Optimization of Acute Kidney Injury (AKI) Time Definitions Using the Electronic Health Record: A First Step in Automating In-Hospital AKI Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
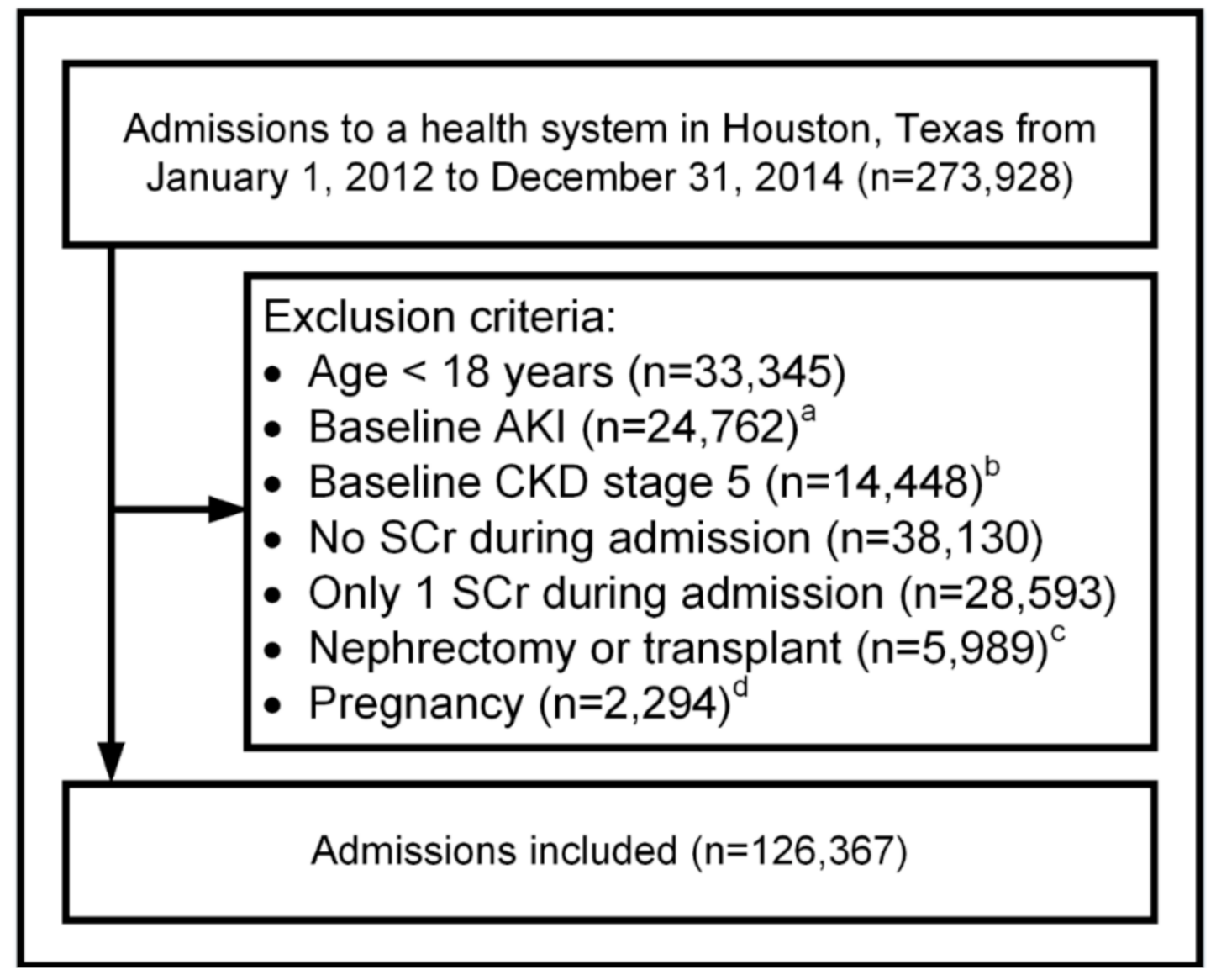
2.2. Patients and Setting
2.3. Demographics and Baseline Comorbidities
2.4. Acute Kidney Injury
2.5. Identification of New Cases of AKI by Expanding Time Windows
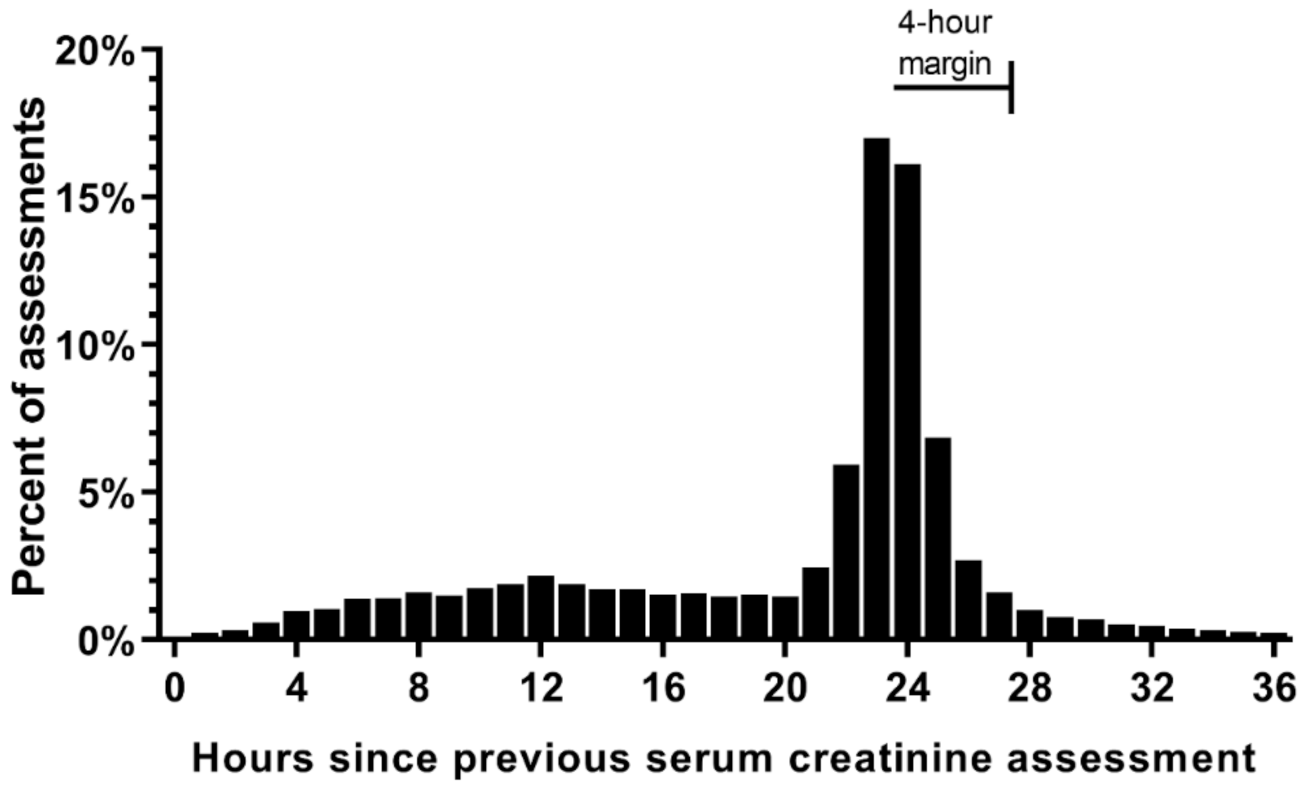
2.6. Observation Windows and Precision Levels
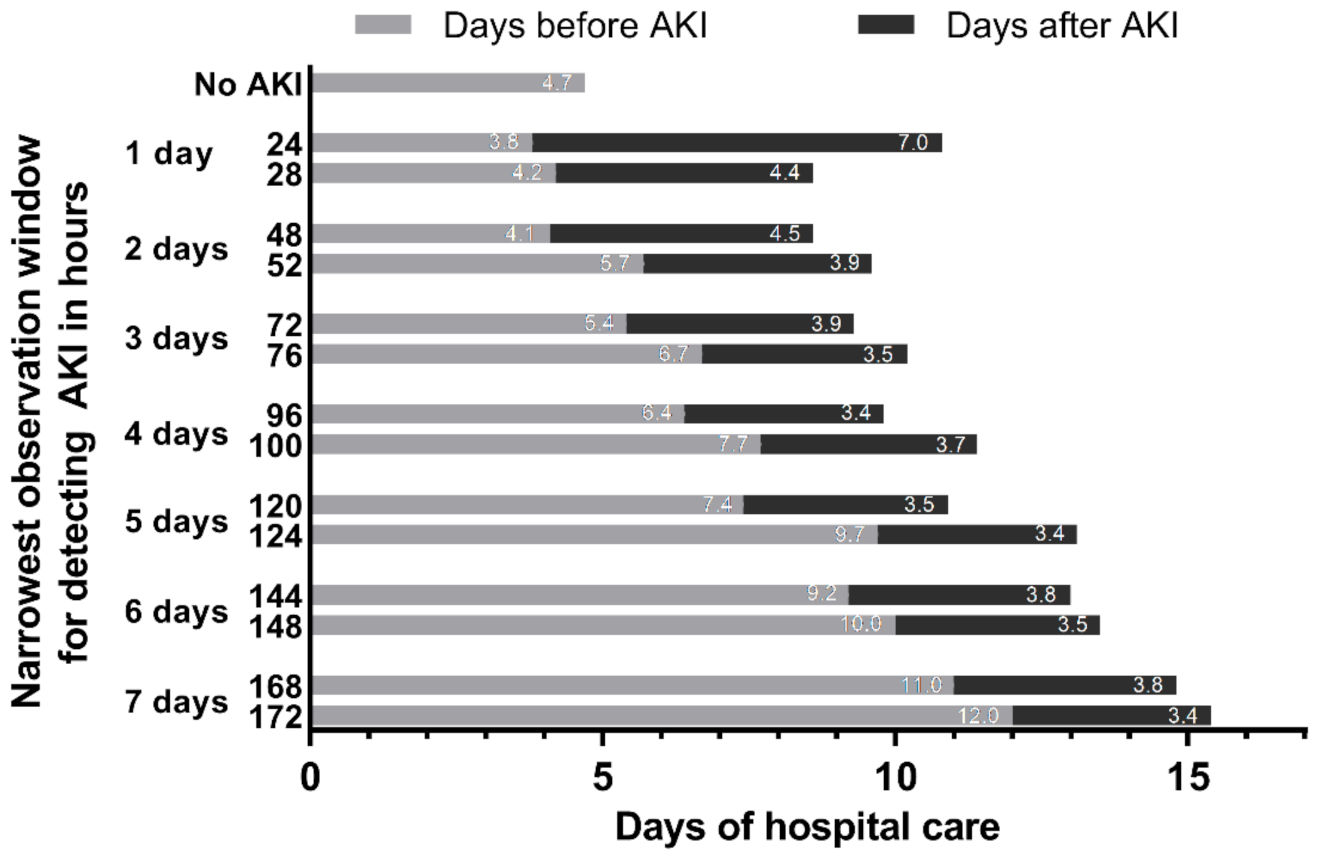
2.7. Narrowest Observation Window for Detecting AKI
2.8. Outcomes of Length of Stay and Mortality
2.9. Statistical Analysis
3. Results
3.1. Patients and Setting
3.2. Identification of New Cases of AKI by Expanding Time Windows
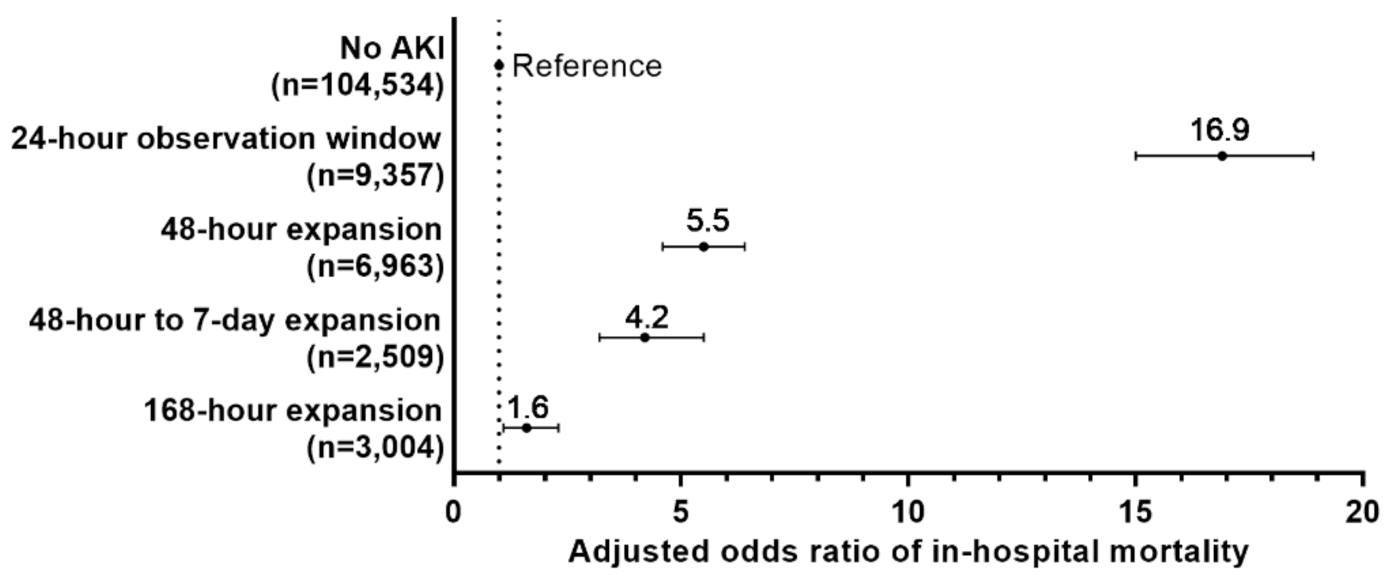
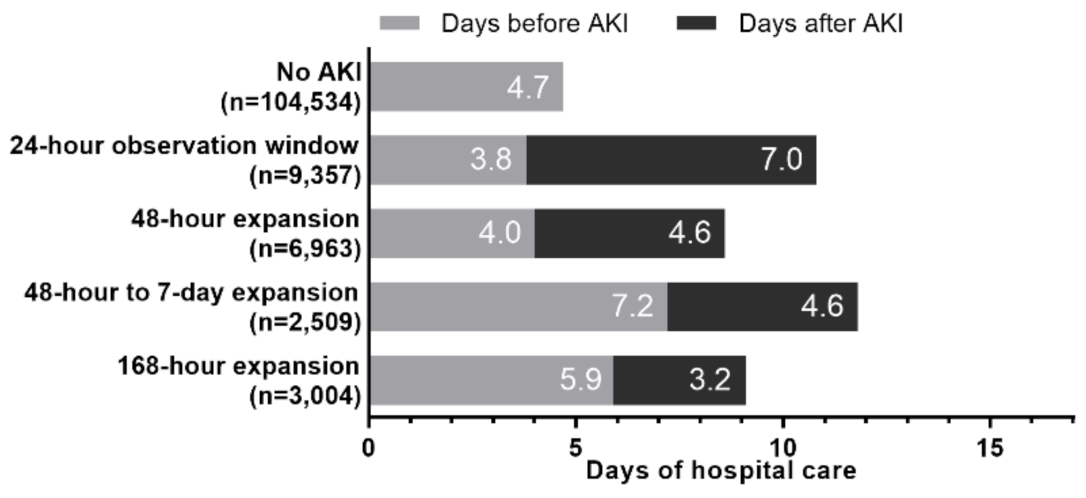
3.3. Outcomes Associated with Time Windows: LOS and Mortality
3.4. SCr Measurement during Routine Care
3.5. Observation Windows and Precision Levels
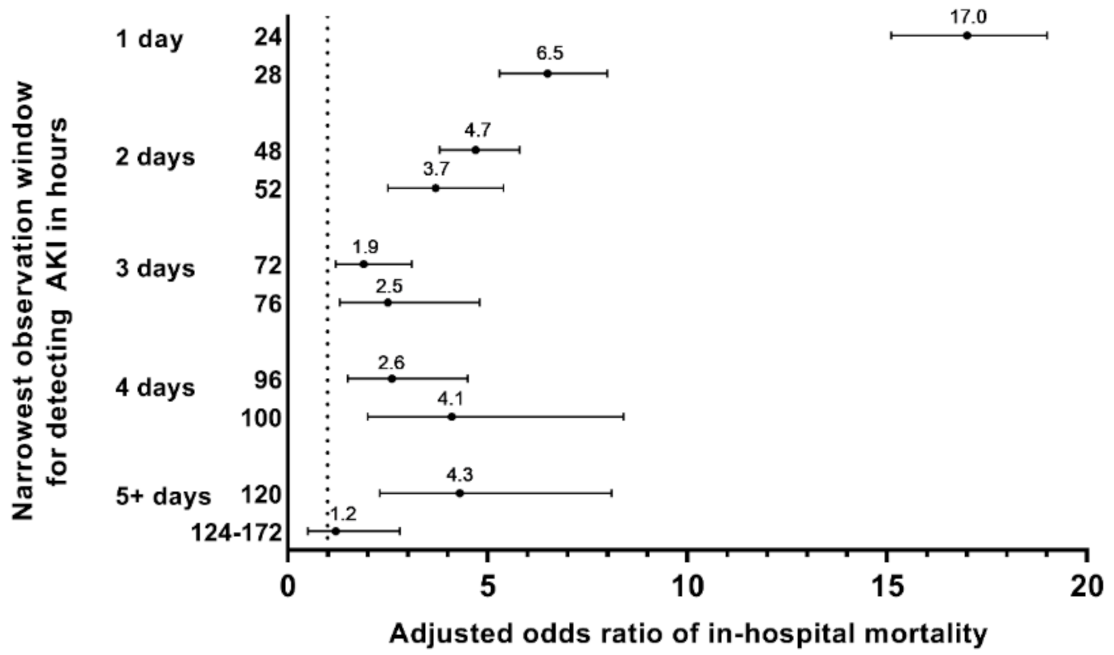
3.6. Narrowest Observation Window for Detecting AKI and Associated Clinical Outcomes
3.7. Sensitivity Analysis of Impact of Death as a Competing Event for Hospital Length of Stay
3.8. Sensitivity Analysis of the SCr Criteria Used for Detection of AKI
4. Discussion
4.1. Impact of Observation Windows
4.2. Impact of Precision Levels
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Remuzzi, G.; Horton, R. Acute renal failure: An unacceptable death sentence globally. Lancet 2013, 382, 2041–2042. [Google Scholar] [CrossRef]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, L.S.; Amdur, R.L.; Shaw, A.D.; Faselis, C.; Palant, C.E.; Kimmel, P.L. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin. J. Am. Soc. Nephrol. 2014, 9, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. on behalf of the Acute Disease Quality Initiative W: Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidney Disease: Improving Global Outcomes Workgroup. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012, 2, 1–138. [Google Scholar] [CrossRef] [Green Version]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute Dialysis Quality Initiative workgroup: Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Network tAKI: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrow, H.G.; Swan, J.T.; Moore, L.W.; Gaber, A.O.; Suki, W.N. Disparate outcomes observed within Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury stage 1. Kidney Int. 2019, 95, 905–913. [Google Scholar] [CrossRef] [PubMed]
- James, M.; Bouchard, J.; Ho, J.; Klarenbach, S.; LaFrance, J.-P.; Rigatto, C.; Wald, R.; Zappitelli, M.; Pannu, N. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 2013, 61, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N. The definitions and staging systems of acute kidney injury and their limitations in practice. Arab J. Nephrol. Transpl. 2013, 6, 145–152. [Google Scholar]
- Palevsky, P.M.; Liu, K.D.; Brophy, P.D.; Chawla, L.S.; Parikh, C.R.; Thakar, C.V.; Tolwani, A.J.; Waikar, S.S.; Weisbord, S.D. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 2013, 61, 649–672. [Google Scholar] [CrossRef] [PubMed]
- The Ad-Hoc Working Group of ERBP; Fliser, D.; Laville, M.; Covic, A.; Fouque, D.; Vanholder, R.; Juillard, L.; Van Biesen, W. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: Part 1: Definitions, conservative management and contrast-induced nephropathy. Nephrol. Dial Transpl. 2012, 27, 4263–4272. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.V.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes Workgroup. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 5–14. [Google Scholar]
- Steyerberg, E.W.; Harrell, F.E., Jr.; Borsboom, G.J.; Eijkemans, M.J.; Vergouwe, Y.; Habbema, J.D. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol. 2001, 54, 774–781. [Google Scholar] [CrossRef]
- Horne, K.L.; Selby, N.M. Recent developments in electronic alerts for acute kidney injury. Curr. Opin. Crit. Care 2015, 21, 479–484. [Google Scholar] [CrossRef] [PubMed]
- National Health System England. UK Renal Registry: Think Kidneys-Acute Kidney Injury. Available online: https://www.thinkkidneys.nhs.uk/aki/ (accessed on 21 November 2018).
- Siew, E.D.; Basu, R.K.; Wunsch, H.; Shaw, A.D.; Goldstein, S.L.; Ronco, C.; Kellum, J.A.; Bagshaw, S.M. ACG: Optimizing administrative datasets to examine acute kidney injury in the era of big data: Workgroup statement from the 15th ADQI Consensus Conference. Can. J. Kidney Health Dis. 2016, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristic | Study Sample (n = 126,367) | |
|---|---|---|
| Age, years | ||
| Mean (SD) | 63 | (18) |
| Median (range) | 64 | (18 to 112) |
| Serum creatinine assessments | ||
| Count of SCr during admission, Median (IQR) | 4 | (3 to 6) |
| SCr per day of hospital care, mean (SD), mg/dL | 1.1 | (0.6) |
| n | % | |
| Hospital type | ||
| Community hospital a | 67,175 | 53.2% |
| Academic medical center | 59,192 | 46.8% |
| Admission type | ||
| Elective | 22,018 | 17.4% |
| Urgent | 63,069 | 49.9% |
| Emergency | 41,119 | 32.5% |
| Unavailable | 144 | 0.1% |
| Sex | ||
| Female | 69,698 | 55.2% |
| Male | 56,669 | 44.8% |
| Race | ||
| Caucasian | 77,373 | 61.2% |
| Black or African American | 24,403 | 19.3% |
| Other | 24,591 | 19.5% |
| Estimated GFR using first SCr b, mL/min/1.73 m2 | ||
| 15 to 29 | 3178 | 2.5% |
| 30 to 44 | 9801 | 7.8% |
| 45 to 59 | 16,762 | 13.3% |
| 60 to 74 | 20,098 | 15.9% |
| 75 to 89 | 24,857 | 19.7% |
| 90 to 105 | 25,276 | 20.0% |
| >105 | 26,395 | 20.9% |
| Chronic comorbid conditions c | ||
| Hypertension | 84,771 | 67.1% |
| Heart disease | 59,863 | 47.4% |
| Congestive heart failure d | 23,317 | 18.5% |
| Diabetes | 38,835 | 30.7% |
| Anemia | 18,706 | 14.8% |
| Cancer | 16,106 | 12.8% |
| Cerebrovascular disease | 13,301 | 10.5% |
| Chronic kidney disease | 11,820 | 9.4% |
| Other kidney/urinary tract disease | 24,703 | 9.0% |
| Peripheral and visceral atherosclerosis | 8167 | 6.5% |
| Liver disease | 7759 | 6.1% |
| Pancreas disease (not diabetes) | 1326 | 0.5% |
| Admission sequence e | ||
| First (index) admission for a patient | 83,938 | 66.4% |
| Second admission for a patient | 21,700 | 17.2% |
| Greater than second admission for a patient | 20,729 | 16.4% |
| Observation Window and Precision Level | AKI Incidence | Mean Length of Stay in Days a | In-Hospital Mortality Incidence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | AKI | No AKI | Unadjusted Difference in Means b | 95% CI | AKI | No AKI | Unadjusted Risk Difference c | 95% CI | |
| 1 day | ||||||||||
| 1 cal day | 2381 | 1.9% | 13.8 | 5.5 | 8.4 * | 8.2 to 8.6 | 21.1% | 1.0% | 20.1% * | 18.4% to 21.7% |
| 24 h | 9357 | 7.3% | 10.8 | 5.2 | 5.6 * | 5.5 to 5.7 | 9.6% | 0.8% | 8.8% * | 8.2% to 9.4% |
| 28 h d | 12,509 | 9.9% | 10.2 | 5.1 | 5.1 * | 5.0 to 5.2 | 8.2% | 0.7% | 7.5% * | 7.0% to 8.0% |
| 2 days | ||||||||||
| 2 cal days | 13,271 | 10.5% | 10.2 | 5.1 | 5.1 * | 5.0 to 5.2 | 8.0% | 0.7% | 7.3% * | 6.9% to 7.8% |
| 48 h | 16,320 | 12.9% | 9.8 | 5.0 | 4.8 * | 4.8 to 4.9 | 6.9% | 0.6% | 6.3% * | 5.9% to 6.7% |
| 52 h d | 17,559 | 13.9% | 9.8 | 4.9 | 4.9 * | 4.8 to 5.0 | 6.6% | 0.6% | 6.0% * | 5.7% to 6.4% |
| 3 days | ||||||||||
| 3 cal days | 17,892 | 14.2% | 9.8 | 4.9 | 4.9 * | 4.8 to 5.0 | 6.5% | 0.6% | 5.9% * | 5.6% to 6.3% |
| 72 h | 19,053 | 15.1% | 9.8 | 4.9 | 4.9 * | 4.8 to 5.0 | 6.2% | 0.6% | 5.6% * | 5.3% to 6.0% |
| 76 h d | 19,649 | 15.6% | 9.8 | 4.8 | 4.9 * | 4.9 to 5.0 | 6.1% | 0.6% | 5.5% * | 5.2% to 5.8% |
| 4 days | ||||||||||
| 4 cal days | 19,831 | 15.7% | 9.8 | 4.8 | 5.0 * | 4.9 to 5.0 | 6.0% | 0.6% | 5.5% * | 5.1% to 5.8% |
| 96 h | 20,440 | 16.2% | 9.8 | 4.8 | 5.0 * | 4.9 to 5.1 | 5.9% | 0.6% | 5.3% * | 5.0% to 5.6% |
| 100 h d | 20,749 | 16.4% | 9.8 | 4.8 | 5.0 * | 5.0 to 5.1 | 5.8% | 0.6% | 5.3% * | 5.0% to 5.6% |
| 5 days | ||||||||||
| 5 cal days | 20,856 | 16.5% | 9.8 | 4.8 | 5.0 * | 5.0 to 5.1 | 5.8% | 0.6% | 5.3% * | 5.0% to 5.6% |
| 120 h | 21,168 | 16.8% | 9.8 | 4.8 | 5.1 * | 5.0 to 5.2 | 5.8% | 0.6% | 5.2% * | 4.9% to 5.5% |
| 124 h d | 21,347 | 16.9% | 9.9 | 4.7 | 5.1 * | 5.0 to 5.2 | 5.7% | 0.6% | 5.2% * | 4.9% to 5.5% |
| 6 days | ||||||||||
| 6 cal days | 21,396 | 16.9% | 9.9 | 4.7 | 5.1 * | 5.0 to 5.2 | 5.7% | 0.5% | 5.2% * | 4.9% to 5.5% |
| 144 h | 21,573 | 17.1% | 9.9 | 4.7 | 5.2 * | 5.1 to 5.2 | 5.7% | 0.6% | 5.1% * | 4.8% to 5.4% |
| 148 h d | 21,960 | 17.2% | 9.9 | 4.7 | 5.2 * | 5.1 to 5.3 | 5.6% | 0.6% | 5.1% * | 4.8% to 5.4% |
| 7 days | ||||||||||
| 7 cal days | 21,718 | 17.2% | 9.9 | 4.7 | 5.2 * | 5.1 to 5.3 | 5.6% | 0.6% | 5.1% * | 4.8% to 5.4% |
| 168 h | 21,833 | 17.3% | 10.0 | 4.7 | 5.2 * | 5.2 to 5.3 | 5.6% | 0.5% | 5.1% * | 4.8% to 5.4% |
| 172 h d | 21,894 | 17.3% | 10.0 | 4.7 | 5.3 * | 5.2 to 5.3 | 5.6% | 0.5% | 5.1% * | 4.7% to 5.4% |
| KDIGO | ||||||||||
| 7 days e | 18,829 | 14.9% | 10.1 | 4.8 | 5.3 * | 5.2 to 5.3 | 6.3% | 0.6% | 5.8% * | 5.4% to 6.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swan, J.T.; Moore, L.W.; Sparrow, H.G.; Frost, A.E.; Gaber, A.O.; Suki, W.N. Optimization of Acute Kidney Injury (AKI) Time Definitions Using the Electronic Health Record: A First Step in Automating In-Hospital AKI Detection. J. Clin. Med. 2021, 10, 3304. https://doi.org/10.3390/jcm10153304
Swan JT, Moore LW, Sparrow HG, Frost AE, Gaber AO, Suki WN. Optimization of Acute Kidney Injury (AKI) Time Definitions Using the Electronic Health Record: A First Step in Automating In-Hospital AKI Detection. Journal of Clinical Medicine. 2021; 10(15):3304. https://doi.org/10.3390/jcm10153304
Chicago/Turabian StyleSwan, Joshua T., Linda W. Moore, Harlan G. Sparrow, Adaani E. Frost, A. Osama Gaber, and Wadi N. Suki. 2021. "Optimization of Acute Kidney Injury (AKI) Time Definitions Using the Electronic Health Record: A First Step in Automating In-Hospital AKI Detection" Journal of Clinical Medicine 10, no. 15: 3304. https://doi.org/10.3390/jcm10153304
APA StyleSwan, J. T., Moore, L. W., Sparrow, H. G., Frost, A. E., Gaber, A. O., & Suki, W. N. (2021). Optimization of Acute Kidney Injury (AKI) Time Definitions Using the Electronic Health Record: A First Step in Automating In-Hospital AKI Detection. Journal of Clinical Medicine, 10(15), 3304. https://doi.org/10.3390/jcm10153304






