The Effects of Calculated Remnant-Like Particle Cholesterol on Incident Cardiovascular Disease: Insights from a General Chinese Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Variables
2.3. Other Variates
2.4. Outcome Ascertainment
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Survival Analyses for Different Levels of RC
3.3. Stratification Analyses
3.4. Dose–Response Analyses of RC with Cardiovascular Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, G.; Wang, Y.; Zeng, Y.; Gao, G.F.; Liang, X.; Zhou, M.; Wan, X.; Yu, S.; Jiang, Y.; Naghavi, M.; et al. Rapid health transition in China, 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 381, 1987–2015. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Interheart Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of ldl cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association between lowering ldl-c and cardiovascular risk reduction among different therapeutic interventions: A systematic review and meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Sandesara, P.B.; Virani, S.S.; Fazio, S.; Shapiro, M.D. The Forgotten Lipids: Triglycerides, Remnant Cholesterol, and Atherosclerotic Cardiovascular Disease Risk. Endocr. Rev. 2019, 40, 537–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Langsted, A.; Madsen, C.M.; Nordestgaard, B.G. Contribution of remnant cholesterol to cardiovascular risk. J. Intern. Med. 2020, 288, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Varbo, A.; Benn, M.; Tybjærg-Hansen, A.; Jørgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G. Remnant Cholesterol as a Causal Risk Factor for Ischemic Heart Disease. J. Am. Coll. Cardiol. 2013, 61, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Varbo, A.; Freiberg, J.J.; Nordestgaard, B.G. Extreme Nonfasting Remnant Cholesterol vs Extreme LDL Cholesterol as Contributors to Cardiovascular Disease and All-Cause Mortality in 90000 Individuals from the General Population. Clin. Chem. 2015, 61, 533–543. [Google Scholar] [CrossRef]
- Elshazly, M.B.; Mani, P.; Nissen, S.; Brennan, D.M.; Clark, I.D.; Martin, S.; Jones, S.R.; Quispe, R.; Donnellan, E.; Nicholls, S.J.; et al. Remnant cholesterol, coronary atheroma progression and clinical events in statin-treated patients with coronary artery disease. Eur. J. Prev. Cardiol. 2020, 27, 1091–1100. [Google Scholar] [CrossRef]
- Lin, A.; Nerlekar, N.; Rajagopalan, A.; Yuvaraj, J.; Modi, R.; Mirzaee, S.; Munnur, R.K.; Seckington, M.; Doery, J.C.; Seneviratne, S.; et al. Remnant cholesterol and coronary atherosclerotic plaque burden assessed by computed tomography coronary angiography. Atherosclerosis 2019, 284, 24–30. [Google Scholar] [CrossRef]
- Masuda, D.; Yamashita, S. Postprandial Hyperlipidemia and Remnant Lipoproteins. J. Atheroscler. Thromb. 2017, 24, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Varbo, A.; Nordestgaard, B.G. Remnant lipoproteins. Curr. Opin. Lipidol. 2017, 28, 300–307. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Zheng, L.; Yang, H.; Sun, Y. Grim status of hypertension in rural China: Results from Northeast China Rural Cardiovascular Health Study 2013. J. Am. Soc. Hypertens. 2015, 9, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Hypertension Control: Report of a WHO Expert Committee; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 1996; Volume 862, pp. 1–83.
- Cao, Y.-X.; Zhang, H.-W.; Jin, J.-L.; Liu, H.-H.; Zhang, Y.; Gao, Y.; Guo, Y.-L.; Wu, N.-Q.; Hua, Q.; Li, Y.-F.; et al. The longitudinal association of remnant cholesterol with cardiovascular outcomes in patients with diabetes and pre-diabetes. Cardiovasc. Diabetol. 2020, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; WHO: Geneva, Switzerland, 2006; pp. 1–3. [Google Scholar]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. Using Standardized Serum Creatinine Values in the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Chuang, S.-Y.; Chen, J.-H.; Yeh, W.-T.; Wu, C.-C.; Pan, W.-H. Hyperuricemia and increased risk of ischemic heart disease in a large Chinese cohort. Int. J. Cardiol. 2012, 154, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, J.; Wang, W.; Zeng, Z.; Cheng, J.; Liu, J.; Sun, J.; Wu, Z. Epidemiological transition of stroke in china: Twenty-one-year observational study from the sino-monica-beijing project. Stroke 2008, 39, 1668–1674. [Google Scholar] [CrossRef] [Green Version]
- Gaye, B.; Canonico, M.; Perier, M.-C.; Samieri, C.; Berr, C.; Dartigues, J.-F.; Tzourio, C.; Elbaz, A.; Empana, J.-P. Ideal Cardiovascular Health, Mortality, and Vascular Events in Elderly Subjects. J. Am. Coll. Cardiol. 2017, 69, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, D.; Kang, S. Physical activity and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2012, 137, 869–882. [Google Scholar] [CrossRef]
- Lee, D.H.; Keum, N.; Hu, F.B.; Orav, E.J.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: Prospective US cohort study. BMJ 2018, 362, k2575. [Google Scholar] [CrossRef] [Green Version]
- Varbo, A.; Benn, M.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated Remnant Cholesterol Causes Both Low-Grade Inflammation and Ischemic Heart Disease, Whereas Elevated Low-Density Lipoprotein Cholesterol Causes Ischemic Heart Disease Without Inflammation. Circulation 2013, 128, 1298–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varbo, A.; Benn, M.; Nordestgaard, B.G. Remnant cholesterol as a cause of ischemic heart disease: Evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol. Ther. 2014, 141, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.B.; Frikke-Schmidt, R.; West, A.S.; Grande, P.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 2013, 34, 1826–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varbo, A.; Freiberg, J.J.; Nordestgaard, B.G. Remnant Cholesterol and Myocardial Infarction in Normal Weight, Overweight, and Obese Individuals from the Copenhagen General Population Study. Clin. Chem. 2018, 64, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, A.-M.K.; Langsted, A.; Varbo, A.; Bang, L.E.; Kamstrup, P.R.; Nordestgaard, B.G. Increased Remnant Cholesterol Explains Part of Residual Risk of All-Cause Mortality in 5414 Patients with Ischemic Heart Disease. Clin. Chem. 2016, 62, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Vallejo-Vaz, A.J.; Fayyad, R.; Boekholdt, M.; Hovingh, G.K.; Kastelein, J.J.; Melamed, S.; Barter, P.; Waters, D.D.; Ray, K.K. Triglyceride-Rich Lipoprotein Cholesterol and Risk of Cardiovascular Events Among Patients Receiving Statin Therapy in the TNT Trial. Circulation 2018, 138, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef]
- Batalla, A.; Reguero, J.R.; Hevia, S.; Cubero, G.I.; Cortina, A. Mild hypercholesterolemia and premature heart disease. J. Am. Coll. Cardiol. 2001, 37, 331–332. [Google Scholar] [CrossRef] [Green Version]
- Khot, U.N.; Khot, M.B.; Bajzer, C.T.; Sapp, S.K.; Ohman, E.M.; Brener, S.J.; Ellis, S.G.; Lincoff, A.M.; Topol, E. Prevalence of Conventional Risk Factors in Patients with Coronary Heart Disease. JAMA 2003, 290, 898–904. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Cogswell, M.E.; Flanders, W.D.; Hong, Y.; Zhang, Z.; Loustalot, F.; Gillespie, C.; Merritt, R.; Hu, F.B. Trends in Cardiovascular Health Metrics and Associations With All-Cause and CVD Mortality Among US Adults. JAMA 2012, 307, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- Laslett, L.J.; Alagona, P., Jr.; Clark, B.A., 3rd; Drozda, J.P., Jr.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the american college of cardiology. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef] [Green Version]
- Spring, B.; Moller, A.C.; Colangelo, L.A.; Siddique, J.; Roehrig, M.; Daviglus, M.L.; Polak, J.F.; Reis, J.P.; Sidney, S.; Liu, K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation 2014, 130, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, F.D.R.; Jukema, J.W.; Da Silva, P.M.; McCormack, T.; Catapano, A.L. Barriers to cardiovascular disease risk scoring and primary prevention in Europe. QJM 2010, 103, 727–739. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Jones, D.M. Cardiovascular risk prediction: Basic concepts, current status, and future directions. Circulation 2010, 121, 1768–1777. [Google Scholar] [CrossRef]
- Garshick, M.S.; Vaidean, G.D.; Vani, A.; Underberg, J.A.; Newman, J.D.; Berger, J.; Fisher, E.A.; Gianos, E. Cardiovascular Risk Factor Control and Lifestyle Factors in Young to Middle-Aged Adults with Newly Diagnosed Obstructive Coronary Artery Disease. Cardiology 2019, 142, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease. The framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef]
- Schaefer, E.J.; McNamara, J.R.; Shah, P.K.; Nakajima, K.; Cupples, L.A.; Ordovas, J.; Wilson, P.W. Elevated Remnant-Like Particle Cholesterol and Triglyceride Levels in Diabetic Men and Women in the Framingham Offspring Study. Diabetes Care 2002, 25, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, P.G.; Jensen, M.T.; Biering-Sørensen, T.; Mogelvang, R.; Galatius, S.; Fritz-Hansen, T.; Rossing, P.; Vilsbøll, T.; Jensen, J.S. Cholesterol remnants and triglycerides are associated with decreased myocardial function in patients with type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 137. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, L.; He, J.; Bi, Y.; Li, M.; Wang, T.; Wang, L.; Jiang, Y.; Dai, M.; Lu, J.; et al. Prevalence and Control of Diabetes in Chinese Adults. JAMA 2013, 310, 948–959. [Google Scholar] [CrossRef]
- Castañer, O.; Pintó, X.; Subirana, I.; Amor, A.J.; Ros, E.; Hernáez, Á.; Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Estruch, R.; et al. Remnant Cholesterol, Not LDL Cholesterol, Is Associated With Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 76, 2712–2724. [Google Scholar] [CrossRef]
- Qin, Z.; Zhou, K.; Li, Y.-P.; Wang, J.-L.; Cheng, W.-J.; Hu, C.-P.; Shi, C.; He, H.; Zhou, Y.-J. Remnant lipoproteins play an important role of in-stent restenosis in type 2 diabetes undergoing percutaneous coronary intervention: A single-centre observational cohort study. Cardiovasc. Diabetol. 2019, 18, 1–9. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 aha/acc/aacvpr/aapa/abc/acpm/ada/ags/apha/aspc/nla/pcna guideline on the management of blood cholesterol: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, E.D.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2019, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.E.; Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Low-Grade Inflammation in the Association between Mild-to-Moderate Hypertriglyceridemia and Risk of Acute Pancreatitis: A Study of More Than 115000 Individuals from the General Population. Clin. Chem. 2019, 65, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Balling, M.; Langsted, A.; Afzal, S.; Varbo, A.; Smith, G.D.; Nordestgaard, B.G. A third of nonfasting plasma cholesterol is in remnant lipoproteins: Lipoprotein subclass profiling in 9293 individuals. Atherosclerosis 2019, 286, 97–104. [Google Scholar] [CrossRef]
- Twickler, T.B.; Dallinga-Thie, G.M.; Cohn, J.S.; Chapman, M.J. Elevated remnant-like particle cholesterol concentration: A characteristic feature of the atherogenic lipoprotein phenotype. Circulation 2004, 109, 1918–1925. [Google Scholar] [CrossRef]
- Shin, H.K.; Kim, Y.K.; Kim, K.Y.; Lee, J.H.; Hong, K.W. Remnant lipoprotein particles induce apoptosis in endothelial cells by nad(p)h oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: Prevention by cilostazol. Circulation 2004, 109, 1022–1028. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Nakano, T.; Tanaka, A. The oxidative modification hypothesis of atherosclerosis: The comparison of atherogenic effects on oxidized LDL and remnant lipoproteins in plasma. Clin. Chim. Acta 2006, 367, 36–47. [Google Scholar] [CrossRef]
- Liu, L.; Wen, T.; Zheng, X.-Y.; Yang, D.-G.; Zhao, S.-P.; Xu, D.-Y.; Lü, G.-H. Remnant-like particles accelerate endothelial progenitor cells senescence and induce cellular dysfunction via an oxidative mechanism. Atherosclerosis 2009, 202, 405–414. [Google Scholar] [CrossRef]
- Kitulwatte, I.D.; Pollanen, M.S. A Comparative Study of Coronary Atherosclerosis in Young and Old. Am. J. Forensic Med. Pathol. 2015, 36, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Kostara, C.; Papathanasiou, A.; Psychogios, N.; Cung, M.T.; Elisaf, M.; Goudevenos, J.; Bairaktari, E.T. NMR-Based Lipidomic Analysis of Blood Lipoproteins Differentiates the Progression of Coronary Heart Disease. J. Proteome Res. 2014, 13, 2585–2598. [Google Scholar] [CrossRef]
- Mazzone, T.; Chait, A.; Plutzky, J. Cardiovascular disease risk in type 2 diabetes mellitus: Insights from mechanistic studies. Lancet 2008, 371, 1800–1809. [Google Scholar] [CrossRef] [Green Version]
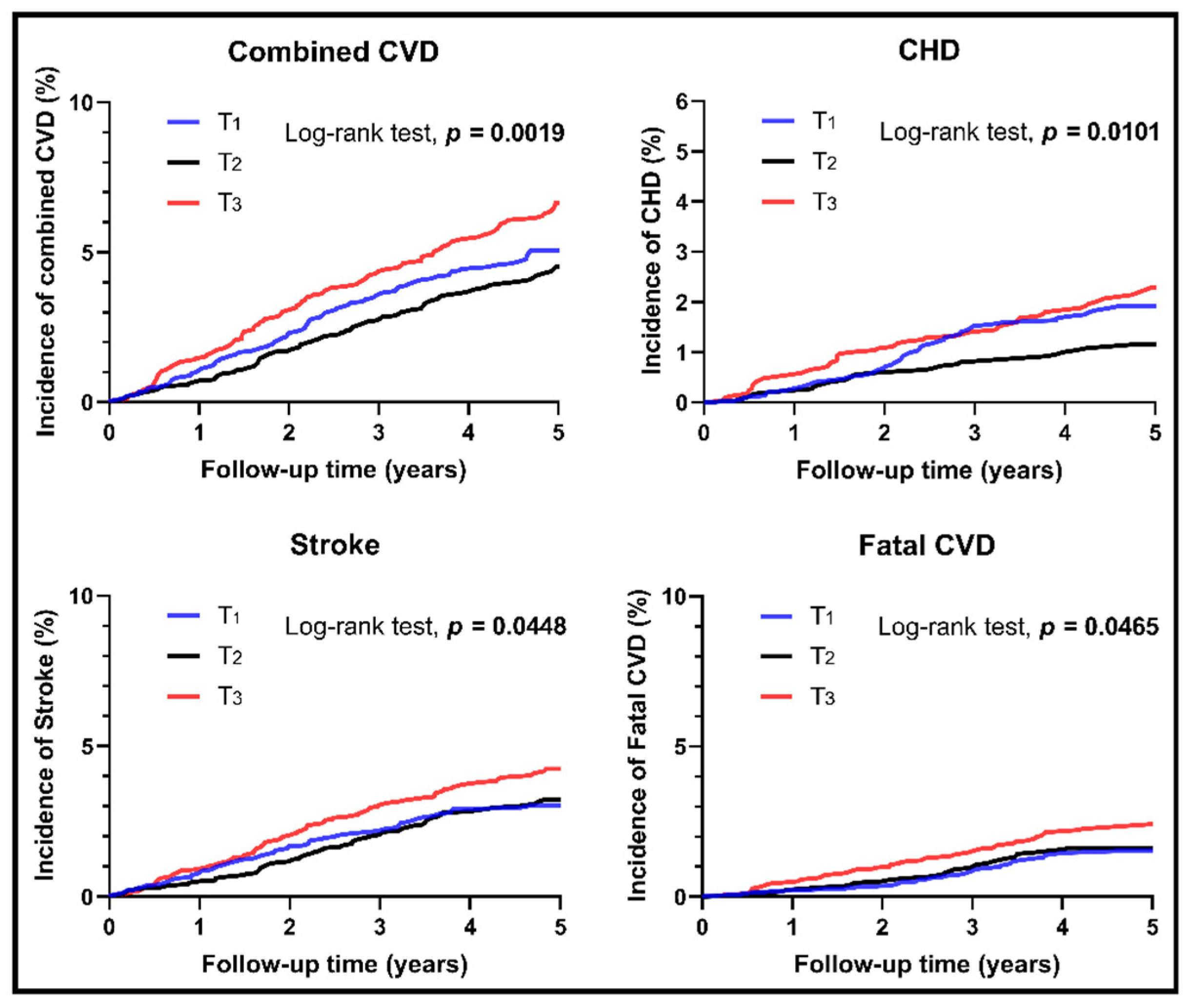

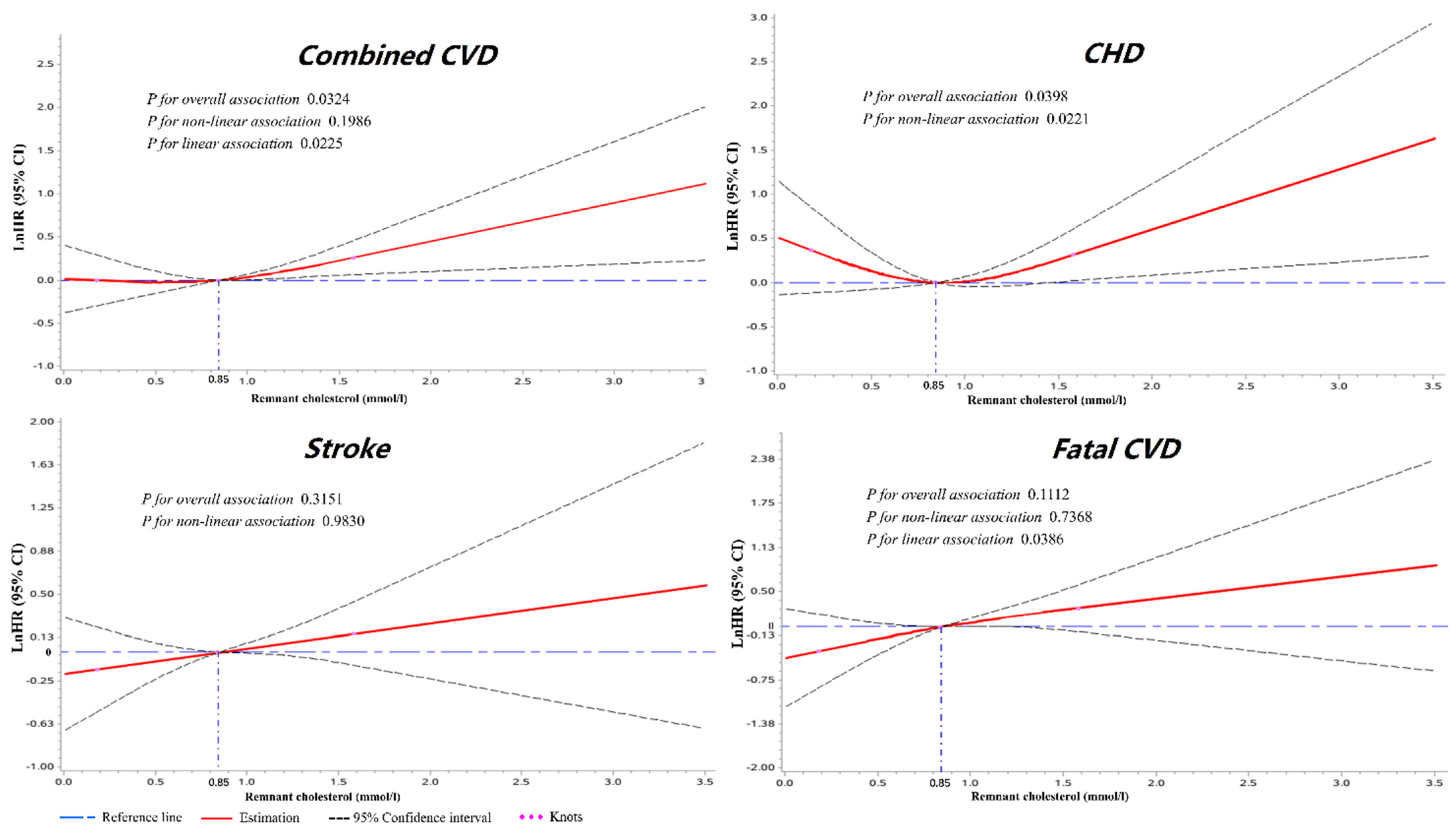
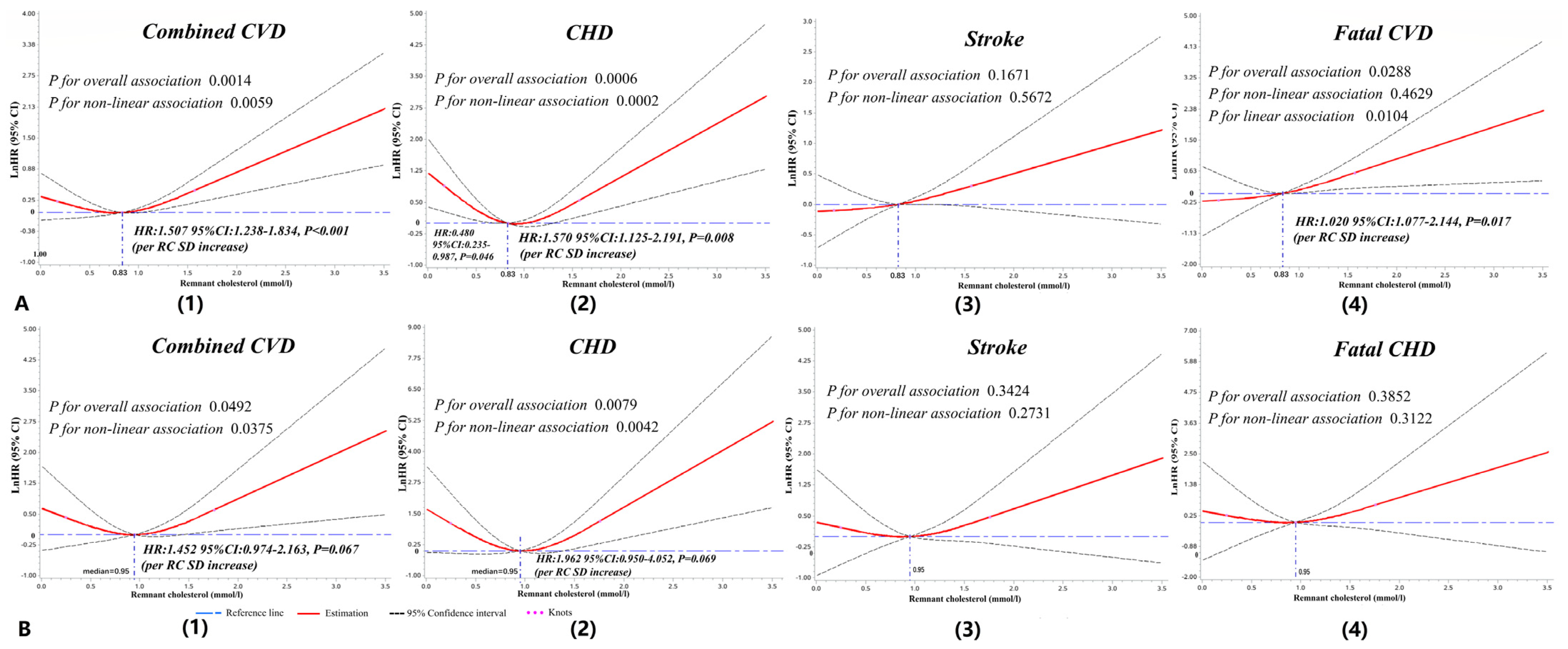
| Total n = 8782 | Remnant Cholesterol | p-Value | p1 | p2 | p3 | |||
|---|---|---|---|---|---|---|---|---|
| Tertile I (n = 2949) | Tertile II (n = 2932) | Tertile III (n = 2901) | ||||||
| Age (year) | 53.2 ± 10.4 | 52.2 ± 10.6 | 52.7 ± 10.4 | 54.7 ± 10.0 | <0.001 | 0.201 | <0.001 | <0.001 |
| Male (%) | 4075 (46.4) | 1432 (48.6) | 1343 (45.8) | 1300 (44.8) | 0.012 | |||
| Ethnicity of Han (%) | 8277 (94.2) | 2664 (90.3) | 2820 (96.2) | 2793 (96.3) | <0.001 | |||
| Current smoking (%) | 3118 (35.5) | 1121 (38.0) | 1022 (34.9) | 975 (33.6) | 0.001 | |||
| Current drinking alcohol (%) | 1992 (22.7) | 755 (25.6) | 619 (21.1) | 618 (21.3) | <0.001 | |||
| Physical activity | ||||||||
| Low | 2964 (33.8) | 819 (27.8) | 1033 (35.2) | 1112 (38.3) | <0.001 | |||
| Medium | 1652 (18.8) | 566 (19.2) | 581 (19.8) | 505 (17.4) | ||||
| High | 4091 (46.6) | 1534 (52.0) | 1290 (44.0) | 1267 (44.0) | ||||
| HTN (%) | 4154 (47.3) | 1141 (38.7) | 965 (32.9) | 911 (31.4) | <0.001 | |||
| DM (%) | 738 (8.4) | 201 (6.8) | 212 (7.2) | 325 (11.2) | <0.001 | |||
| BMI (kg/m2) | 24.7 ± 3.6 | 24.7 ± 3.8 | 24.4 ± 3.6 | 24.8 ± 3.5 | <0.001 | 0.003 | 0.866 | <0.001 |
| SBP (mmHg) | 140.5 ± 22.7 | 144.0 ± 24.3 | 137.1 ± 21.6 | 140.3 ± 21.5 | <0.001 | <0.001 | <0.001 | <0.001 |
| DBP (mmHg) | 81.6 ± 11.5 | 81.1 ± 11.8 | 81.1 ± 11.5 | 82.6 ± 11.3 | <0.001 | 1.0 | <0.001 | <0.001 |
| FBG (mmol/L) | 5.8 ± 1.5 | 5.6 ± 1.4 | 5.8 ± 1.7 | 5.8 ± 1.3 | <0.001 | 0.043 | <0.001 | <0.001 |
| TC (mmol/L) | 5.2 ± 1.0 | 4.7 ± 0.9 | 5.0 ± 0.8 | 5.8 ± 1.0 | <0.001 | <0.001 | <0.001 | <0.001 |
| TG (mmol/L) | 1.4 ± 0.8 | 1.1 ± 0.5 | 1.3 ± 0.7 | 1.8 ± 0.8 | <0.001 | <0.001 | <0.001 | <0.001 |
| HDL-C (mmol/L) | 1.4 ± 0.3 | 1.6 ± 0.4 | 1.3 ± 0.3 | 1.3 ± 0.3 | <0.001 | <0.001 | <0.001 | 0.626 |
| LDL-C (mmol/L) | 2.9 ± 0.8 | 2.8 ± 0.8 | 2.8 ± 0.7 | 3.1 ± 0.8 | <0.001 | 0.429 | <0.001 | <0.001 |
| Estimated eGFR (mL/min/1.73 m2) | 94.1 ± 15.1 | 101.6 ± 13.2 | 92.3 ± 14.3 | 88.2 ± 14.4 | <0.001 | <0.001 | <0.001 | <0.001 |
| Serum uric acid (μmol/L) | 285.6 ± 81.5 | 260.7 ± 73.8 | 284.8 ± 79.5 | 290.4 ± 78.5 | <0.001 | <0.001 | <0.001 | <0.001 |
| RC (mmol/L) | 0.83 ± 0.44 | 0.36 ± 0.16 | 0.84 ± 0.10 | 1.32 ± 0.30 | <0.001 | <0.001 | <0.001 | <0.001 |
| RC Q1 | RC Q2 | RC Q3 | RC Continuous (1-SD †) | |
|---|---|---|---|---|
| Combined CVD | ||||
| n/N | 139/2191 | 117/2932 | 175/2901 | 431/8782 |
| Model 1 | 1.23 (0.96–1.57) | 1.00 (ref) | 1.49 (1.18–1.89) ** | 1.33 (1.09–1.63) ** |
| Model 2 | 1.22 (0.95–1.57) | 1.00 (ref) | 1.49 (1.18–1.89) ** | 1.32 (1.08–1.62) ** |
| Model 3 | 1.16 (0.90–1.50) | 1.00 (ref) | 1.37 (1.07–1.74) * | 1.28 (1.02–1.62) * |
| CHD | ||||
| n/N | 55/2191 | 34/2932 | 61/2901 | 150/8782 |
| Model 1 | 1.77 (1.15–2.74) * | 1.00 (ref) | 1.71 (1.12–2.61) * | 1.17 (0.82–1.67) |
| Model 2 | 1.74 (1.12–2.70) * | 1.00 (ref) | 1.71 (1.12–2.61) * | 1.16 (0.81–1.66) |
| Model 3 | 1.68 (1.08–2.62) * | 1.00 (ref) | 1.63 (1.06–2.53) * | 1.15 (0.76–1.74) |
| Stroke | ||||
| n/N | 88/2191 | 87/2932 | 118/2901 | 293/8782 |
| Model 1 | 1.04 (0.77–1.40) | 1.00 (ref) | 1.30 (0.98–1.72) | 1.31 (1.03–1.67) * |
| Model 2 | 1.04 (0.77–1.41) | 1.00 (ref) | 1.31 (0.98–1.73) | 1.30 (1.02–1.67) * |
| Model 3 | 0.99 (0.73–1.35) | 1.00 (ref) | 1.19 (0.89–1.59) | 1.25 (0.94–1.66) |
| Fatal CVD | ||||
| n/N | 41/2191 | 44/2932 | 63/2901 | 148/8782 |
| Model 1 | 0.97 (0.64–1.49) | 1.00 (ref) | 1.394 (0.95–2.05) | 1.44 (1.05–1.97) * |
| Model 2 | 0.95 (0.62–1.46) | 1.00 (ref) | 1.421 (0.97–2.09) | 1.47 (1.07–2.01) * |
| Model 3 | 0.90 (0.58–1.39) | 1.00 (ref) | 1.37 (0.92–2.05) | 1.51 (1.05–2.17) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, G.; Guo, X.; Ouyang, N.; Li, Z.; Ye, N.; Yu, S.; Yang, H.; Sun, Y. The Effects of Calculated Remnant-Like Particle Cholesterol on Incident Cardiovascular Disease: Insights from a General Chinese Population. J. Clin. Med. 2021, 10, 3388. https://doi.org/10.3390/jcm10153388
Chen Y, Li G, Guo X, Ouyang N, Li Z, Ye N, Yu S, Yang H, Sun Y. The Effects of Calculated Remnant-Like Particle Cholesterol on Incident Cardiovascular Disease: Insights from a General Chinese Population. Journal of Clinical Medicine. 2021; 10(15):3388. https://doi.org/10.3390/jcm10153388
Chicago/Turabian StyleChen, Yanli, Guangxiao Li, Xiaofan Guo, Nanxiang Ouyang, Zhao Li, Ning Ye, Shasha Yu, Hongmei Yang, and Yingxian Sun. 2021. "The Effects of Calculated Remnant-Like Particle Cholesterol on Incident Cardiovascular Disease: Insights from a General Chinese Population" Journal of Clinical Medicine 10, no. 15: 3388. https://doi.org/10.3390/jcm10153388
APA StyleChen, Y., Li, G., Guo, X., Ouyang, N., Li, Z., Ye, N., Yu, S., Yang, H., & Sun, Y. (2021). The Effects of Calculated Remnant-Like Particle Cholesterol on Incident Cardiovascular Disease: Insights from a General Chinese Population. Journal of Clinical Medicine, 10(15), 3388. https://doi.org/10.3390/jcm10153388






