Association between Subclinical Hypothyroidism and Incident Hypertension in Women: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Inclusion Criteria
- (1)
- Case-control studies, cohort studies (prospective or retrospective), and cross-sectional studies that reported the incidence of HTN in females with SCH and those without SCH.
- (2)
- Statistics such as odds ratio or hazard ratio with 95% confidence interval and p-values associated with t-test, Wilcoxon test, or Kruskal–Wallis test, and sufficient raw data for these calculations had to be provided.
2.3. Quality Assessment of the Included Studies
2.4. Definition of Subclinical Hypothyroidism
2.5. Definition of Hypertension
2.6. Middle-Aged and Older Subgroups
2.7. Data Extraction
2.8. Statistical Analysis
3. Results
3.1. Study Search Results
3.2. Description of the Included Studies and Their Quality Assessment
3.3. Quantitative Meta-Analysis Results
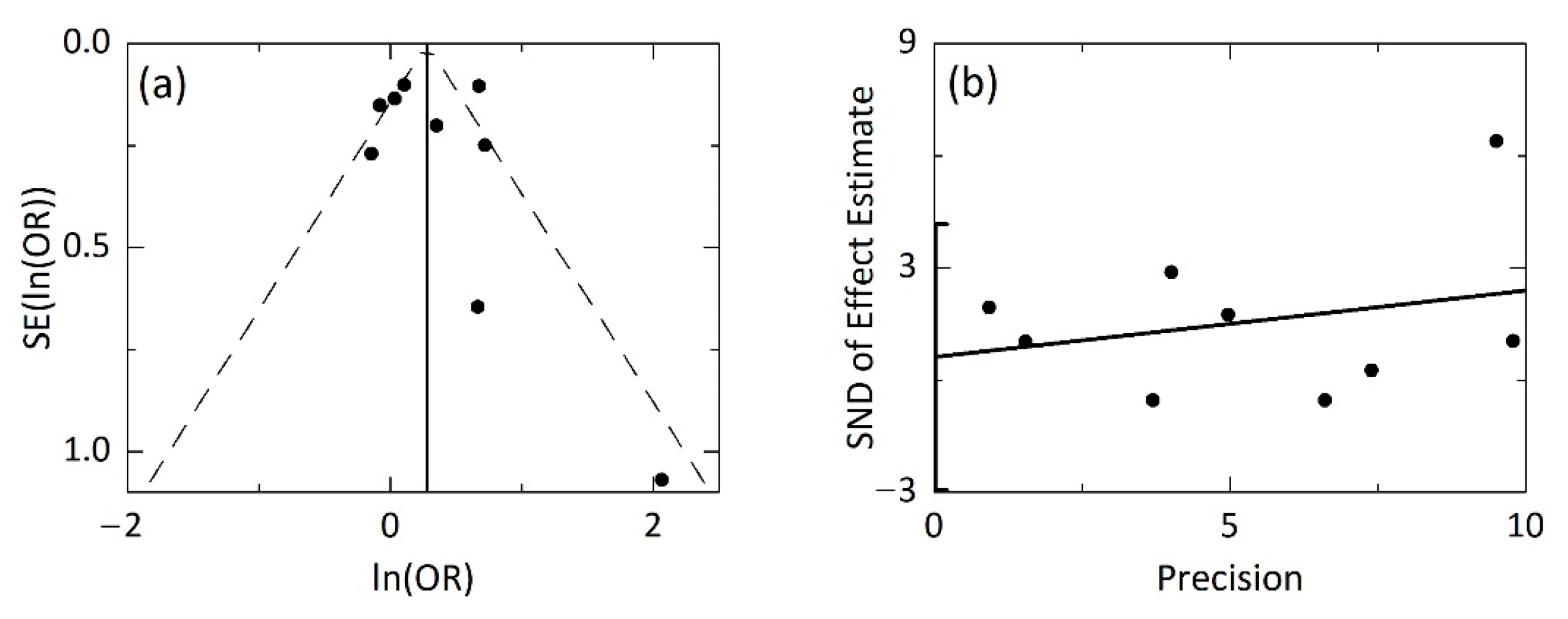
3.4. Publication Bias
3.5. Sensitivity Analysis
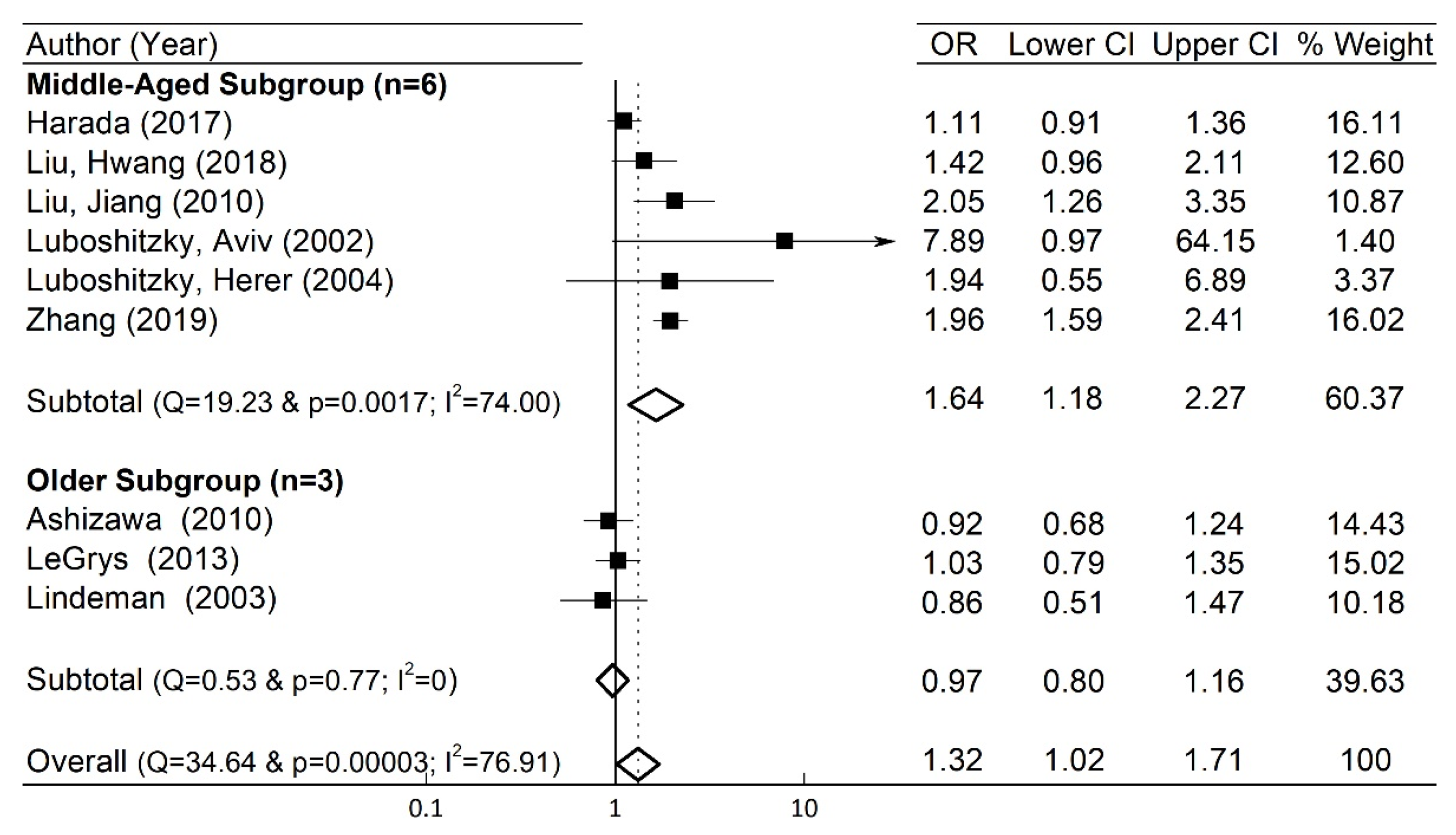
3.6. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pearce, S.H.S.; Brabant, G.; Duntas, L.H.; Monzani, F.; Peeters, R.P.; Razvi, S.; Wemeau, J.L. ETA guideline: Management of subclinical hypothyroidism. Eur. Thyroid. J. 2013, 2, 215–228. [Google Scholar] [CrossRef]
- Canaris, G.J.; Manowitz, N.R.; Mayor, G.; Ridgway, E.C. The Colorado thyroid disease prevalence study. Arch. Intern. Med. 2000, 160, 526–534. [Google Scholar] [CrossRef]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National health and nutrition examination survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Asvold, B.O.; Vatten, L.J.; Bjøro, T. Changes in the prevalence of hypothyroidism: The HUNT study in Norway. Eur. J. Endocrinol. 2013, 169, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.; Behl, A.; Iyer, V.; Joshi, H.; Dholye, J.P.; Varthakavi, P.K. Prevalence, clinical and biochemical profile of subclinical hypothyroidism in normal population in Mumbai. Indian J. Endocrinol. Metab. 2013, 17, 454–459. [Google Scholar] [CrossRef]
- Delitala, A.P.; Pilia, M.G.; Ferreli, L.; Loi, F.; Curreli, N.; Balaci, L.; Schlessinger, D.; Cucca, F. Prevalence of unknown thyroid disorders in a Sardinian cohort. Eur. J. Endocrinol. 2014, 171, 143–149. [Google Scholar] [CrossRef]
- Kim, W.G.; Kim, W.B.; Woo, G.; Kim, H.; Cho, Y.; Kim, T.Y.; Kim, S.W.; Shin, M.H.; Park, J.W.; Park, H.L.; et al. Thyroid stimulating hormone reference range and prevalence of thyroid dysfunction in the Korean population: Korea national health and nutrition examination survey 2013 to 2015. Endocrinol. Metab. 2017, 32, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Gourmelon, R.; Donadio-Andréi, S.; Chikh1, K.; Rabilloud, M.; Kuczewski, E.; Gauchez, A.S.; Charrié, A.; Brard, P.-Y.; Andréani, R.; Bourre, J.-C.; et al. Subclinical hypothyroidism: Is it really subclinical with aging? Aging Dis. 2019, 10, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Floriani, C.; Gencer, B.; Collet, T.H.; Rodondi, N. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur. Heart J. 2018, 39, 503–507. [Google Scholar] [CrossRef]
- Manolis, A.A.; Manolis, T.A.; Melita, H.; Manolis, A.S. Subclinical thyroid dysfunction and cardiovascular consequences: An alarming wake-up call? Trends Cardiovasc. Med. 2020, 30, 57–69. [Google Scholar] [CrossRef]
- Duntas, L.H.; Chiovato, L. Cardiovascular risk in patients with subclinical hypothyroidism. Eur. Endocrinol. 2014, 10, 157–160. [Google Scholar] [CrossRef][Green Version]
- Harada, P.H.N.; Buring, J.E.; Cook, N.R.; Cobble, M.E.; Kulkarni, K.R.; Mora, S. Impact of subclinical hypothyroidism on cardiometabolic biomarkers in women. J. Endocr. Soc. 2017, 1, 113–123. [Google Scholar] [CrossRef]
- Liu, F.H.; Hwang, J.S.; Kuo, C.F.; Ko, Y.S.; Chen, S.T.; Lin, J.D. Subclinical hypothyroidism and metabolic risk factors association: A health examination-based study in northern Taiwan. Biomed. J 2018, 41, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jiang, F.; Shan, Z.; Wang, B.; Wang, J.; Lai, Y.; Chen, Y.; Li, M.; Liu, H.; Li, C.; et al. A cross-sectional survey of relationship between serum TSH level and blood pressure. J. Hum. Hypertens. 2010, 24, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Luboshitzky, R.; Aviv, A.; Herer, P.; Lavie, L. Risk factors for cardiovascular disease in women with subclinical hypothyroidism. Thyroid 2002, 12, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Luboshitzky, R.; Herer, P. Cardiovascular risk factors in middle-aged women with subclinical hypothyroidism. Neuro Endocrinol. Lett. 2004, 25, 262–266. [Google Scholar] [PubMed]
- Zhang, J.; Huang, C.; Meng, Z.; Fan, Y.; Yang, Q.; Zhang, W.; Gao, Y.; Yang, Z.; Cai, H.; Bian, B.; et al. Gender-specific differences on the association of hypertension with subclinical thyroid dysfunction. Int. J. Endocrinol. 2019, 2019, 6053068. [Google Scholar] [CrossRef]
- Ashizawa, K.; Imaizumi, M.; Usa, T.; Tominaga, T.; Sera, N.; Hida, A.; Ejima, E.; Neriishi, K.; Soda, M.; Ichimaru, S.; et al. Metabolic cardiovascular disease risk factors and their clustering in subclinical hypothyroidism. Clin. Endocrinol. 2010, 72, 689–695. [Google Scholar] [CrossRef]
- LeGrys, V.A.; Funk, M.J.; Lorenz, C.E.; Giri, A.; Jackson, R.D.; Manson, J.E.; Schectman, R.; Edwards, T.L.; Heiss, G.; Hartmann, K.E. Subclinical hypothyroidism and risk for incident myocardial infarction among postmenopausal women. J. Clin. Endocrinol. Metab. 2013, 98, 2308–2317. [Google Scholar] [CrossRef][Green Version]
- Lindeman, R.D.; Romero, L.J.; Schade, D.S.; Wayne, I.S.; Baumgartner, R.N.; Garry, P.J. Impact of subclinical hypothyroidism on serum total homocysteine concentrations, the prevalence of coronary heart disease (CHD), and CHD Risk Factors in the New Mexico elder health survey. Thyroid 2003, 13, 595–600. [Google Scholar] [CrossRef]
- Spencer, C.A.; LoPresti, J.S.; Patel, A.; Guttler, R.B.; Eugen, A.; Shen, D.; Gray, D.; Nicoloff, J.T. Applications of new chemiluminometeric thyrotropin assay to subnormal measurement. J. Clin. Endocrinol. Metab. 1990, 70, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Al Eidan, E.; Ur Rahman, S.; Al Qahtani, S.; Al Farhan, A.I.; Abdulmajeed, I. Prevalence of hypothyroidism in adults visiting primary health-care setting in Riyadh. J. Community Hosp. Intern. Med. Percept. 2018, 8, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wang, X.; Peng, W.; Feng, Y.; Tang, W.; Wu, X.; Mao, X.; Bo, R.; Li, W.; Chen, J.; et al. Gender-specific associations between subclinical hypothyroidism and blood pressure in Chinese adults. Endocrine 2009, 36, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.R.; Wartofsky, L. Minimally symptomatic (subclinical) hypothyroidism. Endocrinologist 1997, 7, 44–50. [Google Scholar] [CrossRef]
- Klein, I. Subclinical hypothyroidism—Just a high serum thyrotropin (TSH) concentration or something else? J. Clin. Endocrinol. Metab. 2013, 98, 508–510. [Google Scholar] [CrossRef]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa scale: Comparing reviewers’ to authors assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Sun, J.; Freeman, B.D.; Natanson, C. Meta-Analysis of Clinical Trials. In Principles and Practice of Clinical Research; Gallin, J.I., Ognibene, F.P., Johnson, L.L., Eds.; Elsevier: London, UK, 2018; pp. 317–327. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Harbord, M. Funnel plots in meta-analysis. Stata J. 2004, 4, 127–141. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Pearce, S.H.S.; Zaman, A.; Iervasi, G.; Razvi, S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar]
- Klein, I.; Danzi, S. Thyroid disease and the heart. Curr. Probl. Cardiol. 2016, 41, 65–92. [Google Scholar] [CrossRef]
- Kociol, R.D.; Cooper, L.T.; Fang, J.C.; Moslehi, J.J.; Pang, P.S.; Sabe, M.A.; Shah, R.V.; Sims, D.B.; Thiene, G.; Vardeny, O.; et al. Recognition and initial management of fulminant myocarditis: A scientific statement from the American heart association. Circulation 2020, 141, e69–e92. [Google Scholar] [CrossRef]
- Taddei, S.; Caraccio, N.; Virdis, A.; Dardano, A.; Versari, D.; Ghiadoni, L.; Salvetti, A.; Ferrannini, E.; Monzani, F. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: Beneficial effect of levothyroxine therapy. J. Clin. Endocrinol. Metab. 2003, 88, 3731–3737. [Google Scholar] [CrossRef]
- Duntas, L.H.; Brenta, G. The effect of thyroid disorders on lipid levels and metabolism. Med. Clin. N. Am. 2012, 96, 269–281. [Google Scholar] [CrossRef]
- Saric, M.S.; Jurasic, M.J.; Sovic, S.; Kranjcec, B.; Glivetic, T.; Demarin, V. Dyslipidemia in subclinical hypothyroidism requires assessment of small dense low density lipoprotein cholesterol (sdLDL-C). Rom. J. Intern. Med. 2017, 55, 159–166. [Google Scholar] [CrossRef][Green Version]
- Boucai, L.; Surks, M.I. Reference limits of serum TSH and free T4 are significantly influenced by race and age in an urban outpatient medical practice. Clin. Endocrinol. 2009, 70, 788–793. [Google Scholar] [CrossRef]
- Kahapola-Arachchige, K.M.; Hadlow, N.; Wardrop, R.; Lim, E.M.; Walsh, J.P. Age-specific TSH reference ranges have minimal impact on the diagnosis of thyroid dysfunction. Clin. Endocrinol. 2012, 77, 773–779. [Google Scholar] [CrossRef]
- Surks, M.I.; Hollowell, J.G. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: Implications for the prevalence of subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2007, 92, 4575–4582. [Google Scholar] [CrossRef]
- Waring, A.C.; Arnold, A.M.; Newman, A.B.; Bùzková, P.; Hirsch, C.; Cappola, A.R. Longitudinal changes in thyroid function in the oldest old and survival: The cardiovascular health study all-stars study. J. Clin. Endocrinol. Metab. 2012, 97, 3944–3950. [Google Scholar] [CrossRef] [PubMed]
- Bremner, A.P.; Feddema, P.; Leedman, P.J.; Brown, S.J.; Beilby, J.P.; Lim, E.M.; Wilson, S.G.; O’Leary, P.C.; Walsh, J.P. Age-related changes in thyroid function: A longitudinal study of a community-based cohort. J. Clin. Endocrinol. Metab. 2012, 97, 1554–1562. [Google Scholar] [CrossRef]
- Stott, D.J.; Rodondi, N.; Kearney, P.M.; Ford, I.; Westendorp, R.G.J.; Mooijaart, S.P.; Sattar, N.; Aubert, C.E.; Aujesky, D.; Bauer, D.C.; et al. TRUST Study Group. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N. Engl. J. Med. 2017, 376, 2534–2544. [Google Scholar] [CrossRef]
- Ye, Y.; Xie, H.; Zeng, Y.; Zhao, X.; Tian, Z.; Zhang, S. Association between subclinical hypothyroidism and blood Pressure—A meta-analysis of observable studies. Endocr. Pract. 2014, 20, 150–157. [Google Scholar] [CrossRef]

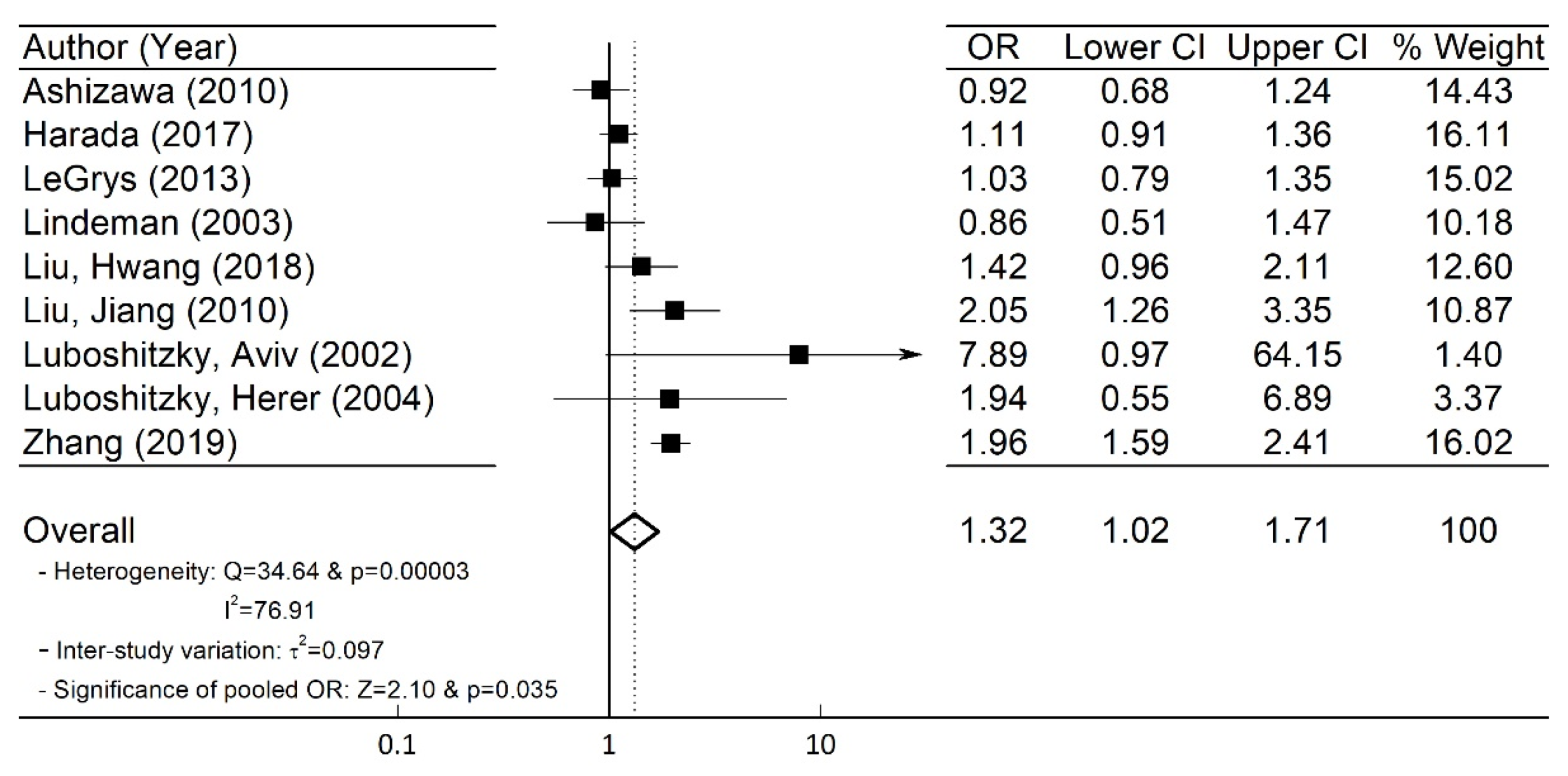

| Author | Country | Published Year | Study Type | SCH (HTN(a)/ No HTN(b)) (1896) | Control (HTN(c)/ No HTN(d)) (20,076) | Mean Age | Odds Ratio (95% CI) | Mean (Reference) TSH Level in SCH (mIU/L) | HTN SBP/DBP (mmHg) |
|---|---|---|---|---|---|---|---|---|---|
| Ashizawa ° | Japan | 2010 | Cross- Sectional | 194 (110/84) | 2134 (1253/881) | 71.5 | NR | 5.98 (>4.5) | >140/90 |
| Harada | U.S. | 2017 | Prospective Cohort | 573 (167/406) | 2571 (694/1877) | 56.9 | NR | NR (>4.2) | >130/85 |
| Legrys ° | U.S. | 2013 | Case Cohort | 282 (85/197) | 3381 (995/2386) | 67.5 | NR | 5.85 (>4.68) | NR |
| Lindeman ° | U.S. | 2003 | Cross- Sectional | 74 (27/47) | 283 (113/170) | 73.9 | NR | NR (>4.7) | >160/95 |
| Liu, Hwang | Taiwan | 2018 | Cross- Sectional | 102 (45/57) | 6323 (2257/4066) | 48.5 | NR | NR (>5.5) | >130/85 |
| Liu, Jiang | China | 2010 | Cross- Sectional | 75 (31/44) | 724 (185/539) | 44.8 | NR | 6.8 (>4.8) | >140/90 |
| Luboshitzky Aviv | Israel | 2002 | Prospective Cohort | 57 (11/46) | 34 (1/33) | 46.8 | NR | 10 (>4.5) | >140/90 |
| Luboshitzky Herer | Israel | 2004 | Prospective Cohort | 44 (15/29) | 9 (4/15) | 51.6 | NR | 9.2 (>4.5) | >140 |
| Zhang | China | 2019 | Cross- Sectional | 495 | 4607 | 48.8 | 1.959 (1.594, 2.407) | NR (>5.0) | >140/90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Prasitlumkum, N.; Randhawa, S.; Banerjee, D. Association between Subclinical Hypothyroidism and Incident Hypertension in Women: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3318. https://doi.org/10.3390/jcm10153318
Kim J, Prasitlumkum N, Randhawa S, Banerjee D. Association between Subclinical Hypothyroidism and Incident Hypertension in Women: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(15):3318. https://doi.org/10.3390/jcm10153318
Chicago/Turabian StyleKim, Jean, Narut Prasitlumkum, Sandeep Randhawa, and Dipanjan Banerjee. 2021. "Association between Subclinical Hypothyroidism and Incident Hypertension in Women: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 15: 3318. https://doi.org/10.3390/jcm10153318
APA StyleKim, J., Prasitlumkum, N., Randhawa, S., & Banerjee, D. (2021). Association between Subclinical Hypothyroidism and Incident Hypertension in Women: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(15), 3318. https://doi.org/10.3390/jcm10153318






