C.; Garcia, G.; Kalil, F.; Ferreira, F.; Carvalhal, M.; Oliveira, R.; Silva, A.; Vasconcelos, I.; Henri, C.; Noya-Rabelo, M. Prognostic value of TIMI score versus GRACE score in ST-segment elevation myocardial infarction. Arq. Bras. Cardiol. 2014, 103, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Huang, S.S.; Lin, S.J. TIMI and GRACE Risk Scores Predict Both Short-Term and Long-Term Outcomes in Chinese Patients with Acute Myocardial Infarction. Acta Cardiol. Sin. 2018, 34, 4–12. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P.; Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 2015, 278, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Westman, P.C.; Lipinski, M.J.; Luger, D.; Waksman, R.; Bonow, R.O.; Wu, E.; Epstein, S.E. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part II. Circulation 2003, 108, 1772–1778. [Google Scholar] [CrossRef]
- Wensley, F.; Gao, P.; Burgess, S.; Kaptoge, S.; Di Angelantonio, E.; Shah, T.; Engert, J.C.; Clarke, R.; Davey-Smith, G.; Nordestgaard, B.G.; et al. Association between C reactive protein and coronary heart disease: Mendelian randomization analysis based on individual participant data. BMJ 2011, 342, d548. [Google Scholar] [CrossRef]
- Eltoft, A.; Arntzen, K.A.; Hansen, J.B.; Wilsgaard, T.; Mathiesen, E.B.; Johnsen, S.H. C-reactive protein in atherosclerosis—A risk marker but not a causal factor? A 13-year population-based longitudinal study: The Tromsø study. Atherosclerosis 2017, 263, 293–300. [Google Scholar] [CrossRef]
- Shrivastava, A.K.; Singh, H.V.; Raizada, A.; Kumar Singh, S. C-reactive protein, inflammation and coronary heart disease. Egypt. Heart J. 2015, 67, 89–97. [Google Scholar] [CrossRef]
- Strang, F.; Schunkert, H. C-reactive protein and coronary heart disease: All said--is not it? Mediat. Inflamm. 2014, 2014, 757123. [Google Scholar] [CrossRef]
- Rubin, J.; Chang, H.J.; Nasir, K.; Blumenthal, R.S.; Blaha, M.J.; Choi, E.K.; Chang, S.A.; Yoon, Y.E.; Chun, E.J.; Choi, S.I.; et al. Association between high-sensitivity C-reactive protein and coronary plaque subtypes assessed by 64-slice coronary computed tomography angiography in an asymptomatic population. Circ. Cardiovasc. Imaging 2011, 4, 201–209. [Google Scholar] [CrossRef][Green Version]
- Carrero, J.J.; Andersson Franko, M.; Obergfell, A.; Gabrielsen, A.; Jernberg, T. hsCRP Level and the Risk of Death or Recurrent Cardiovascular Events in Patients with Myocardial Infarction: A Healthcare-Based Study. J. Am. Heart Assoc. 2019, 8, e012638. [Google Scholar] [CrossRef]
- Polyakova, E.A.; Mikhaylov, E.N. The prognostic role of high-sensitivity C-reactive protein in patients with acute myocardial infarction. J. Geriatr. Cardiol. 2020, 17, 379–383. [Google Scholar] [CrossRef]
- Badimon, L.; Peña, E.; Arderiu, G.; Padró, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G. C-Reactive Protein in Atherothrombosis and Angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Fanola, C.L.; Morrow, D.A.; Cannon, C.P.; Jarolim, P.; Lukas, M.A.; Bode, C.; Hochman, J.S.; Goodrich, E.L.; Braunwald, E.; O’Donoghue, M.L. Interleukin-6 and the Risk of Adverse Outcomes in Patients After an Acute Coronary Syndrome: Observations From the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib-Thrombolysis in Myocardial Infarction 52) Trial. J. Am. Heart Assoc. 2017, 6, e005637. [Google Scholar] [CrossRef] [PubMed]
- Lino, D.O.C.; Freitas, I.A.; Meneses, G.C.; Martins, A.; Daher, E.F.; Rocha, J.; Silva Junior, G.B. Interleukin-6 and adhesion molecules VCAM-1 and ICAM-1 as biomarkers of post-acute myocardial infarction heart failure. Braz. J. Med. Biol. Res. 2019, 52, e8658. [Google Scholar] [CrossRef]
- Lahdentausta, L.; Leskelä, J.; Winkelmann, A.; Tervahartiala, T.; Sorsa, T.; Pesonen, E.; Pussinen, P.J. Serum MMP-9 Diagnostics, Prognostics, and Activation in Acute Coronary Syndrome and Its Recurrence. J. Cardiovasc. Transl. Res. 2018, 11, 210–220. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Feng, Y.; Dong, G.; Wang, Y.; Yang, J. The Role of Matrix Metalloproteinase-9 in Atherosclerotic Plaque Instability. Mediat. Inflamm. 2020, 2020, 3872367. [Google Scholar] [CrossRef]
- Hamed, G.M.; Fattah, M.F. Clinical Relevance of matrix metalloproteinase 9 in patients with acute coronary syndrome. Clin. Appl. Thromb. Hemost. 2015, 21, 705–711. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Sarna, S.; Puolakkainen, M.; Öhlin, H.; Sorsa, T.; Pesonen, E. The balance of serum matrix metalloproteinase-8 and its tissue inhibitor in acute coronary syndrome and its recurrence. Int. J. Cardiol. 2013, 167, 362–368. [Google Scholar] [CrossRef]
- Postadzhiyan, A.S.; Tzontcheva, A.V.; Kehayov, I.; Finkov, B. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and their association with clinical outcome, troponin T and C-reactive protein in patients with acute coronary syndromes. Clin. Biochem. 2008, 41, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Karpasova, E.A.; Diatlova, A.S.; Linkova, N.S.; Bunin, V.A.; Polyakova, V.O.; Krylova, Y.S.; Kvetnoy, I.M. Troponins, Adhesion Molecules, and Interleukins as Diagnostic Markers of CVDs: Expression in Peripheral Tissues. Biol. Bull. Rev. 2020, 10, 296–307. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Peetz, D.; Hafner, G.; Tiret, L.; Meyer, J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001, 104, 1336–1342. [Google Scholar] [CrossRef]
- Doo, Y.C.; Han, S.J.; Park, W.J.; Kim, S.M.; Choi, S.H.; Cho, G.Y.; Hong, K.S.; Han, K.R.; Lee, N.H.; Oh, D.J.; et al. Associations between C-reactive protein and circulating cell adhesion molecules in patients with unstable angina undergoing coronary intervention and their clinical implication. Clin. Cardiol. 2005, 28, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Doğan, C.; Bayram, Z.; Çap, M.; Özkalaycı, F.; Unkun, T.; Erdoğan, E.; Uslu, A.; Acar, R.D.; Guvendi, B.; Akbal, Ö.Y.; et al. Comparison of 30-Day MACE between Immediate versus Staged Complete Revascularization in Acute Myocardial Infarction with Multivessel Disease, and the Effect of Coronary Lesion Complexity. Medicina 2019, 55, 51. [Google Scholar] [CrossRef]
- Agra Bermejo, R.; Cordero, A.; García-Acuña, J.M.; Gómez Otero, I.; Varela Román, A.; Martínez, Á.; Álvarez Rodríguez, L.; Abou-Jokh, C.; Rodríguez-Mañero, M.; Cid Álvarez, B.; et al. Determinants and Prognostic Impact of Heart Failure and Left Ventricular Ejection Fraction in Acute Coronary Syndrome Settings. Rev. Esp. Cardiol. 2018, 71, 820–828. [Google Scholar] [CrossRef]
- Baksyte, G.; Macas, A.; Brazdzionyte, J.; Saferis, V.; Tamosiunas, M.; Krisciukaitis, A. Prognostic markers in the acute phase of myocardial infarction. Crit. Care 2007, 11 (Suppl. S2), P240. [Google Scholar] [CrossRef]
- Wang, J.C.; Normand, S.L.; Mauri, L.; Kuntz, R.E. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation 2004, 110, 278–328. [Google Scholar] [CrossRef]
- Morrow, D.A.; Antman, E.M.; Charlesworth, A.; Cairns, R.; Murphy, S.A.; de Lemos, J.A.; Giugliano, R.P.; McCabe, C.H.; Braunwald, E. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000, 102, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Chotechuang, Y.; Phrommintikul, A.; Kuanprasert, S.; Muenpa, R.; Ruengorn, C.; Patumanond, J.; Chaichuen, T.; Thanachaikun, N.; Benjanuwatra, T.; Sukonthasarn, A. GRACE score and cardiovascular outcomes prediction among the delayed coronary intervention after postfibrinolytic STEMI patients in a limited PCI-capable hospital. Open Heart 2020, 7, e001133. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Fang, L.; Moore, X.L.; Dart, A.M.; Wang, L.M. Systemic inflammatory response following acute myocardial infarction. J. Geriatr. Cardiol. 2015, 12, 305–312. [Google Scholar] [CrossRef]
- Wang, H.; Eitzman, D.T. Acute myocardial infarction leads to acceleration of atherosclerosis. Atherosclerosis 2013, 229, 18–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohamedali, B.; Shroff, A. Impact of smoking status on cardiovascular outcomes following percutaneous coronary intervention. Clin. Cardiol. 2013, 36, 372–377. [Google Scholar] [CrossRef]
- Steele, L.; Palmer, J.; Lloyd, A.; Fotheringham, J.; Iqbal, J.; Grech, E.D. The impact of smoking on mortality after acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: A retrospective cohort outcome study at 3 years. J. Thromb. Thrombolysis 2019, 47, 520–526. [Google Scholar] [CrossRef]
- Aune, E.; Røislien, J.; Mathisen, M.; Thelle, D.S.; Otterstad, J.E. The “smoker’s paradox” in patients with acute coronary syndrome: A systematic review. BMC Med. 2011, 9, 97. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied. Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Kristono, G.A.; Holley, A.S.; Lakshman, P.; Brunton-O’Sullivan, M.M.; Harding, S.A.; Larsen, P.D. Association between inflammatory cytokines and long-term adverse outcomes in acute coronary syndromes: A systematic review. Heliyon 2020, 6, e03704. [Google Scholar] [CrossRef]
- Ong, S.B.; Hernández-Reséndiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gupta, V.K.; Gupta, R.; Arora, S.; Gupta, V. Relationship of high-sensitive C-reactive protein with cardiovascular risk factors, clinical presentation and angiographic profile in patients with acute coronary syndrome: An Indian perspective. Indian Heart J. 2013, 65, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Koosha, P.; Roohafza, H.; Sarrafzadegan, N.; Vakhshoori, M.; Talaei, M.; Sheikhbahaei, E.; Sadeghi, M. High Sensitivity C-Reactive Protein Predictive Value for Cardiovascular Disease: A Nested Case Control from Isfahan Cohort Study (ICS). Glob. Heart 2020, 15, 3. [Google Scholar] [CrossRef]
- Krintus, M.; Kozinski, M.; Stefanska, A.; Sawicki, M.; Obonska, K.; Fabiszak, T.; Kubica, J.; Sypniewska, G. Value of C-reactive protein as a risk factor for acute coronary syndrome: A comparison with apolipoprotein concentrations and lipid profile. Mediat. Inflamm. 2012, 2012, 419804. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.O.; Park, Y.; Seo, J.H.; Jeong, M.H.; Chae, S.C.; Ahn, T.H.; Jang, W.Y.; Kim, W.; Park, E.J.; Choi, B.G.; et al. KAMIR-NIH Registry Investigators. Time-dependent prognostic effect of high sensitivity C-reactive protein with statin therapy in acute myocardial infarction. J. Cardiol. 2019, 74, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Lucci, C.; Cosentino, N.; Genovese, S.; Campodonico, J.; Milazzo, V.; De Metrio, M.; Rondinelli, M.; Riggio, D.; Biondi, M.L.; Rubino, M.; et al. Prognostic impact of admission high-sensitivity C-reactive protein in acute myocardial infarction patients with and without diabetes mellitus. Cardiovasc. Diabetol. 2020, 19, 183. [Google Scholar] [CrossRef]
- Ammirati, E.; Cannistraci, C.V.; Cristell, N.A.; Vecchio, V.; Palini, A.G.; Tornvall, P.; Paganoni, A.M.; Miendlarzewska, E.A.; Sangalli, L.M.; Monello, A.; et al. Identification and predictive value of interleukin-6+ interleukin-10+ and interleukin-6- interleukin-10+ cytokine patterns in ST-elevation acute myocardial infarction. Circ. Res. 2012, 111, 1336–1348. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Roitman-Johnson, B.; Stampfer, M.J.; Allen, J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 1998, 351, 88–92. [Google Scholar] [CrossRef]
- O’Malley, T.; Ludlam, C.A.; Riemermsa, R.A.; Fox, K.A. Early increase in levels of soluble inter-cellular adhesion molecule-1 (sICAM-1); potential risk factor for the acute coronary syndromes. Eur. Heart J. 2001, 22, 1226–1234. [Google Scholar] [CrossRef]
- Haverslag, R.; Pasterkamp, G.; Hoefer, I.E. Targeting adhesion molecules in cardiovascular disorders. Cardiovasc. Hematol. Disord. Drug. Targets. 2008, 8, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Güray, U.; Erbay, A.R.; Güray, Y.; Yilmaz, M.B.; Boyaci, A.A.; Sasmaz, H.; Korkmaz, S.; Kütük, E. Levels of soluble adhesion molecules in various clinical presentations of coronary atherosclerosis. Int. J. Cardiol. 2004, 96, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Turhan, H.; Saydam, G.S.; Erbay, A.R.; Ayaz, S.; Yasar, A.S.; Aksoy, Y.; Basar, N.; Yetkin, E. Increased plasma soluble adhesion molecules; ICAM-1, VCAM-1, and E-selectin levels in patients with slow coronary flow. Int. J. Cardiol. 2006, 108, 224–230. [Google Scholar] [CrossRef]
- Hillis, G.S.; Terregino, C.; Taggart, P.; Killian, A.; Zhao, N.; Kaplan, J.; Dalsey, W.C.; Mangione, A. Soluble intercellular adhesion molecule-1 as a predictor of early adverse events in patients with chest pain compatible with myocardial ischemia. Ann. Emerg. Med. 2001, 38, 223–228. [Google Scholar] [CrossRef]
- Luc, G.; Arveiler, D.; Evans, A.; Amouyel, P.; Ferrieres, J.; Bard, J.M.; Elkhalil, L.; Fruchart, J.C.; Ducimetiere, P. PRIME Study Group. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: The PRIME Study. Atherosclerosis 2003, 170, 169–176. [Google Scholar] [CrossRef]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Opstad, T.B.; Seljeflot, I.; Bøhmer, E.; Arnesen, H.; Halvorsen, S. MMP-9 and Its Regulators TIMP-1 and EMMPRIN in Patients with Acute ST-Elevation Myocardial Infarction: A NORDISTEMI Substudy. Cardiology 2018, 139, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Zhao, Q.; Qu, H.J.; Li, X.M.; Chen, Q.J.; Liu, F.; Chen, B.D.; Yang, Y.N. Usefulness of plasma matrix metalloproteinase-9 levels in prediction of in-hospital mortality in patients who received emergent percutaneous coronary artery intervention following myocardial infarction. Oncotarget 2017, 8, 105809–105818. [Google Scholar] [CrossRef]
- Desperak, P.; Hawranek, M.; Gąsior, P.; Desperak, A.; Lekston, A.; Gąsior, M. Long-term outcomes of patients with multivessel coronary artery disease presenting non-ST-segment elevation acute coronary syndromes. Cardiol. J. 2019, 26, 157–168. [Google Scholar] [CrossRef]
- Van der Schaaf, R.J.; Vis, M.M.; Sjauw, K.D.; Koch, K.T.; Baan, J., Jr.; Tijssen, J.G.; de Winter, R.J.; Piek, J.J.; Henriques, J.P. Impact of multivessel coronary disease on long-term mortality in patients with ST-elevation myocardial infarction is due to the presence of a chronic total occlusion. Am. J. Cardiol. 2006, 98, 1165–1169. [Google Scholar] [CrossRef]
- Karpiński, L.; Płaksej, R.; Kosmala, W.; Witkowska, M. Serum levels of interleukin-6, interleukin-10 and C-reactive protein in relation to left ventricular function in patients with myocardial infarction treated with primary angioplasty. Kardiol. Pol. 2008, 66, 1279–1285. [Google Scholar] [PubMed]
- Swiatkiewicz, I.; Taub, P.R. The usefulness of C-reactive protein for the prediction of post-infarct left ventricular systolic dysfunction and heart failure. Kardiol. Pol. 2018, 76, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Liang, Y.; Chen, P.; Zhang, Y.; Yin, X.; Zhang, M. In-depth proteomics approach reveals novel biomarkers of cardiac remodelling after myocardial infarction: An exploratory analysis. J. Cell Mol. Med. 2020, 24, 10042–10051. [Google Scholar] [CrossRef]
- Groot, H.E.; Al Ali, L.; van der Horst, I.C.C.; Schurer, R.; van der Werf, H.W.; Lipsic, E.; van Veldhuisen, D.J.; Karper, J.C.; van der Harst, P. Plasma interleukin 6 levels are associated with cardiac function after ST-elevation myocardial infarction. Clin. Res. Cardiol. 2019, 108, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Vanhaverbeke, M.; Veltman, D.; Pattyn, N.; De Crem, N.; Gillijns, H.; Cornelissen, V.; Janssens, S.; Sinnaeve, P.R. C-reactive protein during and after myocardial infarction in relation to cardiac injury and left ventricular function at follow-up. Clin. Cardiol. 2018, 41, 1201–1206. [Google Scholar] [CrossRef]
- Tomaniak, M.; Katagiri, Y.; Modolo, R.; de Silva, R.; Khamis, R.Y.; Bourantas, C.V.; Torii, R.; Wentzel, J.J.; Gijsen, F.; van Soest, G.; et al. Vulnerable plaques and patients: State-of-the-art. Eur. Heart J. 2020, 41, 2997–3004. [Google Scholar] [CrossRef]
- Jones, D.P.; Patel, J. Therapeutic Approaches Targeting Inflammation in Cardiovascular Disorders. Biology 2018, 7, 49. [Google Scholar] [CrossRef]
- Shah, S.R.; Abbasi, Z.; Fatima, M.; Ochani, R.K.; Shahnawaz, W.; Asim Khan, M.; Shah, S.A. Canakinumab and cardiovascular outcomes: Results of the CANTOS trial. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 21–22. [Google Scholar] [CrossRef]
- Huang, S.; Frangogiannis, N.G. Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. Br. J. Pharmacol. 2018, 175, 1377–1400. [Google Scholar] [CrossRef]


| Variable | Total n = 225 | Primary End-Point Reached during Follow-Up | p Value | |
|---|---|---|---|---|
| Yes n = 56 | No n = 169 | |||
| Patient demographics | ||||
| Age, y, mean ± SD (median) | 63.7 ± 13.4 (65) | 70 ± 10.5 (71) | 61.7 ± 13.2 (63) | 0.0003 |
| Gender, male n (%) | 152 (67.5%) | 36 (64.2%) | 116 (68.6%) | 0.5 |
| BMI kg/m2 | 28 ± 5.4 | 27.3 ± 5 | 28.3 ± 5.6 | 0.1 |
| Index event characteristics | ||||
| STEMI n (%) | 165 (73.3%) | 34 (20.6%) | 131 (79.3%) | 0.01 |
| NSTEMI n (%) | 60 (26.6%) | 22 (36.6%) | 38 (63.3%) | |
| Time from onset of symptoms to admission, hrs, mean ± SD (median) for total | 12.4 ± 19.5 (8) | 19.6 ± 33.6 (10) | 9.8 ± 9.7 (7) | 0.3 |
| Medical history and comorbidities | ||||
| HTN n (%) | 187 (83.1%) | 50 (89.2%) | 137 (81%) | 0.2 |
| DM n (%) | 60 (26.6%) | 21 (37.5%) | 39 (23.1%) | 0.03 |
| Smoking n (%) | 90 (40%) | 12 (21.4%) | 78 (46.1%) | 0.001 |
| Dyslipidemia n (%) | 69 (17.2%) | 16 (28.5%) | 53 (31.3%) | 0.6 |
| Stroke n (%) | 19 (8.4%) | 7 (12.5%) | 12 (7.1%) | 0.3 |
| Previous MI n (%) | 20 (8.8%) | 8 (14.2%) | 12 (7.1%) | 0.1 |
| PAD n (%) | 11 (4.8%) | 4 (7.1%) | 7 (4.1%) | 0.4 |
| Obesity n (%) | 45 (20%) | 12 (21.4%) | 33 (14.6%) | 0.7 |
| Biochemical analysis for renal, metabolic and myocardial necrosis markers, mean ± SD (median) | ||||
| Peak CK MB (U/L) | 80.9 ± 172.2 (21.9) | 79.8 ± 130.6 (24.2) | 133.6 ± 59.2 (21.9) | 0.6 |
| Creatine kinase (U/L) | 1480 ± 1631 (868) | 1367 ± 1432 (867) | 1516 ± 1692 (868) | 0.7 |
| Total cholesterol (mg/dL) | 187.8 ± 50.5 (186.5) | 175.7 ± 48.7 (175) | 192 ± 50.5 (190.2) | 0.03 |
| Triglycerides (mg/dL) | 168.2 ± 112.5 (139.2) | 167.9 ± 140.5 (124) | 168.3 ± 101.1 (149.5) | 0.1 |
| Glycemia on admission (mg/dL) | 142.6 ± 61.9 (121.5) | 155 ± 67.4 (139) | 138.3 ± 59.6 (117) | 0.01 |
| eGFR (mL/min) | 95.1 ± 37.4 (95.5) | 87.2 ± 39.7 (92.5) | 97.7 ± 36 (97.4) | 0.1 |
| Acute phase complications | ||||
| Ventricular arrhythmias n (%) | 26 (11.5%) | 8 (14.2%) | 18 (10.6%) | 0.6 |
| NOAF n (%) | 39 (17.3%) | 13 (23.2%) | 26 (15.3%) | 0.1 |
| High degree AV conduction abnormalities n (%) | 4 (1.7%) | 1 (1.7%) | 3 (1.7%) | 0.9 |
| Resuscitated CA n (%) | 17 (7.5%) | 6 (10.7%) | 11 (6.5%) | 0.3 |
| Hemodynamic instability n (%) | 21 (9.3%) | 7 (12.5%) | 14 (8.2%) | 0.4 |
| Mechanical complications n (%) | 0 | 0 | 0 | n.a. |
| Composite of all acute complications n (%) | 69 (30.6%) | 21 (37.5%) | 48 (28.4%) | 0.2 |
| STEMI Patients | NSTEMI Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Total n = 165 (73.3%) | Primary End-Point Reached during Follow-Up | p Value | Total n = 60 (26.6%) | Primary End-Point Reached during Follow-Up | p Value | ||
| Yes n = 34 (20.6%) | No n = 131 (79.3%) | Yes n = 22 (36.6%) | No n = 38 (63.3%) | |||||
| Age, y, mean ± SD (median) | 61.4 ± 14.0 (62) | 69 ± 14 (70) | 59.7 ± 13.4 (60) | <0.001 | 69.6 ± 9.4 (71) | 71.1 ± 9.3 (74) | 68.8 ± 9.5 (69) | 0.3 |
| Gender, male n (%) | 123 | 23 (67.5%) | 100 (76.3%) | 0.3 | 29 | 13 (59%) | 16 (42.1%) | 0.3 |
| BMI kg/m2 | 28.1 ± 5.5 (26.9) | 26.5 ± 4.3 (26.2) | 28.4 ± 5.8 (27.1) | 0.03 | 28 ± 5.1 (27.4) | 28.5 ± 5.8 (27.7) | 27.7 ± 4.8 (27.2) | 0.5 |
| Time from onset of symptoms to admission, hrs, mean ± SD (median) | 7.3 ± 3.1 (7) | 9.2 ± 0.2.4 (10) | 6.6 ± 3.1 (6) | 0.01 | 23.5 ± 31.1 (10) | 38.6 ± 54.2 (4.5) | 17 ± 14.9 (12) | 0.4 |
| Medical history and comorbidities | ||||||||
| HTN n (%) | 132 (80%) | 30 (88.2%) | 102 (77.2%) | 0.2 | 55 (91.6%) | 20 (90.9%) | 35 (92.1%) | 0.9 |
| DM n (%) | 34 (20.6%) | 7 (20%) | 28 (21.2%) | 0.9 | 25 (41.6%) | 14 (63.6%) | 11 (28.9%) | 0.01 |
| Smoking n (%) | 78 (29%) | 11 (32.5%) | 67 (51.1%) | 0.05 | 12 (20%) | 1 (4.55%) | 11 (81.8%) | 0.001 |
| Dyslipidemia n (%) | 52 (31.5%) | 10 (29.4%) | 42 (32%) | 0.7 | 17 (28.3%) | 6 (27.2%) | 11 (28.9%) | 0.8 |
| Stroke n (%) | 9 (5.4%) | 3 (8.8%) | 6 (4.5%) | 0.3 | 10 (15.3%) | 4 (18.1%) | 6 (15.7%) | 0.9 |
| Previous MI n (%) | 5 (3%) | 1 (2.9%) | 4 (3.0%) | 0.9 | 15 (16.6%) | 7 (31.8%) | 8 (21.0%) | 0.5 |
| PAD n (%) | 4 (2.4%) | 0 (0%) | 4 (3.0%) | 0.5 | 7 (11.6%) | 4 (18.1%) | 3 (7.8%) | 0.4 |
| Obesity n (%) | 24 (14.5%) | 3 (8.8%) | 21 (16%) | 0.4 | 21 (35%) | 9 (40.9%) | 12 (31.5%) | 0.6 |
| Biochemical analysis for renal, metabolic and myocardial necrosis markers, mean ± SD (median) | ||||||||
| Peak CK MB (U/L) | 103.8 ± 205.5 (22.0) | 105.9 ± 164.2 (24.05) | 101.9 ± 245.8 (21.95) | 0.9 | 32.8 ± 35.8 (19.3) | 40.6 ± 39.4 (28.6) | 44 ± 38.5 (44) | 0.2 |
| Creatine kinase (U/L) | 1781 ± 1756 (1233) | 1751 ± 1613 (1232) | 1789 ± 1796 (1233) | 0.9 | 668.3 ± 795.9 (320) | 800.2 ± 871.1 (580) | 593.4 ± 752.1 (260) | 0.2 |
| Total cholesterol (mg/dL) | 188.7 ± 51.6 (188) | 178.3 ± 50.2 (175) | 191.6 ± 51.8 (189.1) | 0.1 | 185.1 ± 47.57 | 171.8 ± 47.3 | 193.3 ± 46.4 | 0.1 |
| Triglycerides (mg/dL) | 167.7 ± 114 (134) | 162.1 ± 144.4 (112.7) | 169.2 ± 104.6 (147) | 0.1 | 169.6 ± 109.2 | 176.8 ± 137.4 | 165.4 ± 90.2 | 0.4 |
| Glycemia on admission (mg/dL) | 140.5 ± 56.2 (120) | 142.4 ± 53.5 (130) | 140 ± 57.2 (119) | 0.3 | 147.9 ± 75 | 173.8 ± 82 | 132.9 ± 67.3 | 0.02 |
| eGFR (mL/min) | 95 ± 35.8 (95.3) | 89.4 ± 38.7 (95.3) | 96.5 ± 35.1 (95.2) | 0.3 | 95.2 ± 40.7 | 83.6 ± 42.3 | 101.1 ± 39.2 | 0.2 |
| Acute phase complications n (%) | ||||||||
| Ventricular arrhythmias | 20 (12.1%) | 5 (14.7%) | 15 (11.4%) | 0.5 | 7 (11.6%) | 3 (13.6%) | 4 (10.5%) | 0.6 |
| NOAF | 27 (16.3%) | 8 (23.5%) | 19 (14.5%) | 0.3 | 12 (20%) | 5 (22.7%) | 7 (18.4%) | 0.7 |
| High degree AV conduction abnormalities | 3 (1.8%) | 0 (0%) | 3 (2.2%) | 0.9 | 1 (1.6%) | 1 (4.5%) | 0 (0%) | 0.3 |
| Resuscitated CA | 17(10.3%) | 4 (12.1%) | 13 (7.6%) | 0.4 | 3 (5%) | 2 (9.0%) | 1 (2.5%) | 0.2 |
| Hemodynamic instability | 14 (8.48%) | 4 (11.7%) | 10 (9.9%) | 0.7 | 5 (8.3%) | 3 (13.6%) | 2 (5.26%) | 0.3 |
| Mechanical complications | 0 | 0 | 0 | na | 0 | 0 | 0 | na |
| Composite of all acute complications | 52 (31.5%) | 13 (38.2%) | 39 (29.7%) | 0.3 | 17 (28.3%) | 8 (36.3%) | 9 (23.6%) | 0.4 |
| Total Study Population (STEMI + NSTEMI) | STEMI Patients | NSTEMI Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Total n = 225 | Primary End-Point Reached during Follow-Up | p Value | Total n = 165 (73.3%) | Primary End-Point Reached during Follow-Up | p Value | Total n = 60 (26.6%) | Primary End-Point Reached during Follow-Up | p Value | |||
| Yes n = 56 | No n = 169 | Yes n = 34 (20.6%) | No n = 131 (79.3%) | Yes n = 22 (36.6%) | No n = 38 (63.3%) | |||||||
| Serum inflammatory biomarkers, mean ± SD (median) | ||||||||||||
| Hs-CRP (mg/L) | 5.2 ± 4.5 (3.6) | 11.1 ± 13.8 (5.7) | 5.1 ± 4.4 (3.4) | 0.03 | 4.6 ± 3.9 (3.4) | 5.6 ± 5.7 (4.3) | 4.2 ± 3.3 (3.3) | 0.6 | 6.6 ± 5.2 (5.5) | 11.24 ± 11.8 (6.7) | 6.8 ± 5.7 (4.7) | 0.08 |
| Il-6 (pg/mL) | 8.0 ± 5.5 (6.8) | 8.9 ± 7.0 (6.9) | 8.7 ± 6.5 (7.0) | 0.9 | 20 ± 44.9 (8.4) | 34.6 ± 94.6 (8.6) | 16.8 ± 23.3 (8.4) | 0.6 | 16.0 ± 30.6 (7.4) | 12.9 ± 17.1 (5.4) | 17.6 ± 35.8 (7.8) | 0.8 |
| I-CAM (ng/mL) | 250.0 ± 133.9 (215.4) | 452 ± 283 (390.8) | 220.5 ± 104.6 (201.1) | 0.0003 | 342 ± 237.4 (246.4) | 490.1 ± 226.2 (239.4) | 309.1 ± 226.2 (227.6) | 0.006 | 248.4 ± 226.1 (179.5) | 375.7 ± 351.6 (214.8) | 184.7 ± 62.5 (171.7) | 0.1 |
| V-CAM (ng/mL) | 966.5 ± 248.3 (895.2) | 1045 ± 317.7 (895.2) | 953.3 ± 235.0 (901.5) | 0.4 | 1002 ± 339.5 (895.2) | 1274 ± 569.2 (1067) | 938.4 ± 224.9 (894.6) | 0.02 | 845.4 ± 245.8 (927) | 767.2 ± 69.7 (757.8) | 994 ± 255.2 (948.6) | 0.01 |
| E-selectin (ng/mL) | 71.7 ± 30.1 (67.8) | 74.7 ± 28 (72.7) | 70.2 ± 30.8 (64.7) | 0.3 | 72.4 ± 29.8 (68.8) | 78.2 ± 23.3 (73.2) | 71.2 (32.2) | 0.2 | 63.9 ± 33.9 (57.6) | 61 ± 40.6 (49.9) | 66 ± 30.1 (58) | 0.7 |
| MMP-9 (ng/mL) | 1285 ± 843.7 (1117) | 2255 ± 1226 (1937) | 1099 ± 706.1 (1020) | 0.0001 | 1412 ± 1067 (1135) | 2554 ± 1275 (2249) | 1173 ± 856.9 (1101) | 0.001 | 1452 ± 966 (1110) | 1919 ± 1155 (1608) | 1096 ± 683 (846) | 0.09 |
| Imaging markers | ||||||||||||
| LVEF% (Simpson’s biplane) | 44.2 ± 6.5 (45) | 41.4 ± 7.6 (42) | 45.1 ± 6.1 (45) | 0.005 | 44 ± 6.3 (45) | 40.8 ± 7.3 (43) | 44.8 ± 5.7 (45) | 0.01 | 44.4 ± 8.1 (45) | 42.2 ± 8.1 (40) | 46.3 ± 7.8 (45.5) | 0.1 |
| Multivessel CAD n (%) * | 123 (54.6%) | 33 (58.9%) | 90 (53.2%) | 0.4 | 76 (46%) | 15 (44.1%) | 64 (48.8%) | 0.6 | 44 | 18 (81.8%) | 26 (68.4%) | 0.3 |
| Left coronary artery culprit n (%) | 158 (70.2%) | 41 (73.2%) | 117 (69.2%) | 0.5 | 108 (65.4%) | 24 (70.5%) | 84 | 0.4 | 50 (83.3%) | 17 (77.2%) | 33 (86.8%) | 0.4 |
| Right coronary artery culprit n (%) | 67 (29.7%) | 15 (26.7%) | 52 (30.7%) | 0.5 | 57 (34.5%) | 10 (29.4%) | 47 | 0.4 | 10 (16.6%) | 5 (22.7%) | 5 (13.1%) | 0.4 |
| Total Study Population (STEMI + NSTEMI) | ||||||||
|---|---|---|---|---|---|---|---|---|
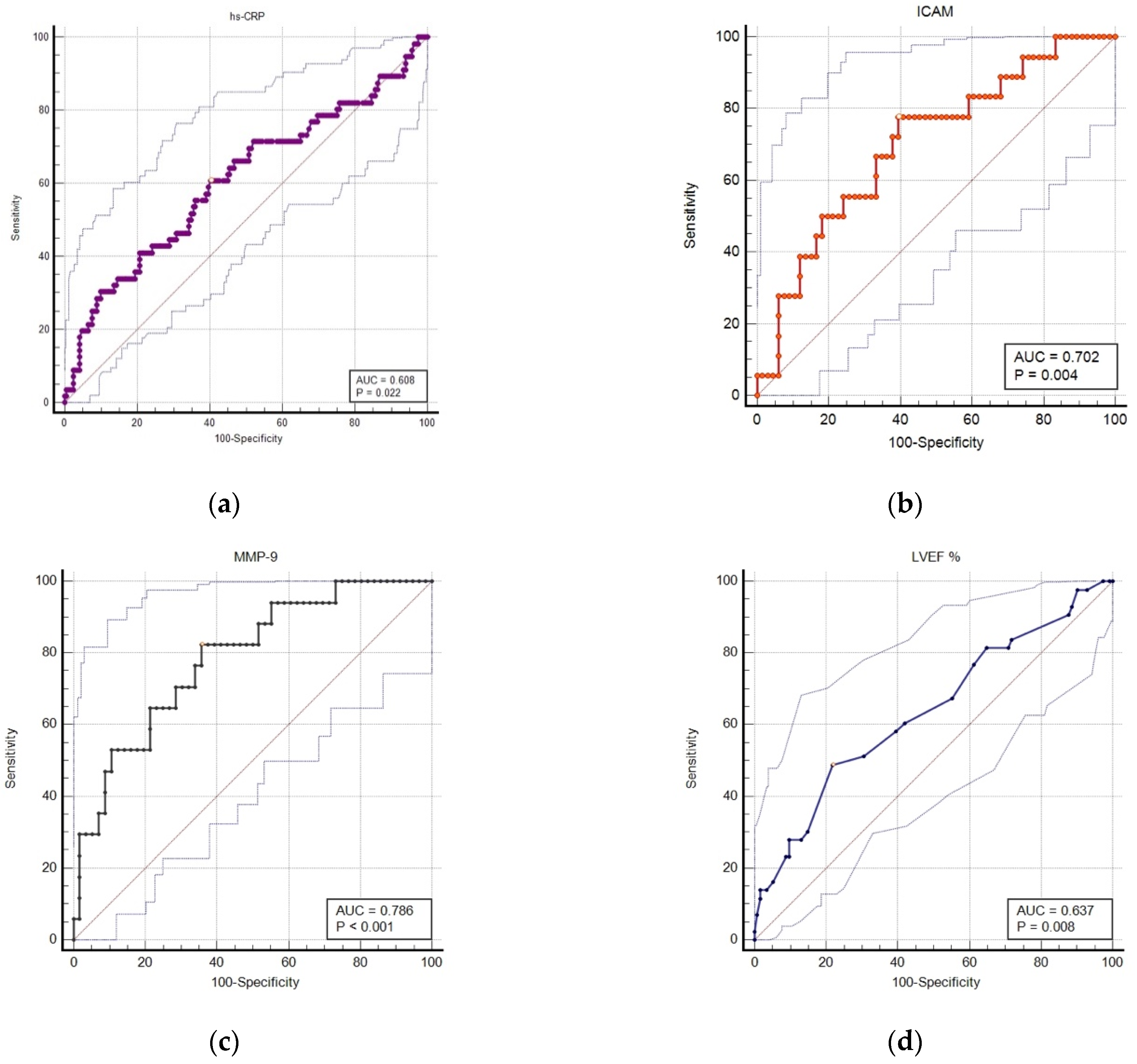
| Parameter | AUC | 95%CI for AUC | z Statistic | Youden Index | Cut off Value for Predicting MACE | Sensitivity % | Specificity % | p Value |
| hs-CRP (mg/L) | 0.608 | 0.54–0.67 | 2.29 | 0.204 | >5.6 | 60.7 | 59.7 | 0.02 |
| I-CAM (ng/mL) | 0.702 | 0.59–0.79 | 2.90 | 0.383 | >239.7 | 77.7 | 60.6 | 0.004 |
| V-CAM (ng/mL) | 0.600 | 0.48–0.70 | 1.29 | 0.20 | >975.4 | 61.1 | 59.1 | 0.6 |
| MMP 9 (ng/mL) | 0.786 | 0.67–0.87 | 4.61 | 0.466 | >1155 | 82.3 | 64.2 | <0.001 |
| LVEF% | 0.637 | 0.55–0.71 | 2.64 | 0.269 | ≤40 | 48.8 | 78.0 | 0.008 |
| STEMI patients | ||||||||
| hs-CRP (mg/L) | 0.572 | 0.49–0.64 | 1.18 | 0.191 | >13.0 | 34.38 | 0.78 | 0.2 |
| I-CAM (ng/mL) | 0.747 | 0.62–0.84 | 3.52 | 0.472 | >239.7 | 91.67 | 1.85 | <0.001 |
| V-CAM (ng/mL) | 0.715 | 0.58–0.82 | 2.45 | 0.355 | >877.9 | 90.91 | 2.13 | 0.01 |
| MMP-9 (ng/mL) | 0.828 | 0.70–0.91 | 4.49 | 0.596 | >1393 | 88.89 | 2.27 | <0.001 |
| LVEF% | 0.652 | 0.56–0.73 | 2.48 | 0.25 | ≤40 | 46.71 | 100.0 | 0.01 |
| NSTEMI patients | ||||||||
| hs-CRP (mg/L) | 0.633 | 0.49–0.75 | 1.69 | 0.27 | >5.7 | 77.2 | 2.6 | 0.09 |
| I-CAM (ng/mL) | 0.667 | 0.41–0.86 | 1.08 | 0.33 | >234.0 | 50 | 8.3 | 0.2 |
| V-CAM (ng/mL) | 0.528 | 0.28–0.76 | 0.18 | 0.25 | ≤852.1 | 50.0 | 91.6 | 0.8 |
| MMP 9 (ng/mL) | 0.729 | 0.48–0.90 | 1.85 | 0.45 | >849 | 87.5 | 8.33 | 0.06 |
| LVEF% | 0.640 | 0.46–0.79 | 1.5 | 2.44 | ≤37 | 29.4 | 95.0 | 0.1 |
| Univariable Analysis | |||
| Variable | OR | 95% CI for OR | p |
| Gender | 0.8 | 0.44–1.51 | 0.5 |
| HTN | 1.9 | 0.78–4.70 | 0.2 |
| DM | 2.0 | 1.06–3.81 | 0.03 |
| Smoking | 0.3 | 0.16–0.63 | 0.001 |
| Dyslipidemia | 0.8 | 0.46–1.67 | 0.6 |
| Stroke | 1.8 | 0.72–4.89 | 0.3 |
| Previous MI | 2.1 | 0.84–5.46 | 0.1 |
| PAD | 1.7 | 0.56–5.89 | 0.4 |
| Obesity | 1.1 | 0.53–2.32 | 0.7 |
| Multivessel CAD | 1.2 | 0.67–2.23 | 0.4 |
| Total cholesterol | 0.9 | 0.98–0.99 | 0.04 |
| Glycemia on admission | 1.0 | 0.99–1.00 | 0.09 |
| LVEF < 40% | 2.7 | 1.07–5.67 | 0.03 |
| hs-CRP (mg/L) | 2.3 | 1.22–4.30 | 0.007 |
| ICAM (ng/mL) | 5.0 | 1.62–15.03 | 0.007 |
| MMP-9 (ng/mL) | 8.4 | 2.22–29.23 | 0.0009 |
| Multivariable Analysis | |||
| Variable | Adjusted OR | 95% CI for adjusted OR | p |
| DM | 4.4 | 0.31–80.14 | 0.2 |
| Smoking | 0.4 | 0.03–4.06 | 0.5 |
| Total cholesterol | 0.9 | 0.98–1.00 | 0.2 |
| LVEF | 0.9 | 0.83–1.04 | 0.2 |
| Acute phase complications * | 1.1 | 0.42–2.92 | 0.7 |
| hs-CRP (mg/L) | 1.5 | 0.62–0.97 | 0.3 |
| ICAM (ng/mL) | 3.2 | 1.11–9.88 | 0.03 |
| MMP-9 (ng/mL) | 3.6 | 1.21–11.49 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opincariu, D.; Rodean, I.; Rat, N.; Hodas, R.; Benedek, I.; Benedek, T. Systemic Vulnerability, as Expressed by I-CAM and MMP-9 at Presentation, Predicts One Year Outcomes in Patients with Acute Myocardial Infarction—Insights from the VIP Clinical Study. J. Clin. Med. 2021, 10, 3435. https://doi.org/10.3390/jcm10153435
Opincariu D, Rodean I, Rat N, Hodas R, Benedek I, Benedek T. Systemic Vulnerability, as Expressed by I-CAM and MMP-9 at Presentation, Predicts One Year Outcomes in Patients with Acute Myocardial Infarction—Insights from the VIP Clinical Study. Journal of Clinical Medicine. 2021; 10(15):3435. https://doi.org/10.3390/jcm10153435
Chicago/Turabian StyleOpincariu, Diana, Ioana Rodean, Nora Rat, Roxana Hodas, Imre Benedek, and Theodora Benedek. 2021. "Systemic Vulnerability, as Expressed by I-CAM and MMP-9 at Presentation, Predicts One Year Outcomes in Patients with Acute Myocardial Infarction—Insights from the VIP Clinical Study" Journal of Clinical Medicine 10, no. 15: 3435. https://doi.org/10.3390/jcm10153435
APA StyleOpincariu, D., Rodean, I., Rat, N., Hodas, R., Benedek, I., & Benedek, T. (2021). Systemic Vulnerability, as Expressed by I-CAM and MMP-9 at Presentation, Predicts One Year Outcomes in Patients with Acute Myocardial Infarction—Insights from the VIP Clinical Study. Journal of Clinical Medicine, 10(15), 3435. https://doi.org/10.3390/jcm10153435







