Abstract
Background: Cardiac rehabilitation (CR) is a requisite component of care for patients with heart failure (HF). We aimed to evaluate the clinical outcomes in outpatients with HF with preserved ejection fraction (HFpEF) compared to those in patients with non-HFpEF who did and did not continue a 5-month CR program. Methods: 173 outpatients with HF who participated in a 5-month CR program were registered. We divided them into two groups: HFpEF (n = 84, EF 63 ± 7%) and non-HFpEF (n = 89, EF 31 ± 11%). We further divided the patients into those who continued the CR program (continued group) and those who did not (discontinued group) in the HFpEF and non-HFpEF groups. The clinical outcomes at 5 months were compared among the groups. Results: There were no significant differences in patient characteristics at baseline between the continued and discontinued groups in the HFpEF and non-HFpEF groups except for % diabetes mellitus in the non-HFpEF group. The rates of all-cause death and hospital admissions in the continued group in both the HFpEF and non-HFpEF groups were significantly lower than those in the discontinued group. The all-cause death and hospital admissions in each group were independently associated with the continuation of the CR program. Conclusions: The continuation of a 5-month CR program was associated with the prevention of all-cause death and hospital admissions in both the HFpEF and non-HFpEF groups.
1. Introduction
Cardiac rehabilitation (CR) is a requisite component of care for patients with heart failure (HF) and a class A recommendation in several guidelines [1,2]. CR improves cardiorespiratory function and quality of life (QOL) and reduces mortality in patients with HF with reduced left ventricular ejection fraction (HFrEF) [3,4]. Although we treat many patients with HF with preserved ejection fraction (HFpEF) [5], patients with HFpEF and HFrEF have almost the same survival rates [6]. Furthermore, no pharmacotherapy has been shown to improve the survival rate in patients with HFpEF [7,8,9,10,11].
We have studied CR for more than eight years and reported some beneficial effects in patients with cardiovascular diseases (CVD) [12,13,14,15,16,17]. We previously reported that a CR program significantly reduced blood pressure (BP) and visit-to-visit variability in BP, and patients with mild to moderate chronic kidney disease (CKD) who participated in our CR program showed an increase in their estimated glomerular filtration rate (eGFR) [14,15]. We also reported that long-term CR in elderly CVD outpatients helped to maintain the anaerobic threshold (AT), left ventricular EF (LVEF), plasma levels of brain natriuretic peptide (BNP) and eGFR for 5 years [16]. These studies indicate that CR is effective in patients with hypertension (HTN), CKD and/or advanced age. Thus, CR may be effective for HFpEF patients caused by various factors, such as HTN, CKD and/or aging.
It is confirmed and clearly evidenced that CR is associated with the prognosis of HFrEF patients, whereas the evidence in HFpEF patients is relatively weak. Recently, it has been reported that CR improves the exercise capacity and QOL of HFpEF patients [18,19,20], and it has been suggested that it also improves the prognosis of these patients [21]. In the real world, many patients begin, but later discontinue, CR for various reasons. We previously reported that continuation of a 5-month CR program was associated with the prevention of all-cause death and hospital admissions in outpatients with CVD including HF [17]. Therefore, we hypothesized that the continuation of CR would have a positive effect on the prognosis for HFpEF patients and aimed to compare the clinical outcomes in HFpEF patients to those in non-HFpEF patients who did and did not continue a 5-month CR program.
2. Materials and Methods
2.1. Study Population and Protocol
This single-center retrospective cohort study evaluated the efficacy of CR in HFpEF and non-HFpEF patients. One hundred and seventy-three outpatients who had HF and participated in a 5-month CR program at Fukuoka University Hospital were enrolled from 2011 to 2019. We divided the patients into two groups according to LVEF at the beginning of the CR program: ≥50% LVEF (n = 84, HFpEF group) and <50% LVEF (n = 89, non-HFpEF group). In addition, we further divided the patients into those who continued the 5-month CR program (continued group) and those who did not (discontinued group). The clinical outcomes were compared between the groups for up to 5 months. The primary endpoint was all-cause death and hospital admission. This study was approved by the ethics committee of Fukuoka University Hospital.
2.2. Exercise Protocol
At the beginning of the CR program, the patients underwent a cardiopulmonary exercise test (CPX) using Cpex1 (Inter Riha, Tokyo, Japan) and a stress test system with an ML9000 electrocardiogram (Fukuda Denshi, Tokyo, Japan). The CR program was the same as in our previous report [15]. In brief, the patients participated in a supervised exercise training program at the hospital’s gym one to three times a week. The exercise workload was based on the AT according to the CPX results. Each session lasted 1 h, beginning with a warm-up exercise for 5 min, followed by 30 min of cycling with an aero bike (75XLIII; Combi, Tokyo, Japan) or walking on a treadmill (TRD-350; Sakai Medical, Tokyo, Japan) at the patient’s indicated exercise intensity and 25 min of cooling down and stretching.
2.3. Data Collection
Patient characteristics, including comorbidities, medications, LVEF and plasma BNP, were assessed at baseline. Patients with low-density lipoprotein cholesterol ≥140 mg/dL, triglyceride ≥150 mg/dL or high-density lipoprotein cholesterol <40 mg/dL and lipid-lowering therapy were diagnosed with dyslipidemia (DL). Patients with systolic and/or diastolic blood pressure (SBP/DBP) ≥140/90 mmHg or who were under antihypertensive treatment were considered to have HTN. Patients who were being treated for diabetes mellitus (DM) or who had symptoms of DM and a hemoglobin A1c ≥6.5% and/or a fasting glucose concentration ≥126 mg/dL were considered to have DM. Otherwise, the results of a 75 g oral glucose tolerance test were used to diagnose DM. Coronary artery disease (CAD) was defined as lumen diameter stenosis >50% in at least one major coronary artery as determined by coronary angiography and as diagnosed by anterior myocardial infarctions. Medications included angiotensin converting enzyme inhibitor (ACE-I)/angiotensin II receptor blockers (ARB), β-blockers, diuretics, calcium channel blockers (CCBs) and statins. LVEF obtained by the modified Simpson’s method was analyzed by a Vivid E9 echocardiogram (GE Healthcare, Tokyo, Japan). BNP was analyzed via enzymatic methods in the clinical laboratory of Fukuoka University Hospital.
2.4. Statistical Analyses
All of the data analyses were performed using the SAS (Statistical Analysis System) Software Package (Ver. 9.4, SAS Institute Inc., Cary, NC, USA) at Fukuoka University (Fukuoka, Japan). Continuous variables with a normal distribution are expressed as mean ± standard deviation, and differences between groups were compared using a Student’s t-test. Differences in categorical variables between groups were compared by chi-square analysis. To identify the factors associated with all-cause death and hospital admission, we performed a Cox regression analysis and obtained hazard ratios. Age, gender, BMI, CAD and medications were also subjected to a multivariate analysis to identify independent factors related to all-cause death and hospital admission. A Kaplan–Meier analysis (log-rank test) was applied to verify the time-dependent occurrence of clinical outcomes in groups stratified according to whether they did, or did not, complete a 5-month CR program. A value of p < 0.05 was considered significant.
3. Results
3.1. Patient Characteristics at Baseline in All Patients and in the HFpEF and Non-HFpEF Groups
The patient characteristics at baseline are shown in Table 1. For all the patients, the mean age was 66.6 ± 13.0 years. The mean age in the HFpEF group (70.0 ± 12.7 years) was higher than that in the non-HFpEF group (63.7 ± 12.1 years, p = 0.001). Although there was no difference in comorbidities except for CAD between the groups, the patients in the HFpEF group had less drug interventions, except for statins, than those in the non-HFpEF group (ARB/ACE-I: p = 0.002; diuretics: p < 0.0001; β-blockers: p = 0.0004; CCB: p = 0.0003; statins: p = 0.6). The BNP at baseline in all the patients was 390 ± 494.9 pg/mL, and that in the HFpEF group was significantly lower than that in the non-HFpEF group (205 ± 230 pg/mL vs. 558 ± 602 pg/mL, p < 0.0001). There was no significant difference in the AT peak VO2 between the groups.

Table 1.
Patient characteristics at baseline in all HF patients and HFpEF and non-HFpEF groups.
3.2. Continuation of CR and All-Cause Death and Hospital Admission in All Patients and in the HFpEF and Non-HFpEF Groups
The continuation of 5 months of CR and all-cause death and hospital admissions in all the patients and in the HFpEF and non-HFpEF groups are shown in Table 2. Of the 88 patients (50.9%) who continued the 5-month CR program, 41 (48.8%) were in the HFpEF group and 47 (52.8%) were in the non-HFpEF group. There was no difference in the % continuation of CR between the HFpEF and non-HFpEF groups (p = 0.65). In addition, all-cause death and hospital admissions occurred in 16 patients (19.0%) in the HFpEF group and 19 patients (21.3%) in the non-HFpEF group. There was no significant difference in all-cause death and hospital admissions between the groups (p = 0.85).

Table 2.
Continuation of CR and all-cause death and hospital admissions in all HF patients and HFpEF and non-HFpEF groups.
3.3. Patient Characteristics at Baseline in the Continued and Discontinued Groups in All HF Patients and in the HFpEF and Non-HFpEF Groups
The patient characteristics at baseline in the continued and discontinued groups in all patients and in the HFpEF and non-HFpEF groups are shown in Table 3. There were no significant differences in patient characteristics at baseline between the continued and discontinued groups in all the patients, or in the HFpEF and non-HFpEF groups except for the %DM in all the patients and the non-HFpEF group. There were also no significant differences in BNP levels at baseline among the groups.

Table 3.
Patient characteristics at baseline in the continued and discontinued groups in all HF patients and HFpEF and non-HFpEF groups.
3.4. Kaplan–Meier Curves for All-Cause Death and Hospital Admission in the Continued and Discontinued Groups in All Patients and in the HFpEF and Non-HFpEF Groups
The mean follow-up periods were 136 ± 33 days in all the patients. During follow-up, there were 35 composite outcomes, including 3 all-cause mortalities (1.7%) and 32 hospital admissions (18.5%).
Figure 1A shows the Kaplan–Meier curves for all-cause death and hospital admissions in the continued and discontinued groups in all the patients and in the HFpEF and non-HFpEF groups. The outcome rate was significantly lower in the continued group (p = 0.0003). The Kaplan–Meier curves for patients in the HFpEF and non-HFpEF groups are shown in Figure 1B,C. The rates of all-cause death and hospital admissions in the continued HFpEF and non-HFpEF groups were significantly lower than those in the respective discontinued groups (HFpEF group: p = 0.0084; non-HFpEF group: p = 0.0126).
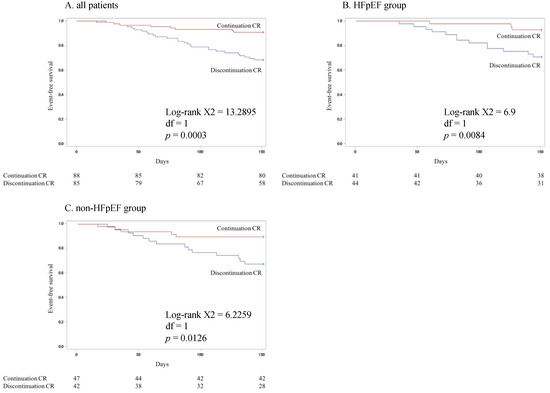
Figure 1.
Kaplan–Meier curves for all-cause death and hospital admissions in the continuation and discontinuation groups in all HF patients (A) and in the HFpEF (B) and non-HFpEF (C) groups.
3.5. Relationships between Various Parameters and All-Cause Death and Hospital Admissions as Assessed by a Univariate Logistic Regression Analysis
Regarding the relationships between various parameters, all-cause death and hospital admissions (Table 4), significant relationships were observed for the continuation of the 5-month CR program (all patients: p = 0.0007; HFpEF patients: p = 0.02; non-HFpEF patients: p = 0.02) and the use of diuretics in all the patients (p = 0.04).

Table 4.
The relationship between various parameters and all-cause death and hospital admission analyzed by a Cox regression analysis in all HF patients and HFpEF and non-HFpEF groups.
In Table 5, Model 1 shows unadjusted data. Models 2 and 3 were adjusted for age and age, gender and BMI, respectively. In addition, Models 4 and 5 were adjusted for age, gender, BMI and CAD and age, gender, BMI, CAD and medications, respectively. For Model 5, all-cause death and hospital admissions were independently associated with continuation of the CR program (all patients: p = 0.0002; HFpEF patients: p = 0.02; non-HFpEF patients: p = 0.046) and diuretics in all the patients (p = 0.03).

Table 5.
The relationship between all-cause death and hospital admissions and its associated factors for continuation of 5-month CR program and in 5-month period in all HF patients (A) and HFpEF (B) and non-HFpEF (C) groups, analyzed by a multivariate logistic regression analysis.
4. Discussion
4.1. Discussion
The continuation of a 5-month CR program was associated with the prevention of all-cause death and hospital admissions in both the HFpEF and non-HFpEF groups. While it is clearly important to introduce CR, these results suggest that it is also important to continue CR in HF patients, including HFpEF patients.
To date, no pharmacological intervention has been demonstrated to improve the prognosis of patients with HFpEF. Recently, it has been suggested that CR may improve the prognosis of these cases [21]. However, that report simply compared a CR group to a non-CR group. In our study, we were able to extract a group that did not continue CR despite having started CR, which was less effective than in patients who were able to continue CR as planned, at least among HFpEF patients. This supports the notion that the prognosis may be improved by the continuation of CR, especially for HFpEF patients, who are difficult to treat pharmacologically.
CR has been reported to reduce peripheral vascular resistance [22], improve endothelial function [23] and reduce sympathetic tone [24] and to significantly decrease SBP [25]. In fact, our previous report also confirmed that CR lowers BP [14]. CR reduced the visit-to-visit variability in BP, which has been shown to be a strong predictor of CVD and stroke independent of mean BP and other CVD risk factors. Since HTN is one of the main causes of HFpEF, it may have contributed to the prevention of all-cause death and hospital admissions in the HFpEF group. In addition, HFpEF is known as a syndromal disease where multiple cardiac and vascular disorders, cardiovascular risk factors and overlapping extracardiac comorbidities may be present in many kinds of combinations. Specifically, multiple cardiac and vascular disorders mean not only ventricular dysfunction but also vascular dysfunction and valvular disease, cardiovascular risk factors, mean HTN and DM, extracardiac comorbidities, mean renal dysfunction, obesity, sarcopenia and so on. It has been reported that CR has various effects such as inducing an anti-inflammatory response [26], as well as effects on skeletal muscle and the mitochondrial ultrastructure [27,28]. It is possible that the improvement of multiple factors, rather than a single factor, with the continuation of the CR program was responsible for the greater effect in HFpEF patients.
The discontinuation of a 5-month CR program was significantly associated with the presence of DM in all the patients and non-HFpEF patients. However, all-cause death and hospital admissions were not associated with the presence of DM. It was unclear why the CR continuation rate of DM patients was low, but an increase in this rate may lead to an improved prognosis. DM patients frequently discontinue their pharmacological treatment; in a previous study, 12% dropped out after the initial visit to the hospital and 33% of the residual cohort dropped out during each subsequent 6-month period [29]. Multifaceted interventions, which include measuring quality-of-care indicators for patients and providing feedback to physicians, were effective in improving the quality of care and the continuation rate for treatment in patients with DM [30]. Such interventions may contribute to improve the continuation rates for pharmacological treatment and CR in DM patients.
There was no significant difference in the rate of continuation between the HFpEF and non-HFpEF groups (p = 0.65). All-cause death and hospital admissions occurred in 16 patients (19.0%) in the HFpEF group and 19 patients (21.3%) in the non-HFpEF group. There were no significant differences in all-cause death and hospital admissions between the groups (p = 0.85). A previous study reported that patients with HFpEF and non-HFpEF had nearly identical prognoses [6], which is consistent with our report. This study reaffirmed that HFpEF patients have a poor prognosis over a short time period.
4.2. Study Limitations
This study has several limitations. First, this was a retrospective study from a single center with a relatively small sample size. Second, we did not perform long-term follow-ups. Third, the patients received different kinds and doses of medications. Fourth, there were many patients whose reasons for CR discontinuation were unknown. Among 85 patients with CR discontinuation, the reasons for discontinuation of CR were the onset of non-cardiac diseases without hospitalization (n = 15), the lowering of motivation (n = 15), problems with access to the hospital (n = 8), being busy with work (n = 4), frailty (n = 3), changing to home rehabilitation (n = 2), self-will (n = 2) and others (n = 3). Thus, 33 patients had unknown reasons for discontinuation. It is undeniable that some patients may be too frail or vulnerable to continue CR, and a worse outcome may result from their patient baseline. Therefore, a large-scale long-term study should be performed to confirm these results.
5. Conclusions
In this study, the continuation of a 5-month CR program was associated with the prevention of all-cause death and hospital admissions in both HFpEF patients and non-HFpEF patients.
Author Contributions
H.M.: data curation, investigation, writing—original draft, and final approval of the manuscript. Y.S.: methodology, investigation, data curation, formal analysis and visualization. Y.Y.: data curation and writing—review and editing. K.M., M.S., T.M., K.K. (Kouji Kaino.), R.T. (Reiko Teshima), N.U., M.F., R.T. (Rie Tazawa) and H.N.: data curation and investigation. K.K. (Ken Kitajima.): data curation and writing—review and editing. S.K.: data curation and investigation. K.F.: conceptualization, investigation, writing—original draft, project administration and final approval of the manuscript. S.-i.M.: conceptualization, investigation, writing—review and editing, supervision, project administration and final approval of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of Fukuoka University School of Medicine.
Informed Consent Statement
This study was a retrospective observational study, carried out by the opt-out methods of our university website.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef]
- Belardinelli, R.; Georgiou, D.; Cianci, G.; Purcaro, A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure. Circulation 1999, 99, 1173–1182. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Davos, C.; Francis, D.P.; Coats, A.J. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004, 328, 189. [Google Scholar] [CrossRef]
- Oktay, A.A.; Rich, J.D.; Shah, S.J. The emerging epidemic of heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2013, 10, 401–410. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.; Roger, V.L.; Redfield, M.M. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Cleland, J.G.; Tendera, M.; Adamus, J.; Freemantle, N.; Polonski, L.; Taylor, J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur. Heart J. 2006, 27, 2338–2345. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Swedberg, K.; Granger, C.B.; Held, P.; Mcmurray, J.; Michelson, E.L.; Olofsson, B.; Östergren, J.; Yusuf, S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM-Overall programme. Lancet 2003, 362, 759–766. [Google Scholar] [CrossRef]
- Massie, B.M.; Carson, P.E.; Mcmurray, J.; Komajda, M.; McKelvie, R.; Zile, M.R.; Anderson, S.; Donovan, M.; Iverson, E.; Staiger, C.; et al. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008, 359, 2456–2467. [Google Scholar] [CrossRef]
- Yamamoto, K.; Origasa, H.; Hori, M. Effects of carvedilol on heart failure with preserved ejection fraction: The Japanese Diastolic Heart Failure Study (J-DHF). Eur. J. Heart Fail. 2013, 15, 110–118. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Miura, S.-I.; Fujimi, K.; Ishida, T.; Matsuda, T.; Fujita, M.; Ura, Y.; Kaino, K.; Sakamoto, M.; Horita, T.; et al. Assessment of various parameters using simple non-invasive tests in patients with cardiovascular diseases with or without cardiac rehabilitation. IJC Heart Vasc. 2016, 12, 63–67. [Google Scholar] [CrossRef]
- Futami, M.; Fujimi, K.; Ueda, T.; Matsuda, T.; Fujita, M.; Kaino, K.; Sakamoto, M.; Horita, T.; Koyoshi, R.; Arimura, T.; et al. Cardiac rehabilitation in patients with cardiovascular disease leads various hemodynamic parameters obtained using simple non-invasive tests to their appropriate levels. IJC Heart Vasc. 2017, 17, 23–29. [Google Scholar] [CrossRef]
- Ishida, T.; Miura, S.-I.; Fujimi, K.; Ueda, T.; Ueda, Y.; Matsuda, T.; Sakamoto, M.; Arimura, T.; Shiga, Y.; Kitajima, K.; et al. Visit-to-visit variability and reduction in blood pressure after a 3-month cardiac rehabilitation program in patients with cardiovascular disease. Int. Heart J. 2016, 57, 607–614. [Google Scholar] [CrossRef]
- Fujimi, K.; Miura, S.-I.; Matsuda, T.; Fujita, M.; Ura, Y.; Kaino, K.; Sakamoto, M.; Horita, T.; Arimura, T.; Shiga, Y.; et al. Influence of a cardiac rehabilitation program on renal function in patients with cardiovascular disease in a one-year follow-up. Cardiol. Res. 2015, 6, 311–315. [Google Scholar] [CrossRef][Green Version]
- Kitajima, K.; Fujimi, K.; Matsuda, T.; Fujita, M.; Kaino, K.; Teshima, R.; Ujifuku, Y.; Horita, T.; Sakamoto, M.; Arimura, T.; et al. Possibility of cardio-renal protection by long-term cardiac rehabilitation in elderly patients with cardiovascular diseases. Intern. Med. 2019, 58, 2133–2138. [Google Scholar] [CrossRef]
- Fujimi, K.; Imaizumi, T.; Suematsu, Y.; Kitajima, K.; Ueda, T.; Ishida, T.; Futami, M.; Ujifuku, Y.; Matsuda, T.; Sakamoto, M.; et al. Differential prognostic impact between completion and non-completion of a 5-month cardiac rehabilitation program in outpatients with cardiovascular diseases. Int. J. Cardiol. 2019, 292, 13–18. [Google Scholar] [CrossRef]
- Fukuta, H.; Goto, T.; Wakami, K.; Kamiya, T.; Ohte, N. Effects of exercise training on cardiac function, exercise capacity, and quality of life in heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2019, 24, 535–547. [Google Scholar] [CrossRef]
- Taylor, R.S.; Davies, E.J.; Dalal, H.M.; Davis, R.; Doherty, P.; Cooper, C.; Holland, D.; Jolly, K.; Smart, N. Effects of exercise training for heart failure with preserved ejection fraction: A systematic review and meta-analysis of comparative studies. Int. J. Cardiol. 2012, 162, 6–13. [Google Scholar] [CrossRef]
- Chan, E.; Giallauria, F.; Vigorito, C.; Smart, N.A. Exercise training in heart failure patients with preserved ejection fraction: A systematic review and meta-analysis. Monaldi Arch. Chest Dis. 2016, 86, 759. [Google Scholar] [CrossRef]
- Kamiya, K.; Sato, Y.; Takahashi, T.; Tsuchihashi-Makaya, M.; Kotooka, N.; Ikegame, T.; Takura, T.; Yamamoto, T.; Nagayama, M.; Goto, Y.; et al. Multidisciplinary cardiac rehabilitation and long-term prognosis in patients with heart failure. Circ. Heart Fail. 2020, 13, 456–466. [Google Scholar] [CrossRef]
- Hambrecht, R.; Gielen, S.; Linke, A.; Fiehn, E.; Yu, J.; Walther, C.; Schoene, N.; Schuler, G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure. JAMA 2000, 283, 3095–3101. [Google Scholar] [CrossRef]
- Hambrecht, R.; Fiehn, E.; Weigl, C.; Gielen, S.; Hamann, C.; Kaiser, R.; Yu, J.; Adams, V.; Niebauer, J.; Schuler, G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 1998, 98, 2709–2715. [Google Scholar] [CrossRef]
- European Heart Failure Training Group. Experience from controlled trials of physical training in chronic heart failure. Protocol and patient factors in effectiveness in the improvement in exercise tolerance. Eur. Heart J. 1998, 19, 466–475. [Google Scholar] [CrossRef]
- Taylor, R.S.; Brown, A.; Ebrahim, S.; Jolliffe, J.; Noorani, H.; Rees, K.; Skidmore, B.; Stone, J.A.; Thompson, D.; Oldridge, N. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am. J. Med. 2004, 116, 682–692. [Google Scholar] [CrossRef]
- Kasapis, C.; Thompson, P.D. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Gielen, S.; Adams, V.; Möbius-Winkler, S.; Linke, A.; Erbs, S.; Yu, J.; Kempf, W.; Schubert, A.; Schuler, G.; Hambrecht, R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J. Am. Coll. Cardiol. 2003, 42, 861–868. [Google Scholar] [CrossRef]
- Hambrecht, R.; Fiehn, E.; Yu, J.; Niebauer, J.; Weigl, C.; Hilbrich, L.; Adams, V.; Riede, U.; Schuler, G. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J. Am. Coll. Cardiol. 1997, 29, 1067–1073. [Google Scholar] [CrossRef]
- Graber, A.L.; Davidson, P.; Brown, A.W.; McRae, J.R.; Woolridge, K. Dropout and Relapse During Diabetes Care. Diabetes Care 1992, 15, 1477–1483. [Google Scholar] [CrossRef]
- Hayashino, Y.; Suzuki, H.; Yamazaki, K.; Goto, A.; Izumi, K.; Noda, M. A cluster randomized trial on the effect of a multifaceted intervention improved the technical quality of diabetes care by primary care physicians: The Japan Diabetes Outcome Intervention Trial-2 (J-DOIT 2). Diabet. Med. 2015, 33, 599–608. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).