Management of Corneal Clouding in Patients with Mucopolysaccharidosis
Abstract
1. Introduction
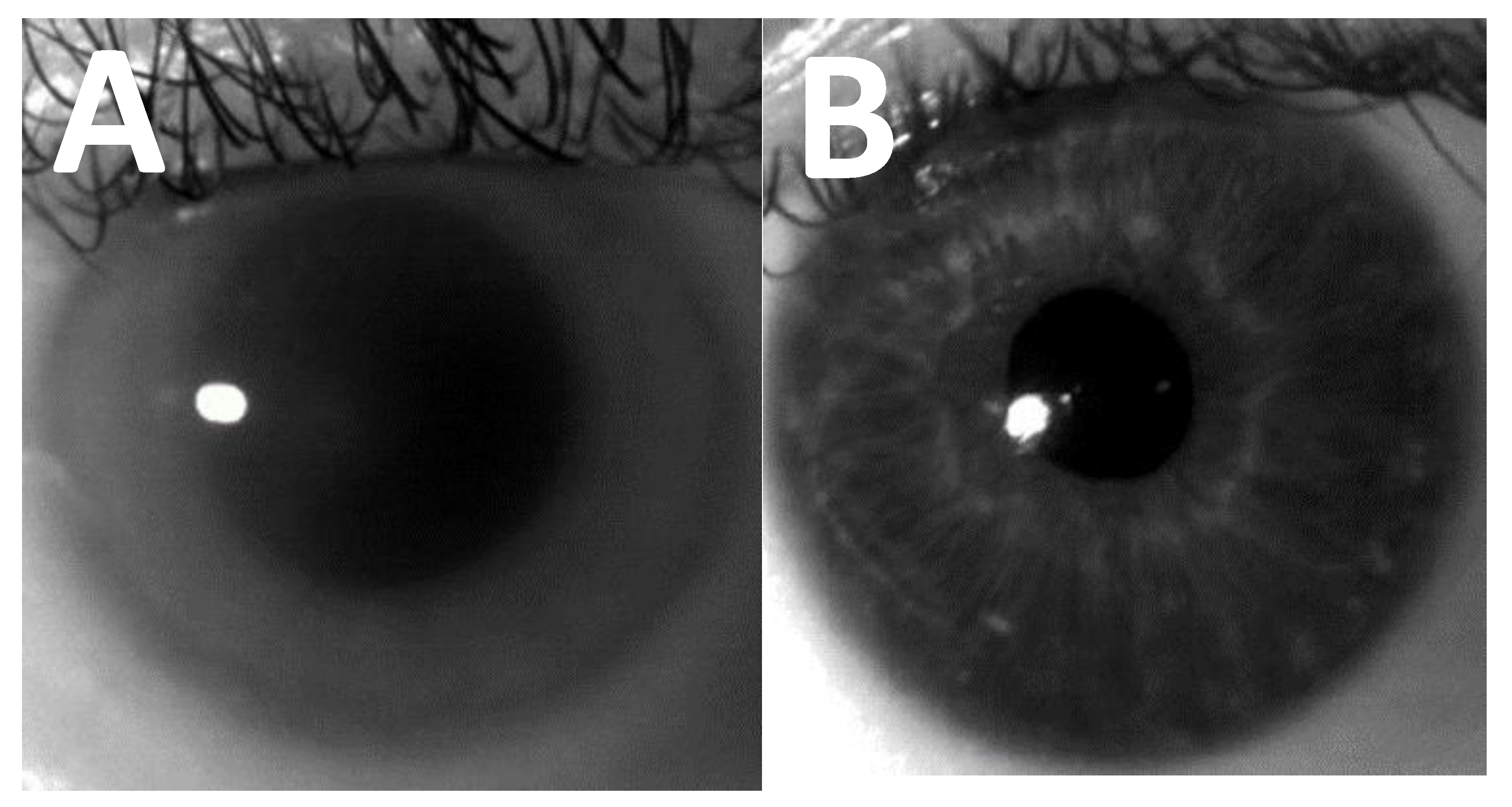
2. Corneal Clouding in MPS
3. Systemic Therapies and Their Effect on Corneal Clouding
3.1. Enzyme Replacement Therapy (ERT)
3.2. Hematopoietic Stem-Cell Transplantation (HSCT)
4. Surgical Treatment for Corneal Clouding
- The effect of visual impairment on the patient’s daily activities and quality of life, and the wishes of the patient to improve their vision;
- The exclusion of other ocular factors (retinopathy or optic neuropathy) as a cause of visual impairment;
- The condition of the ocular surface; dryness or vascularization of the cornea;
- The general health of the patient and their suitability for anesthesia.
4.1. Pre-Operative Planning for Keratoplasty
4.2. Penetrating Keratoplasty (PK)
4.3. Deep Anterior Lamellar Keratoplasty (DALK)
4.4. Postoperative Management
5. Future Corneal Clouding Treatment Options
5.1. Gene Therapy
5.2. Substrate Reduction Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ashworth, J.L.; Biswas, S.; Wraith, E.; Lloyd, I.C. Mucopolysaccharidoses and the Eye. Surv. Ophthalmol. 2006, 51, 1–17. [Google Scholar] [CrossRef]
- Pastores, G.M.; Arn, P.; Beck, M.; Clarke, J.T.; Guffon, N.; Kaplan, P.; Muenzer, J.; Norato, D.Y.; Shapiro, E.; Thomas, J.; et al. The MPS I registry: Design, methodology, and early findings of a global disease registry for monitoring patients with Mucopolysaccharidosis Type I. Mol. Genet. Metab. 2007, 91, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.G.; Ashworth, J.L. Ocular manifestations as key features for diagnosing mucopolysaccharidoses. Rheumatology 2011, 50, v34–v40. [Google Scholar] [CrossRef]
- Ashworth, J.; Flaherty, M.; Pitz, S.; Ramlee, A. Assessment and diagnosis of suspected glaucoma in patients with mucopolysaccharidosis. Acta Ophthalmol. 2015, 93, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Del Longo, A.; Piozzi, E.; Schweizer, F. Ocular features in mucopolysaccharidosis: Diagnosis and treatment. Ital. J. Pediatr. 2018, 44, 125. [Google Scholar] [CrossRef]
- Ashworth, J.L.; Kruse, F.E.; Bachmann, B.; Tormene, A.P.; Suppiej, A.; Parini, R.; Guffon, N. Ocular manifestations in the mucopolysaccharidoses—A review. Clin. Exp. Ophthalmol. 2010, 38, 12–22. [Google Scholar] [CrossRef]
- Nassar, A.; Tabbara, K.F.; Aljurf, M. Ocular manifestations of graft-versus-host disease. Saudi, J. Ophthalmol. 2013, 27, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Teramoto, K.; Moshirfar, M. Ocular manifestations and management recommendations of lysosomal storage disorders I: Mucopolysaccharidoses. Clin. Ophthalmol. 2015, 9, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Ponzin, D.; Ashworth, J.L.; Fahnehjelm, K.T.; Summers, C.G.; Harmatz, P.R.; Scarpa, M. Diagnosis and management of ophthalmological features in patients with mucopolysaccharidosis. Br. J. Ophthalmol. 2011, 95, 613–619. [Google Scholar] [CrossRef]
- Ganesh, A.; Bruwer, Z.; Al-Thihli, K. An update on ocular involvement in mucopolysaccharidoses. Curr. Opin. Ophthalmol. 2013, 24, 379–388. [Google Scholar] [CrossRef]
- Fahnehjelm, K.T.; Malm, G.; Winiarski, J.; Törnquist, A.-L. Ocular findings in four children with mucopolysaccharidosis I-Hurler (MPS I-H) treated early with haematopoietic stem cell transplantation. Acta Ophthalmol. Scand. 2006, 84, 781–785. [Google Scholar] [CrossRef]
- Michelacci, Y.M. Collagens and proteoglycans of the corneal extracellular matrix. Braz. J. Med. Biol. Res. 2003, 36, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Müller, L.J.; Pels, E.; Schurmans, L.R.; Vrensen, G.F. A new three-dimensional model of the organization of proteoglycans and collagen fibrils in the human corneal stroma. Exp. Eye Res. 2004, 78, 493–501. [Google Scholar] [CrossRef]
- Fahnehjelm, K.T.; Ashworth, J.L.; Pitz, S.; Olsson, M.; Törnquist, A.L.; Lindahl, P.; Summers, C.G. Clinical guidelines for diagnosing and managing ocular manifestations in children with mucopolysaccharidosis. Acta Ophthalmol. 2012, 90, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Grupcheva, C.; Craig, J.P.; McGhee, C.N. In Vivo Microstructural Analysis of the Cornea in Scheie’s Syndrome. Cornea 2003, 22, 76–79. [Google Scholar] [CrossRef]
- Tomatsu, S.; Pitz, S.; Hampel, U. Ophthalmological Findings in Mucopolysaccharidoses. J. Clin. Med. 2019, 8, 1467. [Google Scholar] [CrossRef]
- Fahnehjelm, K.T.; Chen, E.; Winiarski, J. Corneal hysteresis in mucopolysaccharidosis I and VI. Acta Ophthalmol. 2012, 90, 445–448. [Google Scholar] [CrossRef]
- Elflein, H.M.; Hofherr, T.; Berisha-Ramadani, F.; Weyer, V.; Lampe, C.; Beck, M.; Pitz, S. Measuring corneal clouding in patients suffering from mucopolysaccharidosis with the Pentacam densitometry programme. Br. J. Ophthalmol. 2013, 97, 829–833. [Google Scholar] [CrossRef]
- Hampe, C.; Wesley, J.; Lund, T.; Orchard, P.; Polgreen, L.; Eisengart, J.; McLoon, L.; Cureoglu, S.; Schachern, P.; McIvor, R. Mucopolysaccharidosis Type I: Current Treatments, Limitations and Prospects for Improvement. Biomolecules 2021, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Aldenhoven, M.; Wynn, R.F.; Orchard, P.J.; O’Meara, A.; Veys, P.; Fischer, A.; Valayannopoulos, V.; Neven, B.; Rovelli, A.; Prasad, V.K.; et al. Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: An international multicenter study. Blood 2015, 125, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Poe, M.D.; Chagnon, S.L.; Escolar, M.L. Early treatment is associated with improved cognition in Hurler syndrome. Ann. Neurol. 2014, 76, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Muenzer, J. Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. Mol. Genet. Metab. 2014, 111, 63–72. [Google Scholar] [CrossRef]
- Laraway, S.; Breen, C.; Mercer, J.; Jones, S.; Wraith, J.E. Does early use of enzyme replacement therapy alter the natural history of mucopolysaccharidosis I? Experience in three siblings. Mol. Genet. Metab. 2013, 109, 315–316. [Google Scholar] [CrossRef]
- Clarke, L.A.; Wraith, J.E.; Beck, M.; Kolodny, E.H.; Pastores, G.M.; Muenzer, J.; Rapoport, D.; Berger, K.; Sidman, M.; Kakkis, E.D.; et al. Long-term Efficacy and Safety of Laronidase in the Treatment of Mucopolysaccharidosis I. Pediatrics 2009, 123, 229–240. [Google Scholar] [CrossRef]
- Giugliani, R.; Muschol, N.; Keenan, H.A.; Dant, M.; Muenzer, J. Improvement in time to treatment, but not time to diagnosis, in patients with mucopolysaccharidosis type I. Arch. Dis. Child. 2021, 106, 674–679. [Google Scholar] [CrossRef]
- De Ru, M.H.; Boelens, J.J.; Das, A.M.; Jones, S.A.; Van Der Lee, J.H.; Mahlaoui, N.; Mengel, E.; Offringa, M.; O’Meara, A.; Parini, R.; et al. Enzyme Replacement Therapy and/or Hematopoietic Stem Cell Transplantation at diagnosis in patients with Mucopolysaccharidosis type I: Results of a European consensus procedure. Orphanet. J. Rare Dis. 2011, 6, 55. [Google Scholar] [CrossRef]
- McGill, J.J.; Inwood, A.C.; Coman, D.; Lipke, M.L.; De Lore, D.; Swiedler, S.J.; Hopwood, J.J. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age-a sibling control study. Clin. Genet. 2010, 77, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Laraway, S.; Mercer, J.; Jameson, E.; Ashworth, J.; Hensman, P.; Jones, S. Outcomes of Long-Term Treatment with Laronidase in Patients with Mucopolysaccharidosis Type I. J. Pediatr. 2016, 178, 219–226. [Google Scholar] [CrossRef]
- Gaffke, L.; Pierzynowska, K.; Podlacha, M.; Brokowska, J.; Węgrzyn, G. Changes in cellular processes occurring in mucopolysaccharidoses as underestimated pathomechanisms of these diseases. Cell Biol. Int. 2021, 45, 498–506. [Google Scholar] [CrossRef]
- Pitz, S.; Ogun, O.; Bajbouj, M.; Arash, L.; Schulze-Frenking, G.; Beck, M. Ocular Changes in Patients With Mucopolysaccharidosis I Receiving Enzyme Replacement Therapy. Arch. Ophthalmol. 2007, 125, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Wraith, J.E. The first 5 years of clinical experience with laronidase enzyme replacement therapy for mucopolysaccharidosis I. Expert Opin. Pharmacother. 2005, 6, 489–506. [Google Scholar] [CrossRef]
- Kakkis, E.D.; Muenzer, J.; Tiller, G.E.; Waber, L.; Belmont, J.; Passage, M.; Izykowski, B.; Phillips, J.; Doroshow, R.; Walot, I.; et al. Enzyme-Replacement Therapy in Mucopolysaccharidosis I. N. Engl. J. Med. 2001, 344, 182–188. [Google Scholar] [CrossRef]
- Pitz, S.; Ogun, O.; Arash, L.; Miebach, E.; Beck, M. Does enzyme replacement therapy influence the ocular changes in type VI mucopolysaccharidosis? Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 975–980. [Google Scholar] [CrossRef]
- Sarfraz, M.W.; Smith, M.; Jones, S.; Ashworth, J. Progression of eye disease over 15 years in a patient with mucopolysaccharidosis type VI on enzyme replacement therapy. BMJ Case Rep. 2021, 14, e238544. [Google Scholar] [CrossRef]
- Aslam, T.; Shakir, S.; Wong, J.; Au, L.; Ashworth, J. Use of iris recognition camera technology for the quantification of corneal opacification in mucopolysaccharidoses. Br. J. Ophthalmol. 2012, 96, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Aslam, T.; Ashworth, J. Use of new imaging in detecting and monitoring ocular manifestations of the mucopolysaccharidoses. Acta Ophthalmol. 2016, 94, e676–e682. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Aslam, T.; Jones, S.; Ashworth, J. Objective Quantification of Changes in Corneal Clouding Over Time in Patients With Mucopolysaccharidosis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 954–958. [Google Scholar] [CrossRef][Green Version]
- Summers, C.G.; Fahnehjelm, K.T.; Pitz, S.; Guffon, N.; Koseoglu, S.T.; Harmatz, P.; Scarpa, M. Systemic therapies for mucopolysaccharidosis: Ocular changes following haematopoietic stem cell transplantation or enzyme replacement therapy—A review. Clin. Exp. Ophthalmol. 2010, 38, 34–42. [Google Scholar] [CrossRef]
- Fahnehjelm, K.T.; Törnquist, A.-L.; Olsson, M.; Winiarski, J. Visual outcome and cataract development after allogeneic stem-cell transplantation in children. Acta Ophthalmol. Scand. 2007, 85, 724–733. [Google Scholar] [CrossRef]
- Fahnehjelm, K.T.; Winiarski, J.; Törnquist, A.-L. Dry-eye syndrome after allogeneic stem-cell transplantation in children. Acta Ophthalmol. 2008, 86, 253–258. [Google Scholar] [CrossRef]
- Guffon, N.; Pettazzoni, M.; Pangaud, N.; Garin, C.; Lina-Granade, G.; Plault, C.; Mottolese, C.; Froissart, R.; Fouilhoux, A. Long term disease burden post-transplantation: Three decades of observations in 25 Hurler patients successfully treated with hematopoietic stem cell transplantation (HSCT). Orphanet. J. Rare Dis. 2021, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gullingsrud, E.O. Ocular abnormalities in the mucopolysaccharidoses after bone marrow transplantation Longer follow-up. Ophthalmology 1998, 105, 1099–1105. [Google Scholar] [CrossRef]
- Vellodi, A.; Young, E.P.; Cooper, A.; Wraith, J.E.; Winchester, B.; Meaney, C.; Ramaswami, U.; Will, A. Bone marrow transplantation for mucopolysaccharidosis type I: Experience of two British centres. Arch. Dis. Child. 1997, 76, 92–99. [Google Scholar] [CrossRef]
- Fahnehjelm, K.T.; Olsson, M.; Chen, E.; Hengstler, J.; Naess, K.; Winiarski, J. Children with mucopolysaccharidosis risk progressive visual dysfunction despite haematopoietic stem cell transplants. Acta Paediatr. 2018, 107, 1995–2003. [Google Scholar] [CrossRef]
- Broek, B.T.V.D.; van Egmond-Ebbeling, M.B.; Achterberg, J.A.; Boelens, J.J.; Vlessert, I.C.; Prinsen, H.C.; van Doorn, J.; van Hasselt, P.M. Longitudinal Analysis of Ocular Disease in Children with Mucopolysaccharidosis I after Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 928–935. [Google Scholar] [CrossRef]
- Bothun, E.D.; Decanini, A.; Summers, C.G.; Orchard, P.J.; Tolar, J. Outcome of Penetrating Keratoplasty for Mucopolysaccharidoses. Arch. Ophthalmol. 2011, 129, 138–144. [Google Scholar] [CrossRef][Green Version]
- Tan, D.T.; Dart, J.K.; Holland, E.J.; Kinoshita, S. Corneal transplantation. Lancet 2012, 379, 1749–1761. [Google Scholar] [CrossRef]
- Pinello, L.; Busin, M.; Fontana, L.; Dua, H.S. Application of (lamellar) keratoplasty and limbal stem cell transplantation for corneal clouding in the mucopolysaccharidoses—A review. Clin. Exp. Ophthalmol. 2010, 38, 52–62. [Google Scholar] [CrossRef]
- Ayyala, R.S. Penetrating Keratoplasty and Glaucoma. Surv. Ophthalmol. 2000, 45, 91–105. [Google Scholar] [CrossRef]
- Chaurasia, S.; Price, F.W.; Gunderson, L.; Price, M. Descemet’s Membrane Endothelial Keratoplasty. Ophthalmology 2014, 121, 454–458. [Google Scholar] [CrossRef]
- Jones, S.M.; Fajgenbaum, M.A.; Hollick, E.J. Endothelial cell loss and complication rates with combined Descemets stripping endothelial keratoplasty and cataract surgery in a UK centre. Eye 2015, 29, 675–680. [Google Scholar] [CrossRef][Green Version]
- Matoba, A.; Oie, Y.; Tanibuchi, H.; Winegarner, A.; Nishida, K. Anterior segment optical coherence tomography and in vivo confocal microscopy in cases of mucopolysaccharidosis. Am. J. Ophthalmol. Case Rep. 2020, 19, 100728. [Google Scholar] [CrossRef]
- Ramesh, P.V.; Jha, K.N.; Srikanth, K. Comparison of Central Corneal Thickness using Anterior Segment Optical Coherence Tomography Versus Ultrasound Pachymetry. J. Clin. Diagn. Res. 2017, 11, NC08–NC11. [Google Scholar] [CrossRef] [PubMed]
- Mullaney, P.; Awad, A.H.; Millar, L. Glaucoma in mucopolysaccharidosis 1-H/S. J. Pediatr. Ophthalmol. Strabismus 1996, 33, 127–131. [Google Scholar] [CrossRef]
- Quigley, H.A.; Maumenee, A.E.; Stark, W.J. Acute glaucoma in systemic mucopolysaccharidosis I-S. Am. J. Ophthalmol. 1975, 80, 70–72. [Google Scholar] [CrossRef]
- Zhang, J.R.; Wang, J.H.; Lin, H.Z.; Lee, Y.C. Anterior Chamber Angles in Different Types of Mucopolysaccharidoses. Am. J. Ophthalmol. 2020, 212, 175–184. [Google Scholar] [CrossRef]
- Caruso, R.C.; Kaiser-Kupfer, M.I.; Muenzer, J.; Ludwig, I.H.; Zasloff, M.A.; Mercer, P.A. Electroretinographic Findings in the Mucopolysaccharidoses. Ophthalmology 1986, 93, 1612–1616. [Google Scholar] [CrossRef]
- Price, F.W.; Price, M.; Grandin, J.C.; Kwon, R. Deep anterior lamellar keratoplasty with femtosecond-laser zigzag incisions. J. Cataract. Refract. Surg. 2009, 35, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Ohden, K.L.; Pitz, S.; Ashworth, J.; Magalhães, A.; Marinho, D.R.; Lindahl, P.; Fahnehjelm, K.T.; Summers, C.G. Outcomes of keratoplasty in the mucopolysaccharidoses: An international perspective. Br. J. Ophthalmol. 2017, 101, 909–912. [Google Scholar] [CrossRef]
- Williams, K.A.; Esterman, A.J.; Bartlett, C.; Holland, H.; Hornsby, N.B.; Coster, D.J. How effective is penetrating corneal transplantation? Factors influencing long-term outcome in multivariate analysis. Transplantation 2006, 81, 896–901. [Google Scholar] [CrossRef]
- Borderie, V.M.; Boëlle, P.Y.; Touzeau, O.; Allouch, C.; Boutboul, S.; Laroche, L. Predicted long-term outcome of corneal transplantation. Ophthalmology 2009, 116, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Käsmann-Kellner, B.; Weindler, J.; Pfau, B.; Ruprecht, K. Ocular Changes in Mucopolysaccharidosis IV A (Morquio A Syndrome) and Long-Term Results of Perforating Keratoplasty. Ophthalmology 1999, 213, 200–205. [Google Scholar] [CrossRef]
- Bergwerk, K.; Falk, R.E.; Glasgow, B.J.; Rabinowitz, Y.S. Corneal transplantation in a patient with mucopolysaccharidosis type VII (Sly disease). Ophthalmic Genet. 2000, 21, 17–20. [Google Scholar] [CrossRef]
- Naumann, G.O.; Rummelt, V. Clearing of the para-transplant host cornea after perforating keratoplasty in Maroteaux-Lamy syndrome (type VI-A mucopolysaccharidosis). Klin. Mon. Fur. Augenheilkd. 1993, 203, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, G.; Raber, I.; Yanoff, M.; Haskins, M. Reciprocal corneal transplantation fails to correct mucopolysaccharidosis VI corneal storage. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2702–2713. [Google Scholar]
- Nanavaty, M.A.; Vijjan, K.S.; Yvon, C. Deep anterior lamellar keratoplasty: A surgeon’s guide. J. Curr. Ophthalmol. 2018, 30, 297–310. [Google Scholar] [CrossRef]
- Christo, C.G.; Van Rooij, J.; Geerards, A.J.; Remeijer, L.; Beekhuis, W.H. Suture-related Complications Following Keratoplasty. Cornea 2001, 20, 816–819. [Google Scholar] [CrossRef]
- Tandon, R.; Singh, R.; Gupta, N.; Vanathi, M. Corneal transplantation in the modern era. Indian J. Med Res. 2019, 150, 7–22. [Google Scholar] [CrossRef]
- Al-Mahmood, A.M.; Al-Swailem, S.A.; Edward, D. Glaucoma and Corneal Transplant Procedures. J. Ophthalmol. 2012, 2012, 1–9. [Google Scholar] [CrossRef][Green Version]
- Chua, A.; Chua, M.J.; Kam, P. Recent advances and anaesthetic considerations in corneal transplantation. Anaesth. Intensive Care 2018, 46, 162–170. [Google Scholar] [CrossRef]
- Reinhart, W.J.; Musch, D.; Jacobs, D.; Lee, W.B.; Kaufman, S.C.; Shtein, R. Deep Anterior Lamellar Keratoplasty as an Alternative to Penetrating Keratoplasty: A Report by the American Academy of Ophthalmology. Ophthalmology 2011, 118, 209–218. [Google Scholar] [CrossRef]
- Abdelaal, A.M.; Alqassimi, A.H.; Malak, M.; Hijazi, H.T.; Hadrawi, M.; Khan, M.A. Indications of Keratoplasty and Outcomes of Deep Anterior Lamellar Keratoplasty Compared to Penetrating Keratoplasty. Cureus 2021, 13, e13825. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska-Bil, D.; Czarnota-Nowakowska, B.; Krysik, K.; Lyssek-Boroń, A.; Dobrowolski, D.; Grabarek, B.; Wylęgała, E. Comparison of Long-Term Outcomes of the Lamellar and Penetrating Keratoplasty Approaches in Patients with Keratoconus. J. Clin. Med. 2021, 10, 2421. [Google Scholar] [CrossRef] [PubMed]
- Espandar, L.; Carlson, A.N. Lamellar Keratoplasty: A Literature Review. J. Ophthalmol. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Hos, D.; Matthaei, M.; Bock, F.; Maruyama, K.; Notara, M.; Clahsen, T.; Hou, Y.; Le, V.N.H.; Salabarria, A.-C.; Horstmann, J.; et al. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog. Retin. Eye Res. 2019, 73, 100768. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Bursztyn, L.L.; Golesic, E.; Mather, R.; Tingey, D.P. Comparison of intraocular pressure post penetrating keratoplasty vs Descemet’s stripping endothelial keratoplasty. Can. J. Ophthalmol. 2016, 51, 19–24. [Google Scholar] [CrossRef] [PubMed]
- De Macedo, J.P.; de Oliveira, L.A.; Hirai, F.; De Sousa, L.B. Femtosecond laser-assisted deep anterior lamellar keratoplasty in phototherapeutic keratectomy versus the big-bubble technique in keratoconus. Int. J. Ophthalmol. 2018, 11, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.A.; Elalfy, M.S.; Said, D.G.; Dua, H.S. Deep anterior lamellar keratoplasty—Triple procedure: A useful clinical application of the pre-Descemet’s layer (Dua’s layer). Eye 2015, 29, 323–326. [Google Scholar] [CrossRef][Green Version]
- Ricardo, J.R.D.S.; Medhi, J.; Pineda, R. Indications for and Outcomes of Deep Anterior Lamellar Keratoplasty in Mucopolysaccharidoses. J. Pediatr. Ophthalmol. Strabismus 2013, 50, 376–381. [Google Scholar] [CrossRef]
- Jhanji, V.; Sharma, N.; Vajpayee, R.B. Intraoperative perforation of Descemet’s membrane during “big bubble” deep anterior lamellar keratoplasty. Int. Ophthalmol. 2010, 30, 291–295. [Google Scholar] [CrossRef]
- Karimian, F.; Feizi, S. Deep Anterior Lamellar Keratoplasty: Indications, Surgical Techniques and Complications. Middle East Afr. J. Ophthalmol. 2010, 17, 28–37. [Google Scholar] [CrossRef]
- Basak, S.K.; Basak, S. Complications and management in Descemet’s stripping endothelial keratoplasty: Analysis of consecutive 430 cases. Indian J. Ophthalmol. 2014, 62, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.L.; Tuft, S.J.; Dart, J.K. Patterns of Rejection after Deep Lamellar Keratoplasty. Ophthalmology 2006, 113, 556–560. [Google Scholar] [CrossRef]
- Perera, C.; Jhanji, V.; Lamoureux, E.; Pollock, G.; Favilla, I.; Vajpayee, R.B. Clinical presentation, risk factors and treatment outcomes of first allograft rejection after penetrating keratoplasty in early and late postoperative period. Eye 2012, 26, 711–717. [Google Scholar] [CrossRef]
- Tan, D.; Ang, M.; Arundhati, A.; Khor, W.-B. Development of Selective Lamellar Keratoplasty within an Asian Corneal Transplant Program: The Singapore Corneal Transplant Study (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2015, 113. [Google Scholar]
- Feizi, S.; Zare, M. Current Approaches for Management of Postpenetrating Keratoplasty Astigmatism. J. Ophthalmol. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Miyadera, K.; Conatser, L.; Llanga, T.A.; Carlin, K.; O’Donnell, P.; Bagel, J.; Song, L.; Kurtzberg, J.; Samulski, R.J.; Gilger, B.; et al. Intrastromal Gene Therapy Prevents and Reverses Advanced Corneal Clouding in a Canine Model of Mucopolysaccharidosis I. Mol. Ther. 2020, 28, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Kamata, Y.; Okuyama, T.; Kosuga, M.; O’Hira, A.; Kanaji, A.; Sasaki, K.; Yamada, M.; Azuma, N. Adenovirus-Mediated Gene Therapy for Corneal Clouding in Mice with Mucopolysaccharidosis Type VII. Mol. Ther. 2001, 4, 307–312. [Google Scholar] [CrossRef]
- Vance, M.; Llanga, T.; Bennett, W.; Woodard, K.; Murlidharan, G.; Chungfat, N.; Asokan, A.; Gilger, B.; Kurtzberg, J.; Samulski, R.J.; et al. AAV Gene Therapy for MPS1-associated Corneal Blindness. Sci. Rep. 2016, 6, 22131. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.L.K.; Thomas, B.J.; Wilkinson, A.S.; Fletcher, J.M.; Byers, S. Inhibition of Glycosaminoglycan Synthesis Using Rhodamine B in a Mouse Model of Mucopolysaccharidosis Type IIIA. Pediatr. Res. 2006, 60, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, M.; Castellucci, M.; Guarrasi, G.; Terrasi, M.; La Blasca, T.; Mule’, G. Retinal and choroidal vasculature changes associated with chronic kidney disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Jakóbkiewicz-Banecka, J.; Piotrowska, E.; Narajczyk, M.; Barańska, S.; Węgrzyn, G. Genistein-mediated inhibition of glycosaminoglycan synthesis, which corrects storage in cells of patients suffering from mucopolysaccharidoses, acts by influencing an epidermal growth factor-dependent pathway. J. Biomed. Sci. 2009, 16, 26. [Google Scholar] [CrossRef] [PubMed]
| Disease | Corneal Opacity | Retinopathy | Optic Nerve Abnormalities | Glaucoma |
|---|---|---|---|---|
| MPS IH Hurler | Very common, mild to severe | Moderate, thickened ELM, parafoveal thinning, parafoveal retinal folds, bulls eye retinopathy | Common, mild to moderate | Uncommon, mild |
| MPS IH/S Hurler–Scheie | Very common, mild to severe | Moderate, retinal pigment epithelial degeneration | Common, mild to moderate | Uncommon, mild |
| MPS IS Scheie | Very common, mild to severe | Moderate | Quite common | Uncommon, mild |
| MPS II Hunter | Rare | Moderate, Pigmented retinopathy | Moderate | Uncommon |
| MPS III Sanfilippo A-D | Not usually significant | Moderate to severe, with pigmentary retinal degeneration | Rare | Rare |
| MPS IV Morquio | Some cases, usually mild | Pigmentary retinopathy | Some cases reported | Some cases reported |
| MPS VI Maroteau x-Lamy | Very common, often severe | Very rare, pigmented retinopathy, parafoveal retinal folds | Common | Unknown frequency |
| MPS VII Sly | Mild to moderate, can be severe | Unknown frequency | Quite common | Unknown frequency |
| MPS IX Natowicz | Unknown frequency | Unknown frequency | Unknown frequency | Unknown frequency |
| MPS Type | Enzyme Defect | Glycosaminoglycan | Inheritance |
|---|---|---|---|
| MPS IH Hurler | α-L-Iduronidase | Dermatan sulphate, Heparin sulphate | AR |
| MPS IH/S Hurler–Scheie | α-L-Iduronidase | Dermatan sulphate, Heparin sulphate | AR |
| MPS IS Scheie | α-L-Iduronidase | Dermatan sulphate, Heparin sulphate | AR |
| MPS II Hunter | Iduronate-2-sulfatase | Dermatan sulphate, Heparin sulphate | X-linked |
| MPS IIIA Sanfilippo A | Heparan sulfamidase | Heparin sulphate | AR |
| MPS IIIB Sanfilippo B | N-Acetyl-α-D-glucosaminidase | Heparin sulphate | AR |
| MPS IIIC Sanfilippo C | Acetyl-CoA:αglucosaminidase N-acetyltransferase | Heparin sulphate | AR |
| MPS IIID Sanfilippo D | N-Acetylglucosamine-6-sulfatase | Heparin sulphate | AR |
| MPS IV Morquio | N-Acetylgalactosamine-6-sulfatase | Keratin sulphate | AR |
| MPS VI Maroteaux-Lamy | N-acetylgalactosamine-4-sulfatase | Dermatan sulphate, | AR |
| MPS VII Sly | β-D-Glucuronidase | Dermatan sulphate, Heparin sulphate, Chondroitin sulphate | AR |
| MPS IX Natowicz | Hyaluronidase | Chondroitin sulphate | AR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGrath, O.; Au, L.; Ashworth, J. Management of Corneal Clouding in Patients with Mucopolysaccharidosis. J. Clin. Med. 2021, 10, 3263. https://doi.org/10.3390/jcm10153263
McGrath O, Au L, Ashworth J. Management of Corneal Clouding in Patients with Mucopolysaccharidosis. Journal of Clinical Medicine. 2021; 10(15):3263. https://doi.org/10.3390/jcm10153263
Chicago/Turabian StyleMcGrath, Orlaith, Leon Au, and Jane Ashworth. 2021. "Management of Corneal Clouding in Patients with Mucopolysaccharidosis" Journal of Clinical Medicine 10, no. 15: 3263. https://doi.org/10.3390/jcm10153263
APA StyleMcGrath, O., Au, L., & Ashworth, J. (2021). Management of Corneal Clouding in Patients with Mucopolysaccharidosis. Journal of Clinical Medicine, 10(15), 3263. https://doi.org/10.3390/jcm10153263






