Prognostic Effects of Vasomotor Reactivity during Targeted Temperature Management in Post-Cardiac Arrest Patients: A Retrospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
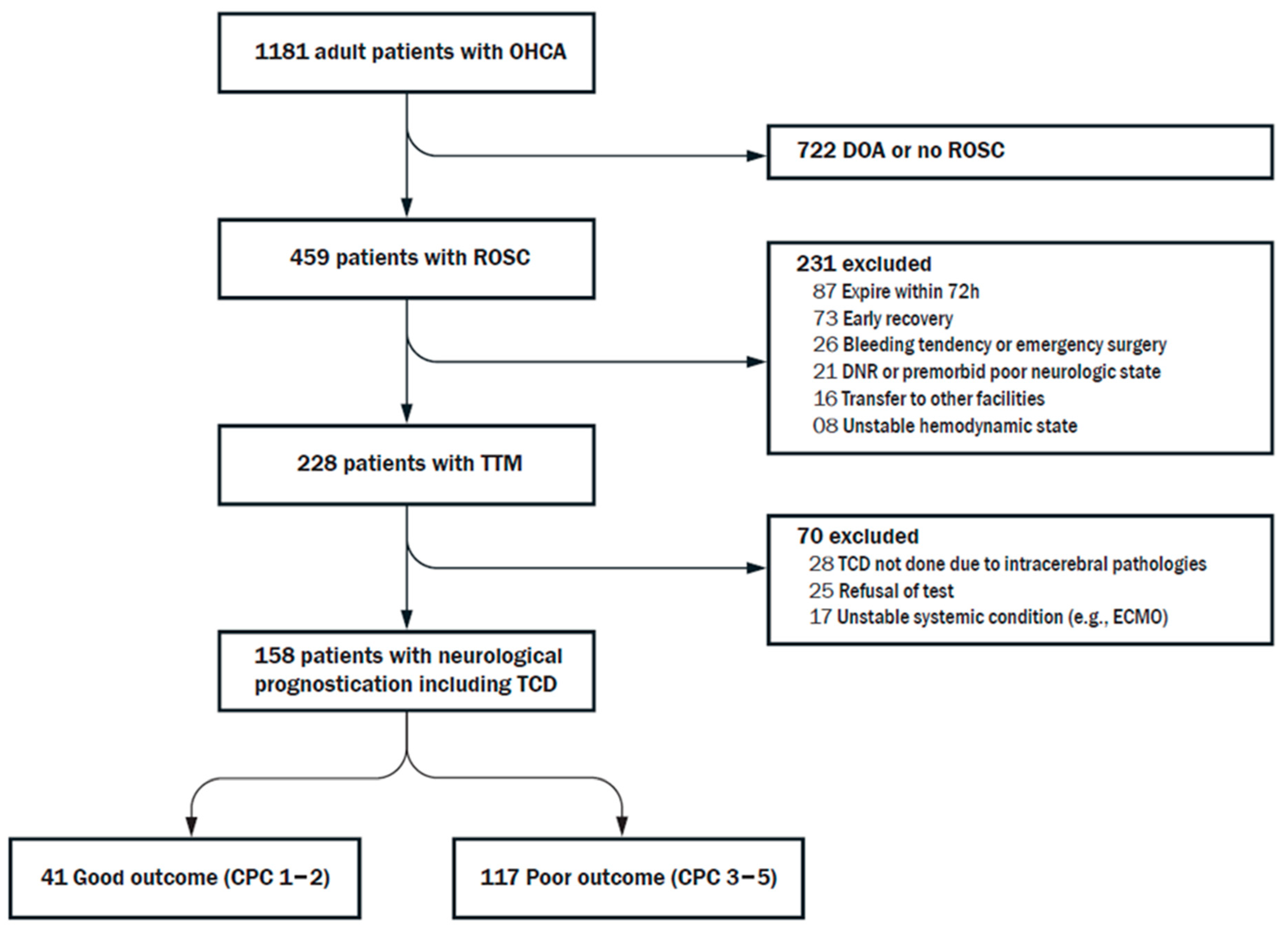
2.1. Study Population
2.2. TTM and Management
2.3. Multimodal Assessment for Neurologic Prognostication
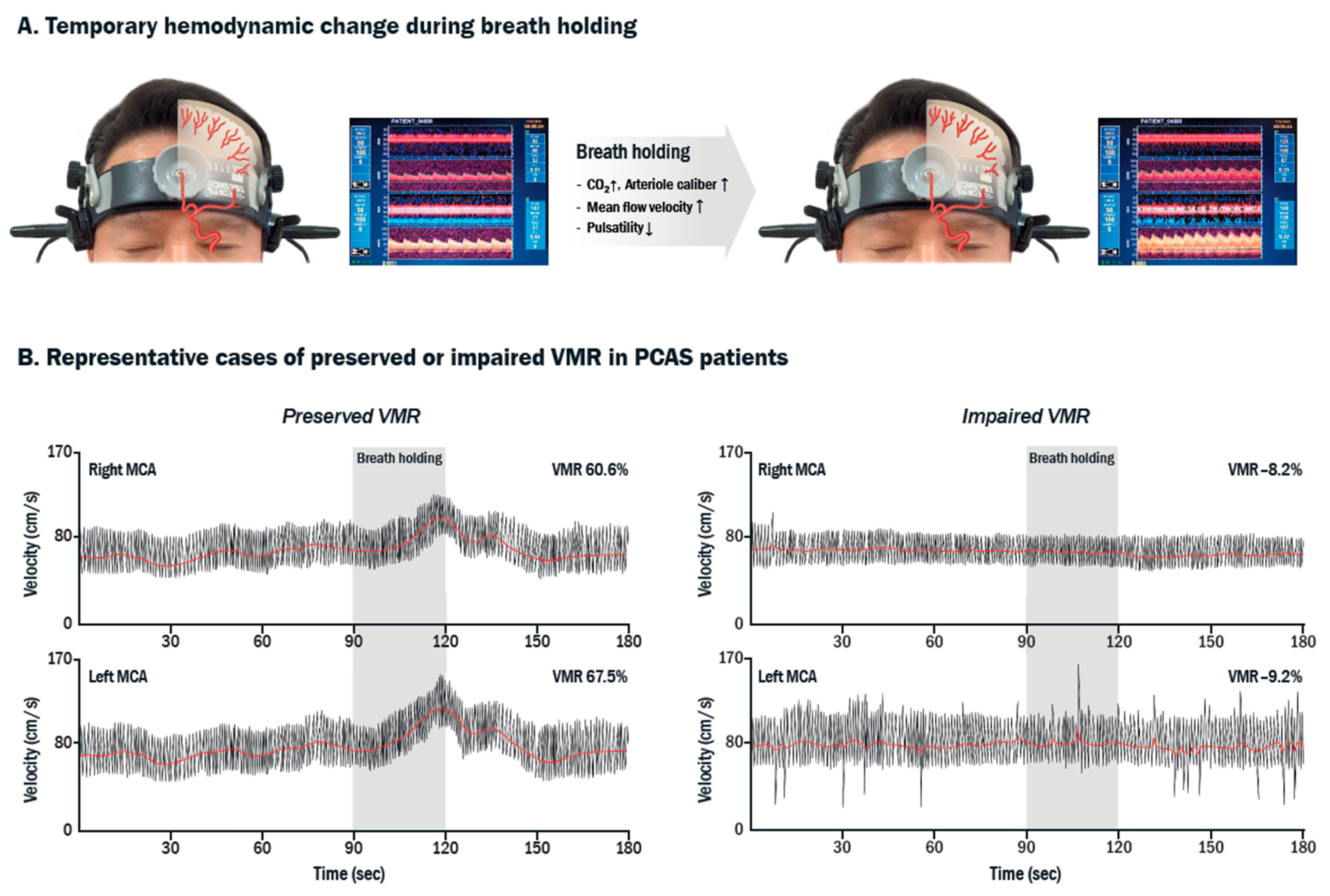
2.4. TCD-VMR
2.5. Outcome Assessment
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Associations of VMR with Outcome
3.3. Prognostic Performance of VMR for Predicting Good Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keenan, S.P.; Dodek, P.; Martin, C.; Priestap, F.; Norena, M.; Wong, H. Variation in length of intensive care unit stay after cardiac arrest: Where you are is as important as who you are. Crit. Care Med. 2007, 35, 836–841. [Google Scholar] [CrossRef]
- Westhall, E.; Rossetti, A.O.; van Rootselaar, A.F.; Wesenberg Kjaer, T.; Horn, J.; Ullen, S.; Friberg, H.; Nielsen, N.; Rosen, I.; Aneman, A.; et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology 2016, 86, 1482–1490. [Google Scholar] [CrossRef]
- Nakashima, R.; Hifumi, T.; Kawakita, K.; Okazaki, T.; Egawa, S.; Inoue, A.; Seo, R.; Inagaki, N.; Kuroda, Y. Critical care management focused on optimizing brain function after cardiac arrest. Circ. J. 2017, 81, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Rossetti, A.O.; Rabinstein, A.A.; Oddo, M. Neurological prognostication of outcome in patients in coma after cardiac arrest. Lancet Neurol. 2016, 15, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Wijdicks, E.F.; Hijdra, A.; Young, G.B.; Bassetti, C.L.; Wiebe, S. Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006, 67, 203–210. [Google Scholar] [CrossRef]
- Panchal, A.R.; Bartos, J.A.; Cabanas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef]
- Nolan, J.P.; Sandroni, C.; Bottiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef]
- Geocadin, R.G.; Wijdicks, E.; Armstrong, M.J.; Damian, M.; Mayer, S.A.; Ornato, J.P.; Rabinstein, A.; Suarez, J.I.; Torbey, M.T.; Dubinsky, R.M.; et al. Practice guideline summary: Reducing brain injury following cardiopulmonary resuscitation: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2017, 88, 2141–2149. [Google Scholar] [CrossRef]
- Westhall, E. Electroencephalography as a prognostic tool after cardiac arrest. Semin. Neurol. 2017, 37, 48–59. [Google Scholar] [CrossRef]
- Xiong, L.; Liu, X.; Shang, T.; Smielewski, P.; Donnelly, J.; Guo, Z.N.; Yang, Y.; Leung, T.; Czosnyka, M.; Zhang, R.; et al. Impaired cerebral autoregulation: Measurement and application to stroke. J. Neurol. Neurosurg. Psychiatry 2017, 88, 520–531. [Google Scholar] [CrossRef]
- Silvestrini, M.; Vernieri, F.; Pasqualetti, P.; Matteis, M.; Passarelli, F.; Troisi, E.; Caltagirone, C. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000, 283, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Gelb, A.W. Regulation of cerebral autoregulation by carbon dioxide. Anesthesiology 2015, 122, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Topcuoglu, M.A. Transcranial Doppler ultrasound in neurovascular diseases: Diagnostic and therapeutic aspects. J. Neurochem. 2012, 123, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.M.; Joo, I.S.; Huh, K.; Sheen, S.S. Simultaneous vasomotor reactivity testing in the middle cerebral and basilar artery with suboccipital probe fixation device. J. Neuroimaging 2010, 20, 83–86. [Google Scholar] [CrossRef]
- Hossmann, K.A. Viability thresholds and the penumbra of focal ischemia. Ann. Neurol. 1994, 36, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.S. Cerebral perfusion and stroke. J. Neurol. Neurosurg. Psychiatry 2004, 75, 353–361. [Google Scholar] [CrossRef]
- Ameloot, K.; Genbrugge, C.; Meex, I.; Jans, F.; Boer, W.; Vander Laenen, M.; Ferdinande, B.; Mullens, W.; Dupont, M.; Dens, J.; et al. An observational near-infrared spectroscopy study on cerebral autoregulation in post-cardiac arrest patients: Time to drop ‘one-size-fits-all’ hemodynamic targets? Resuscitation 2015, 90, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.; Bindra, J.; Chuan, A.; Jaeger, M.; Aneman, A. Are changes in cerebrovascular autoregulation following cardiac arrest associated with neurological outcome? results of a pilot study. Resuscitation 2015, 96, 192–198. [Google Scholar] [CrossRef]
- Callaway, C.W.; Donnino, M.W.; Fink, E.L.; Geocadin, R.G.; Golan, E.; Kern, K.B.; Leary, M.; Meurer, W.J.; Peberdy, M.A.; Thompson, T.M.; et al. Part 8: Post–cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015, 132, S465–S482. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Lee, S.E.; Choi, J.Y.; Gho, Y.R.; Chae, M.K.; Park, E.J.; Choi, M.H.; Hong, J.M. Useful computed tomography score for estimation of early neurologic outcome in post-cardiac arrest patients with therapeutic hypothermia. Circ. J. 2017, 81, 1628–1635. [Google Scholar] [CrossRef] [Green Version]
- Markus, H.S.; Harrison, M.J. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke 1992, 23, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Muller, M.; Voges, M.; Piepgras, U.; Schimrigk, K. Assessment of cerebral vasomotor reactivity by transcranial Doppler ultrasound and breath-holding. A comparison with acetazolamide as vasodilatory stimulus. Stroke 1995, 26, 96–100. [Google Scholar] [CrossRef]
- Herrera, C.R.; Beltramini, G.C.; Avelar, W.M.; Lima, F.O.; Li, L.M. Cerebral vasomotor reactivity assessment using Transcranial Doppler and MRI with apnea test. Braz. J. Med. Biol. Res. 2016, 49, e5437. [Google Scholar] [CrossRef] [Green Version]
- Eder, K.E.; Haussen, D.C.; Searls, D.E.; Henninger, N. Reverberating TCD flow pattern in brain death. Neurology 2012, 79, e79. [Google Scholar] [CrossRef] [Green Version]
- Pickering, J.W.; Endre, Z.H. New metrics for assessing diagnostic potential of candidate biomarkers. Clin. J. Am. Soc. Nephrol. 2012, 7, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Junejo, R.T.; Braz, I.D.; Lucas, S.J.; van Lieshout, J.J.; Phillips, A.A.; Lip, G.Y.; Fisher, J.P. Neurovascular coupling and cerebral autoregulation in atrial fibrillation. J. Cereb. Blood Flow Metab. 2020, 40, 1647–1657. [Google Scholar] [CrossRef]
- Samagh, N.; Bhagat, H.; Jangra, K. Monitoring cerebral vasospasm: How much can we rely on transcranial Doppler. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 12–18. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 158) | Good CPC (n = 41) | Poor CPC (n = 117) | p-Value | |
|---|---|---|---|---|
| Age, years | 60 (48–72) | 56 (41–62) | 61 (51–73) | 0.015 |
| Sex, male, n (%) | 103 (65.2) | 32 (78.1) | 71 (60.7) | 0.045 |
| Witness arrest, n (%) | 109 (69.0) | 31 (75.6) | 78 (66.7) | 0.287 |
| Bystander CPR, n (%) | 114 (72.2) | 29 (70.7) | 85 (72.65) | 0.814 |
| Time to ROSC, min | 25 (15–38) | 15 (11–26) | 26 (17–40) | <0.001 |
| Initial rhythm, n (%) | <0.001 | |||
| Shockable | 41 (26.0) | 28 (68.3) | 13 (11.1) | |
| Non-shockable | 117 (74.1) | 13 (31.7) | 104 (88.9) | |
| Cause of cardiac arrest, n (%) | <0.001 | |||
| Cardiogenic | 60 (38.0) | 31 (75.6) | 29 (24.8) | |
| Other medical | 44 (27.8) | 4 (9.8) | 40 (34.2) | |
| Asphyxia | 41 (26.0) | 4 (9.8) | 37 (31.6) | |
| Other | 13 (8.2) | 2 (4.9) | 11 (9.4) | |
| Comorbidities, n (%) | ||||
| Hypertension | 63 (39.9) | 15 (36.6) | 48 (41.0) | 0.617 |
| Diabetes | 48 (30.4) | 11 (26.8) | 37 (31.6) | 0.566 |
| Cardiac diseases | 31 (19.6) | 10 (24.4) | 21 (18.0) | 0.371 |
| Clinical parameters, n (%) | ||||
| No pupillary light reflex bilaterally | 68 (43.0) | 3 (7.3) | 65 (55.6) | <0.001 |
| GCS motor score | 1.8 ± 1.3 | 2.6 ± 1.8 | 1.5 ± 1.0 | <0.001 |
| GCS motor score ≥2 | 128 (81.0) | 16 (39.0) | 14 (12.0) | <0.001 |
| Serologic marker | ||||
| Initial S100, µg/L | 3.04 (1.23–5.89) | 0.94 (0.46–2.58) | 3.85 (2.13–6.74) | <0.001 |
| 24 h S100, µg/L | 0.76 (0.16–5.21) | 0.11 (0.07–0.18) | 2.24 (0.46–7.38) | <0.001 |
| Imaging parameters | ||||
| Time from ROSC to CT, h | 1.6 (1.0–2.3) | 1.40 (1.0–2.1) | 1.7 (1.0–2.6) | 0.579 |
| GWR | 1.18 ± 0.08 | 1.23 ± 0.06 | 1.16 ± 0.08 | <0.001 |
| ASPECTS-b, points | 7 (2–14) | 14 (8–18) | 5 (2–11) | <0.001 |
| TCD parameters | ||||
| Time from ROSC to TCD, h | 29.7 (23.0–46.6) | 31.90 (22.7–42.1) | 28.8 (23.1–46.9) | 0.852 |
| Bilateral poor temporal window, n (%) | 14 (8.9) | 2 (4.9) | 12 (10.3) | 0.223 |
| Baseline mean flow velocity, cm/s | 63.4 ± 27.1 | 51.9 ± 21.1 | 69.4 ± 28.1 | 0.001 |
| Baseline mean pulsatility index | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.9 ± 0.3 | 0.704 |
| Mean VMR, % | 35.1 ± 37.4 | 54.4 ± 33.0 | 25.1 ± 35.8 | <0.001 |
| Reverberating flow, n (%) | 29 (18.4) | 0 (0.00) | 29 (24.8) | <0.001 |
| Electrophysiological parameters after TTM, n (%) | ||||
| No EEG reactivity (n = 146) | 126/146 (86.3) | 18/32 (56.3) | 108/114 (94.7) | <0.001 |
| Absence of bilateral N20 (n = 123) | 67/123 (54.5) | 0/32 (0.0) | 67/91 (73.6) | <0.001 |
| Variables | Univariate Analysis | p-Value | Multivariable Analysis | p-Value |
|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |||
| Baseline demographics | ||||
| Age (per 1 year increase) | 0.97 (0.95–1.00) | 0.020 | 0.93 (0.87–0.98) | 0.009 |
| Shockable rhythm | 17.23 (7.19–41.32) | <0.001 | 14.17 (2.00–100.41) | 0.008 |
| Cardiogenic cause | 9.41 (4.11–21.51) | <0.001 | 12.80 (1.81–90.42) | 0.011 |
| Time to ROSC (per 1 min increase) | 0.95 (0.92–0.98) | <0.001 | 1.01 (0.95–1.07) | 0.723 |
| Early prognostic parameters | ||||
| Presence of pupillary light reflex | 15.83 (4.63–54.21) | <0.001 | 2.16 (0.28–16.65) | 0.459 |
| GCS motor score ≥2 | 4.71 (2.03–10.91) | <0.001 | 1.10 (0.16–7.66) | 0.926 |
| GWR ≥1.17 | 6.07 (2.49–14.81) | <0.001 | 15.74 (1.11–223.89) | 0.042 |
| ASPECTS-b ≥7 | 10.35 (3.79–28.29) | <0.001 | 9.32 (1.07–81.26) | 0.043 |
| Initial S100 <2.37 | 6.18 (2.78–13.73) | <0.001 | 1.06 (0.24–4.77) | 0.940 |
| Mean VMR ≥30.3% | 17.42 (4.92–61.63) | <0.001 | 122.80 (8.66–1741.91) | <0.001 |
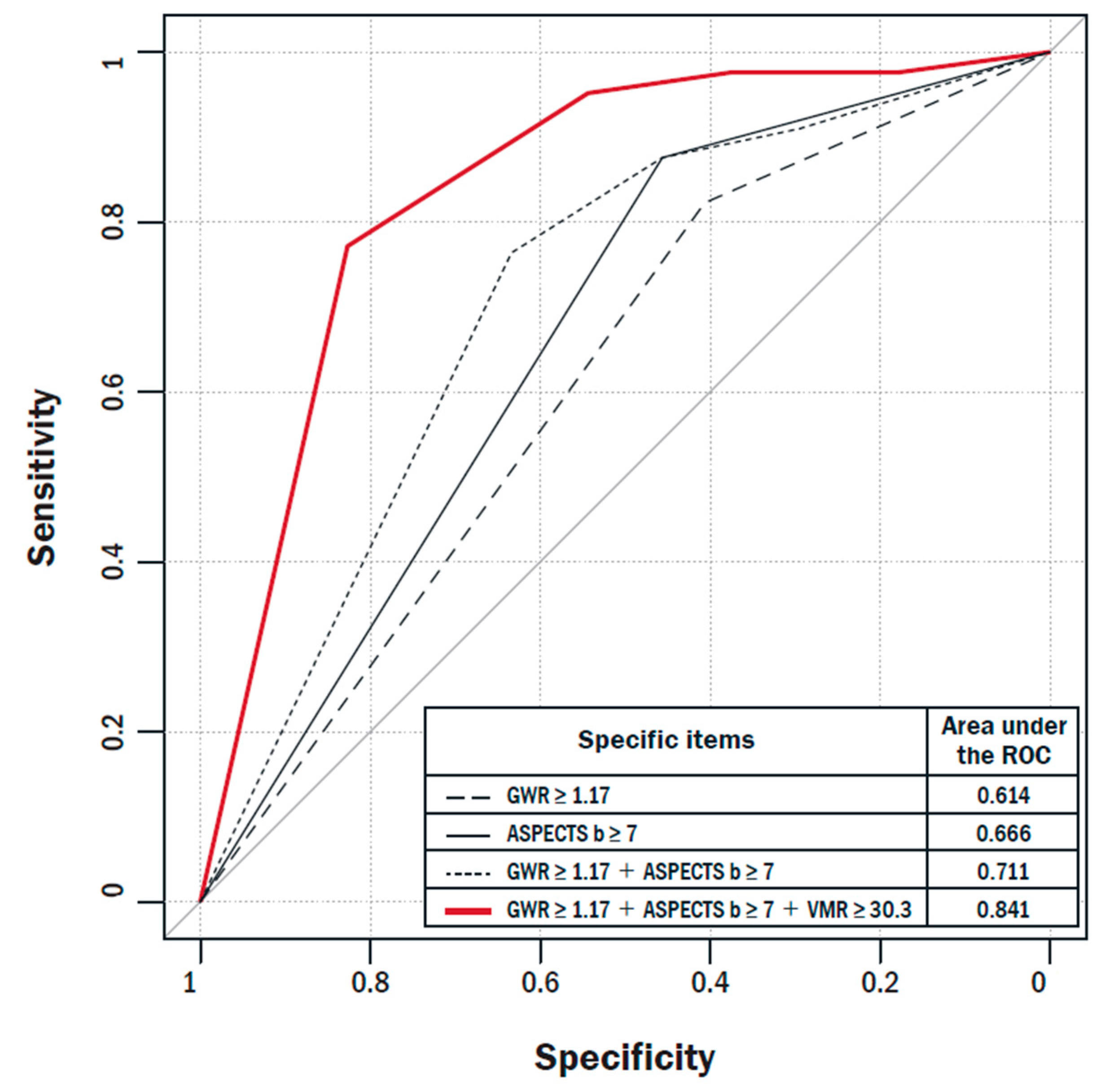
| AUC | Difference in AUC | Standard Error of Difference in AUC | 95% CI of Difference in AUC | p-Value (Difference in AUCs) | Relative IDI (%) | p-Value (Relative IDI) | NRI | p-Value (NRI) | Valid n | |
|---|---|---|---|---|---|---|---|---|---|---|
| GWR ≥ 1.17 | 0.614 | 0.205 | 0.043 | 0.121–0.289 | <0.001 | Ref. | Ref. | 115 | ||
| GWR ≥ 1.17 + VMR ≥ 30.3 | 0.819 | 0.28 | <0.001 | 0.36 | 0.001 | 115 | ||||
| ASPECTS-b ≥ 7 | 0.666 | 0.170 | 0.041 | 0.090–0.250 | <0.001 | Ref. | Ref. | 115 | ||
| ASPECTS-b ≥ 7 + VMR ≥ 30.3 | 0.837 | 0.24 | <0.001 | 0.25 | 0.018 | 115 | ||||
| GWR ≥ 1.17 + ASPECTS-b ≥ 7 | 0.711 | 0.131 | 0.040 | 0.052–0.209 | 0.001 | Ref. | Ref. | 115 | ||
| GWR ≥ 1.17 + ASPECTS-b ≥ 7 + VMR ≥ 30.3 | 0.841 | 0.22 | <0.001 | 0.20 | 0.041 | 115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.H.; Lee, S.E.; Choi, J.Y.; Lee, S.-J.; Kim, D.S.; Chae, M.K.; Park, E.J.; Hong, J.M. Prognostic Effects of Vasomotor Reactivity during Targeted Temperature Management in Post-Cardiac Arrest Patients: A Retrospective Observational Study. J. Clin. Med. 2021, 10, 3386. https://doi.org/10.3390/jcm10153386
Choi MH, Lee SE, Choi JY, Lee S-J, Kim DS, Chae MK, Park EJ, Hong JM. Prognostic Effects of Vasomotor Reactivity during Targeted Temperature Management in Post-Cardiac Arrest Patients: A Retrospective Observational Study. Journal of Clinical Medicine. 2021; 10(15):3386. https://doi.org/10.3390/jcm10153386
Chicago/Turabian StyleChoi, Mun Hee, Sung Eun Lee, Jun Young Choi, Seong-Joon Lee, Da Sol Kim, Minjung Kathy Chae, Eun Jung Park, and Ji Man Hong. 2021. "Prognostic Effects of Vasomotor Reactivity during Targeted Temperature Management in Post-Cardiac Arrest Patients: A Retrospective Observational Study" Journal of Clinical Medicine 10, no. 15: 3386. https://doi.org/10.3390/jcm10153386
APA StyleChoi, M. H., Lee, S. E., Choi, J. Y., Lee, S.-J., Kim, D. S., Chae, M. K., Park, E. J., & Hong, J. M. (2021). Prognostic Effects of Vasomotor Reactivity during Targeted Temperature Management in Post-Cardiac Arrest Patients: A Retrospective Observational Study. Journal of Clinical Medicine, 10(15), 3386. https://doi.org/10.3390/jcm10153386






