Forecasting Progressive Trends in Keratoconus by Means of a Time Delay Neural Network
Abstract
:1. Introduction
2. Materials and Methods
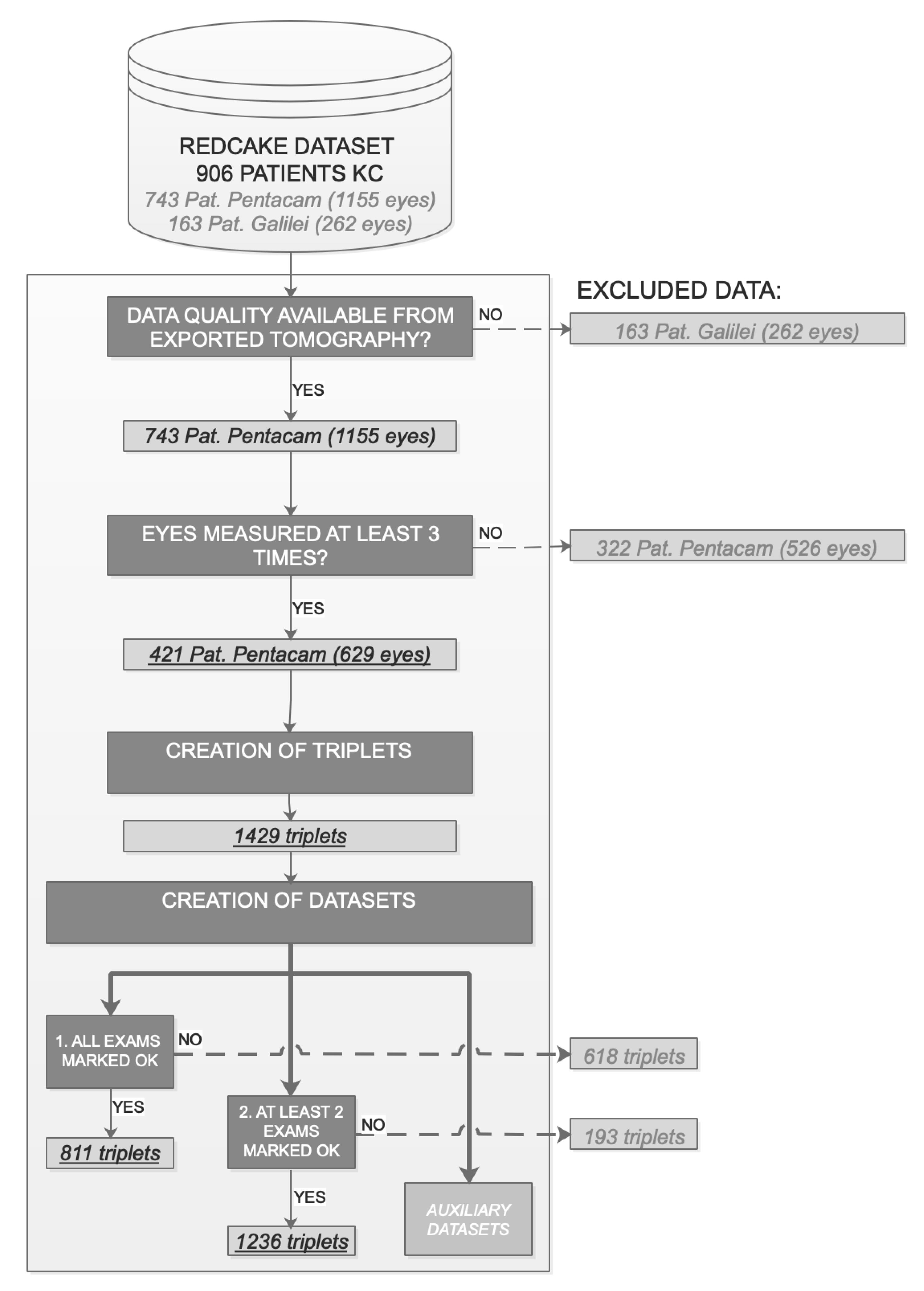
2.1. Patients
2.2. Corneal Parameters Included
2.3. Suspect Progressive KC Definition
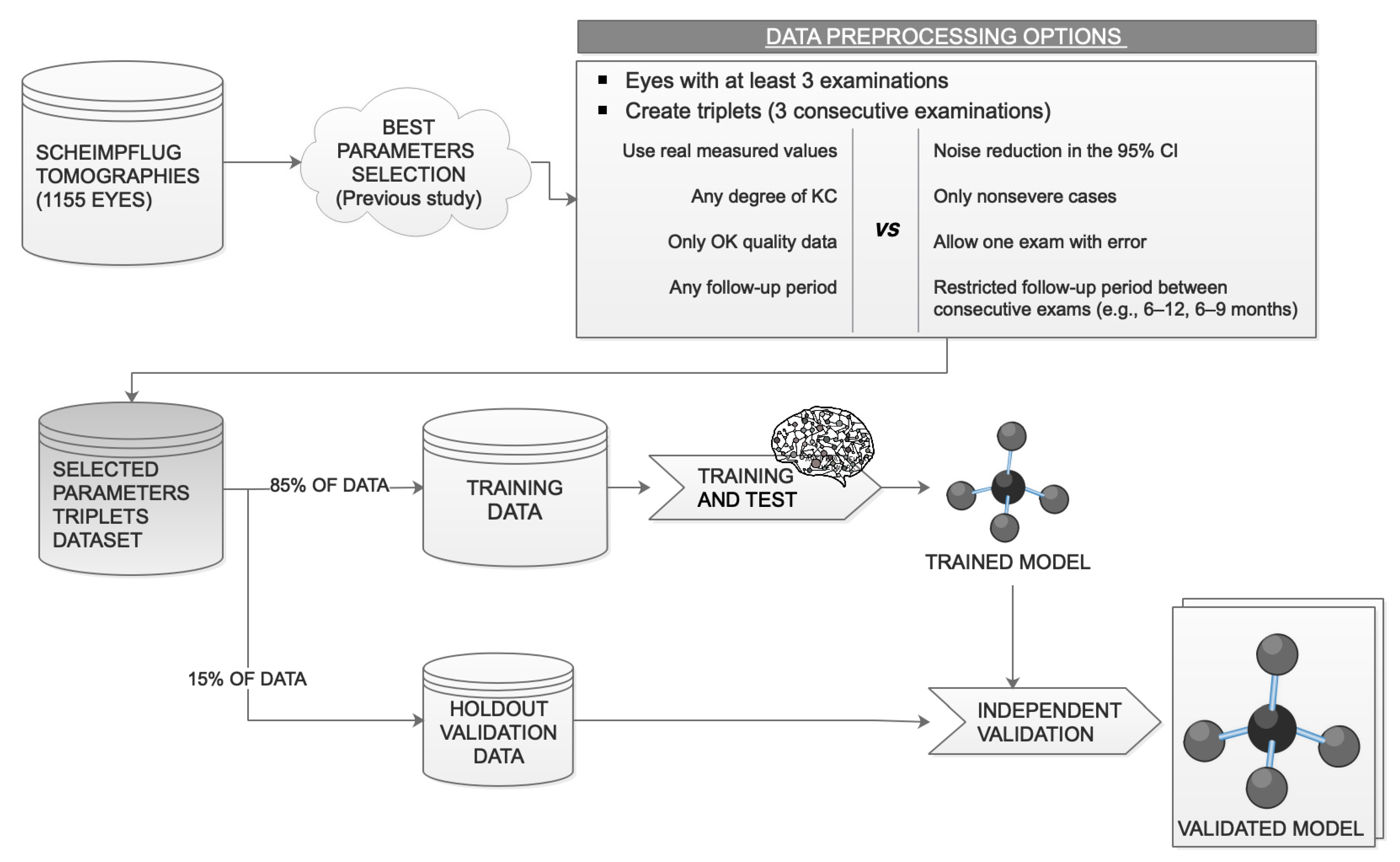
2.4. Data Preprocessing
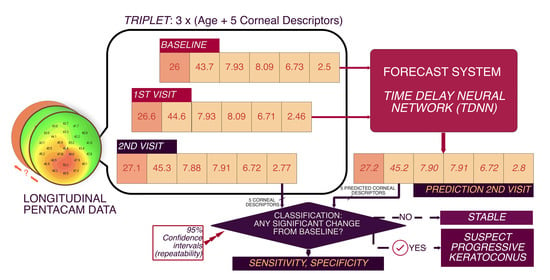
2.4.1. Data Maximization Design: Triplets
2.4.2. Data Quality and Robustness to Errors
2.4.3. Noise Reduction and Normalization
2.5. Network Architecture
2.6. Data Split: Training and Test Datasets
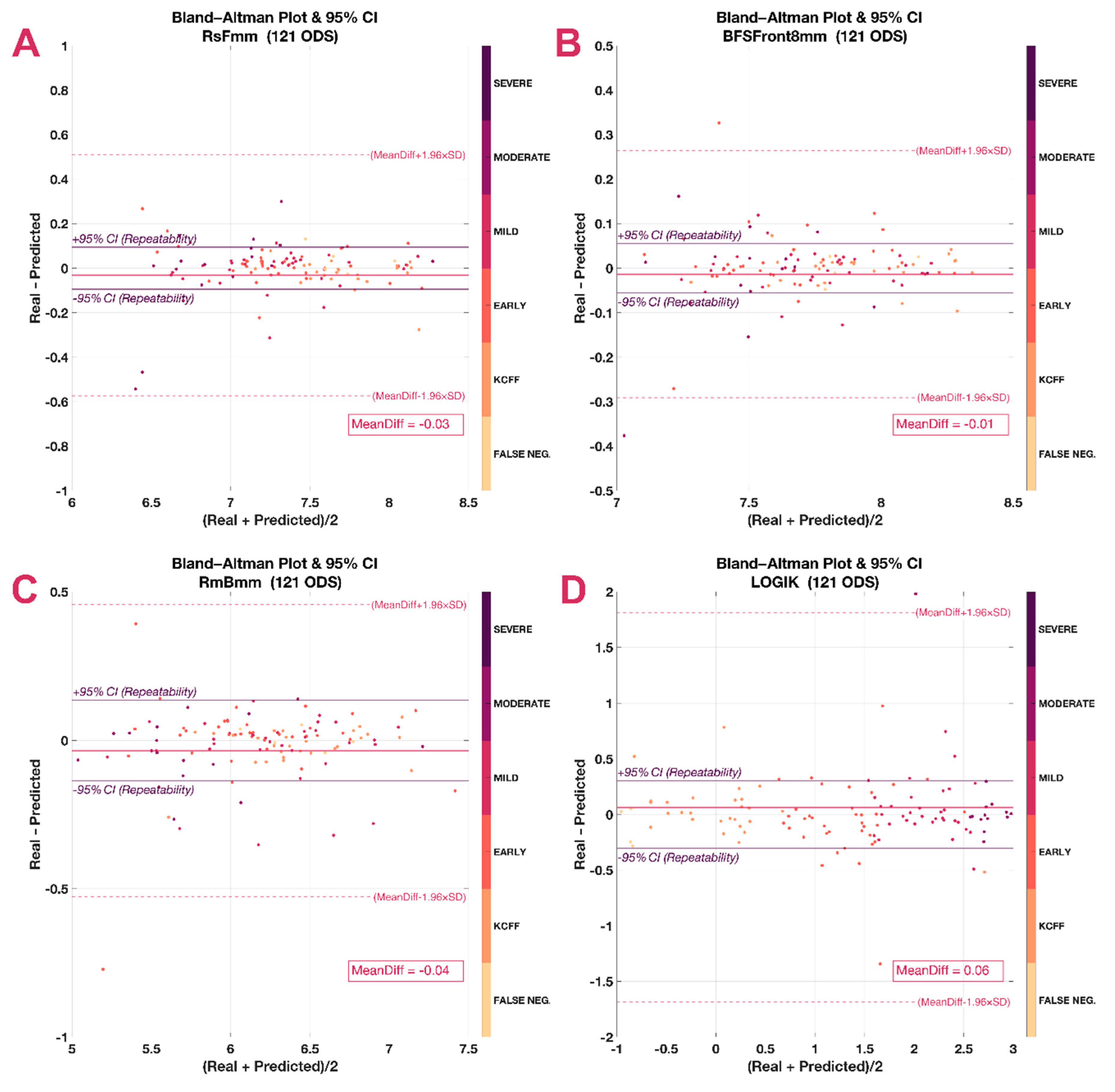
2.7. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
- EVICR.net European Vision Institute Clinical Research NetworkAIBILI, Azinhaga de Santa Comba, Celas, 3000-548 Coimbra, Portugal.
- Centre A—University Medical Center of Johannes Gutenberg–University (Germany)Katrin Lorenz, Katharina Bell, Anna Beck
- Centre B—Ghent University Hospital (Belgium)Bart Leroy, Elke Kreps
- Centre C—Antwerp University Hospital (Belgium)Jos Rozema, Marta Jiménez-García, Sorcha Ní Dhubhghaill, Ikram Issarti, Alejandra Consejo, Carina Koppen
- Centre D—Fondation Asile des Aveugles, Jules Gonin Eye Hospital (Switzerland)Kattayoon Hashemi
- Centre E—University Eye Clinic Maastricht (the Netherlands)Rudy MMA Nuijts, Magali Vandevenne, Frank JHM van den Biggelaar
- Centre F—Braga Hospital (Portugal)Tiago Monteiro, Rui Freitas
- Centre H—Justus Liebig University Giessen (Germany)Birgit Lorenz, Ekaterina Sokolenko, Francesco Luciani, Christine Mais
- Centre I—Tel Aviv Sourasky Medical Center (Israel)David Varssano, Eyal Cohen
Conflicts of Interest
References
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Flockerzi, E.; Daas, L.; Seitz, B. Structural changes in the corneal subbasal nerve plexus in keratoconus. Acta Ophthalmol. 2020, 98, 928–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-García, M.; Ní Dhubhghaill, S.; Consejo, A.; Hershko, S.; Koppen, C.; Rozema, J.J. Scheimpflug Densitometry in Keratoconus: A New Method of Visualizing the Cone. Cornea 2021, 40, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Moran, S.; Gomez, L.; Zuber, K.; Gatinel, D. A Case-Control Study of Keratoconus Risk Factors. Cornea 2020, 39, 697–701. [Google Scholar] [CrossRef]
- Roberts, C.J.; Dupps, W.J. Biomechanics of corneal ectasia and biomechanical treatments. J. Cataract Refract. Surg. 2014, 40, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Kreps, E.O.; Jimenez-Garcia, M.; Issarti, I.; Claerhout, I.; Koppen, C.; Rozema, J.J. Repeatability of the Pentacam HR in Various Grades of Keratoconus. Am. J. Ophthalmol. 2020, 219, 154–162. [Google Scholar] [CrossRef]
- Duncan, J.K.; Belin, M.W.; Borgstrom, M. Assessing progression of keratoconus: Novel tomographic determinants. Eye Vis. 2016, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Romano, V.; Vinciguerra, R.; Arbabi, E.M.; Hicks, N.; Rosetta, P.; Vinciguerra, P.; Kaye, S.B. Progression of Keratoconus in Patients While Awaiting Corneal Cross-linking: A Prospective Clinical Study. J. Refract. Surg. 2018, 34, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Rabinowitz, Y.S.; Rasheed, K.; Yang, H. Longitudinal study of the normal eyes in unilateral keratoconus patients. Ophthalmology 2004, 111, 440–446. [Google Scholar] [CrossRef]
- Vinciguerra, R.; Pagano, L.; Borgia, A.; Montericcio, A.; Legrottaglie, E.F.; Piscopo, R.; Rosetta, P.; Vinciguerra, P. Corneal Cross-linking for Progressive Keratoconus: Up to 13 Years of Follow-up. J. Refract. Surg. 2020, 36, 838–843. [Google Scholar] [CrossRef]
- Ferdi, A.C.; Nguyen, V.; Gore, D.M.; Allan, B.D.; Rozema, J.J.; Watson, S.L. Keratoconus Natural Progression. Ophthalmology 2019, 126, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, R.; Belin, M.W.; Borgia, A.; Piscopo, R.; Montericcio, A.; Confalonieri, F.; Legrottaglie, E.F.; Rosetta, P.; Vinciguerra, P. Evaluating keratoconus progression prior to crosslinking: Maximum keratometry vs the ABCD grading system. J. Cataract Refract. Surg. 2021, 47, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Klyce, S.D. Chasing the suspect: Keratoconus. Br. J. Ophthalmol. 2009, 93, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.; Krauthammer, M. Monitoring of Keratoconus Progression. In Controversies in the Management of Keratoconus; Barbara, A., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 29–38. ISBN 978-3-319-98031-7. [Google Scholar]
- Pagano, L.; Gadhvi, K.A.; Borroni, D.; Iselin, K.C.; Vinciguerra, R.; Tzamalis, A.; Kaye, S.B.; Romano, V. Bilateral Keratoconus Progression: Immediate Versus Delayed Sequential Bilateral Corneal Cross-linking. J. Refract. Surg. 2020, 36, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Klyce, S.D.; Smolek, M.K. Neural network classification of corneal topography. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1327–1335. [Google Scholar]
- Zéboulon, P.; Debellemanière, G.; Bouvet, M.; Gatinel, D. Corneal Topography Raw Data Classification Using a Convolutional Neural Network. Am. J. Ophthalmol. 2020, 219, 33–39. [Google Scholar] [CrossRef]
- Issarti, I.; Consejo, A.; Jiménez-García, M.; Kreps, E.O.; Koppen, C.; Rozema, J.J. Logistic index for keratoconus detection and severity scoring (Logik). Comput. Biol. Med. 2020, 122, 103809. [Google Scholar] [CrossRef]
- Issarti, I.; Consejo, A.; Jiménez-García, M.; Hershko, S.; Koppen, C.; Rozema, J.J. Computer aided diagnosis for suspect keratoconus detection. Comput. Biol. Med. 2019, 109, 33–42. [Google Scholar] [CrossRef]
- Lopes, B.T.; Ramos, I.C.; Salomão, M.Q.; Guerra, F.P.; Schallhorn, S.C.; Schallhorn, J.M.; Vinciguerra, R.; Vinciguerra, P.; Price, F.W.J.; Price, M.O.; et al. Enhanced Tomographic Assessment to Detect Corneal Ectasia Based on Artificial Intelligence. Am. J. Ophthalmol. 2018, 195, 223–232. [Google Scholar] [CrossRef]
- Kim, H.; Shin, K. A hybrid approach based on neural networks and genetic algorithms for detecting temporal patterns in stock markets. Appl. Soft Comput. 2007, 7, 569–576. [Google Scholar] [CrossRef]
- Ramkumar, S.; Kumar, K.S.; Emayavaramban, G. A feasibility study on eye movements using electrooculogram based HCI. Proc. Int. Conf. Intell. Sustain. Syst. ICISS 2017, 2018, 380–383. [Google Scholar] [CrossRef]
- Saad, A.; Gatinel, D. Evaluation of total and corneal wavefront high order aberrations for the detection of forme fruste keratoconus. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2978–2992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-García, M.; Ní Dhubhghaill, S.; Koppen, C.; Varssano, D.; Rozema, J.J. Baseline Findings in the Retrospective Digital Computer Analysis of Keratoconus Evolution (REDCAKE) Project. Cornea 2021, 40, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-García, M.; Kreps, E.O.; Ní Dhubhghaill, S.; Koppen, C.; Rozema, J.J. Determining the Most Suitable Tomography-Based Parameters to Describe Progression in Keratoconus. The Retrospective Digital Computer Analysis of Keratoconus Evolution Project. Eye Contact Lens Sci. Clin. Pract. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Rocha-de-Lossada, C.; Prieto-Godoy, M.; Sánchez-González, J.; Romano, V.; Borroni, D.; Rachwani-Anil, R.; Alba-Linero, C.; Peraza-Nieves, J.; Kaye, S.B.; Rodríguez-Calvo-de-Mora, M. Tomographic and aberrometric assessment of first-time diagnosed paediatric keratoconus based on age ranges: A multicentre study. Acta Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Asroui, L.; Mehanna, C.-J.; Salloum, A.; Chalhoub, R.M.; Roberts, C.J.; Awwad, S.T. Repeatability of Zone Averages Compared to Single Point Measurements of Maximal Curvature in Keratoconus. Am. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistics Notes: Measurement error. BMJ 1996, 313, 744. [Google Scholar] [CrossRef]
- Oh, C.; Han, S.; Jeong, J. Time-Series Data Augmentation based on Interpolation. Procedia Comput. Sci. 2020, 175, 64–71. [Google Scholar] [CrossRef]
- Gudivada, V.N.; Ding, J.; Apon, A. Data Quality Considerations for Big Data and Machine Learning: Going Beyond Data Cleaning and Transformations. Int. J. Adv. Softw. 2017, 10, 1–20. [Google Scholar]
- Liu, C.; Liu, Y.; Yang, B.; Wang, D.; Sun, J. A 2D multistage median filter to reduce random seismic noise. Geophysics 2006, 71. [Google Scholar] [CrossRef]
- Hersh, P.S.; Stulting, R.D.; Muller, D.; Durrie, D.S.; Rajpal, R.K.; Binder, P.S.; Donnenfeld, E.D.; Durrie, D.; Hardten, D.; Hersh, P.; et al. United States Multicenter Clinical Trial of Corneal Collagen Crosslinking for Keratoconus Treatment. Ophthalmology 2017, 124, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, F.; Kling, S.; Gilardoni, F.; Hafezi, N.; Hillen, M.; Abrishamchi, R.; Gomes, J.A.P.; Mazzotta, C.; Randleman, J.B.; Torres-Netto, E.A. Individualized Corneal Cross-linking With Riboflavin and UV-A in Ultrathin Corneas: The Sub400 Protocol. Am. J. Ophthalmol. 2021, 224, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Godefrooij, D.A.; Mangen, M.J.J.; Chan, E.; O’Brart, D.P.S.; Imhof, S.M.; de Wit, G.A.; Wisse, R.P.L. Cost-Effectiveness Analysis of Corneal Collagen Crosslinking for Progressive Keratoconus. Ophthalmology 2017, 124, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, R.L.; Berdahl, J.P.; Donnenfeld, E.D.; Thompson, V.; Kratochvil, D.; Wong, C.; Falvey, H.; Lytle, G.; Botteman, M.F.; Carter, J.A. Corneal cross-linking versus conventional management for keratoconus: A lifetime economic model. J. Med. Econ. 2020. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Giacomin, N.T.; Bueno, R.L.; Ghanem, R.C.; Moraes, H.V.; Santhiago, M.R. Accelerated corneal collagen crosslinking: Technique, efficacy, safety, and applications. J. Cataract Refract. Surg. 2016, 42, 1826–1835. [Google Scholar] [CrossRef]
- Ferdi, A.; Nguyen, V.; Kandel, H.; Tan, J.C.K.; Arnalich-Montiel, F.; Abbondanza, M.; Watson, S. Predictors of progression in untreated keratoconus: A Save Sight Keratoconus Registry study. Br. J. Ophthalmol. 2021, 1–6. [Google Scholar] [CrossRef]
- Goh, Y.W.; Gokul, A.; Yadegarfar, M.E.; Vellara, H.; Shew, W.; Patel, D.; McGhee, C.N.J.; Ziaei, M. Prospective Clinical Study of Keratoconus Progression in Patients Awaiting Corneal Cross-linking. Cornea 2020, 39, 1256–1260. [Google Scholar] [CrossRef]
- Wonneberger, W.; Sterner, B.; MacLean, U.; Claesson, M.; Zetterberg, M. Repeated Same-Day Versus Single Tomography Measurements of Keratoconic Eyes for Analysis of Disease Progression. Cornea 2018, 37, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Brunner, M.; Czanner, G.; Vinciguerra, R.; Romano, V.; Ahmad, S.; Batterbury, M.; Britten, C.; Willoughby, C.E.; Kaye, S.B. Improving precision for detecting change in the shape of the cornea in patients with keratoconus. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gomes, J.A.P.; Tan, D.; Rapuano, C.J.; Belin, M.W.; Ambrósio, R.; Guell, J.L.; Malecaze, F.; Nishida, K.; Sangwan, V.S. Global Consensus on Keratoconus and Ectatic Diseases. Cornea 2015, 34, 359–369. [Google Scholar] [CrossRef]
- Shajari, M.; Steinwender, G.; Herrmann, K.; Kubiak, K.B.; Pavlovic, I.; Plawetzki, E.; Schmack, I.; Kohnen, T. Evaluation of keratoconus progression. Br. J. Ophthalmol. 2019, 103, 551–557. [Google Scholar] [CrossRef]
- McAlinden, C.; Khadka, J.; Pesudovs, K. Statistical methods for conducting agreement (comparison of clinical tests) and precision (repeatability or reproducibility) studies in optometry and ophthalmology. Ophthalmic Physiol. Opt. 2011, 31, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.E.; Lin, S.C. Using a time delay neural network approach to diagnose the out-of-control signals for a multivariate normal process with variance shifts. Mathematics 2019, 7, 959. [Google Scholar] [CrossRef] [Green Version]
- Barnett, M.; Carrasquillo, K.G.; Schornack, M.M. Clinical outcomes of scleral lens fitting with a data-driven, quadrant-specific design: Multicenter review. Optom. Vis. Sci. 2020, 97, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulos, A.J.; Vingopoulos, F.; Sideri, A.M. Long-Term Stability with the Athens Protocol (Topography-Guided Partial PRK Combined with Cross-Linking) in Pediatric Patients with Keratoconus. Cornea 2019, 38, 1049–1057. [Google Scholar] [CrossRef] [PubMed]



| OPTION 1: Triplets without Error (n = 811; 121 Used for External Validation) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | AVG | |
| SENS | 66.0% | 77.5% | 70.4% | 74.1% | 69.6% | 75.6% | 66.0% | 60.0% | 82.6% | 66.0% | 70.8% |
| SPEC | 84.5% | 76.5% | 80.6% | 73.0% | 85.3% | 73.7% | 83.1% | 84.5% | 80.0% | 84.5% | 80.6% |
| PPV | 75.0% | 62.0% | 74.5% | 71.7% | 74.4% | 63.0% | 73.3% | 73.2% | 71.7% | 75.0% | 71.4% |
| NPV | 77.9% | 87.3% | 77.1% | 75.4% | 82.1% | 83.6% | 77.6% | 75.0% | 88.2% | 77.9% | 80.2% |
| OPTION 2: Triplets with 1 Error Allowed (n = 1236, 169 Used for External Validation) | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | AVG | |
| SENS | 70.1% | 69.6% | 70.6% | 66.7% | 68.1% | 75.0% | 77.3% | 71.6% | 77.8% | 73.4% | 72.0% |
| SPEC | 82.9% | 83.3% | 82.1% | 84.9% | 83.5% | 75.2% | 72.8% | 74.7% | 67.9% | 72.2% | 78.0% |
| PPV | 81.3% | 78.6% | 80.0% | 72.4% | 75.4% | 67.1% | 64.6% | 68.8% | 59.0% | 69.9% | 71.7% |
| NPV | 72.3% | 75.8% | 73.4% | 81.1% | 77.9% | 81.7% | 83.3% | 77.2% | 83.7% | 75.6% | 78.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-García, M.; Issarti, I.; Kreps, E.O.; Ní Dhubhghaill, S.; Koppen, C.; Varssano, D.; Rozema, J.J.; on behalf of The REDCAKE Study Group. Forecasting Progressive Trends in Keratoconus by Means of a Time Delay Neural Network. J. Clin. Med. 2021, 10, 3238. https://doi.org/10.3390/jcm10153238
Jiménez-García M, Issarti I, Kreps EO, Ní Dhubhghaill S, Koppen C, Varssano D, Rozema JJ, on behalf of The REDCAKE Study Group. Forecasting Progressive Trends in Keratoconus by Means of a Time Delay Neural Network. Journal of Clinical Medicine. 2021; 10(15):3238. https://doi.org/10.3390/jcm10153238
Chicago/Turabian StyleJiménez-García, Marta, Ikram Issarti, Elke O. Kreps, Sorcha Ní Dhubhghaill, Carina Koppen, David Varssano, Jos J. Rozema, and on behalf of The REDCAKE Study Group. 2021. "Forecasting Progressive Trends in Keratoconus by Means of a Time Delay Neural Network" Journal of Clinical Medicine 10, no. 15: 3238. https://doi.org/10.3390/jcm10153238
APA StyleJiménez-García, M., Issarti, I., Kreps, E. O., Ní Dhubhghaill, S., Koppen, C., Varssano, D., Rozema, J. J., & on behalf of The REDCAKE Study Group. (2021). Forecasting Progressive Trends in Keratoconus by Means of a Time Delay Neural Network. Journal of Clinical Medicine, 10(15), 3238. https://doi.org/10.3390/jcm10153238