The Value of Anterior Segment Optical Coherence Tomography in Different Types of Corneal Infections: An Update
Abstract
:1. Introduction
2. Corneal Infections
3. Anterior Segment Optical Coherence Tomography
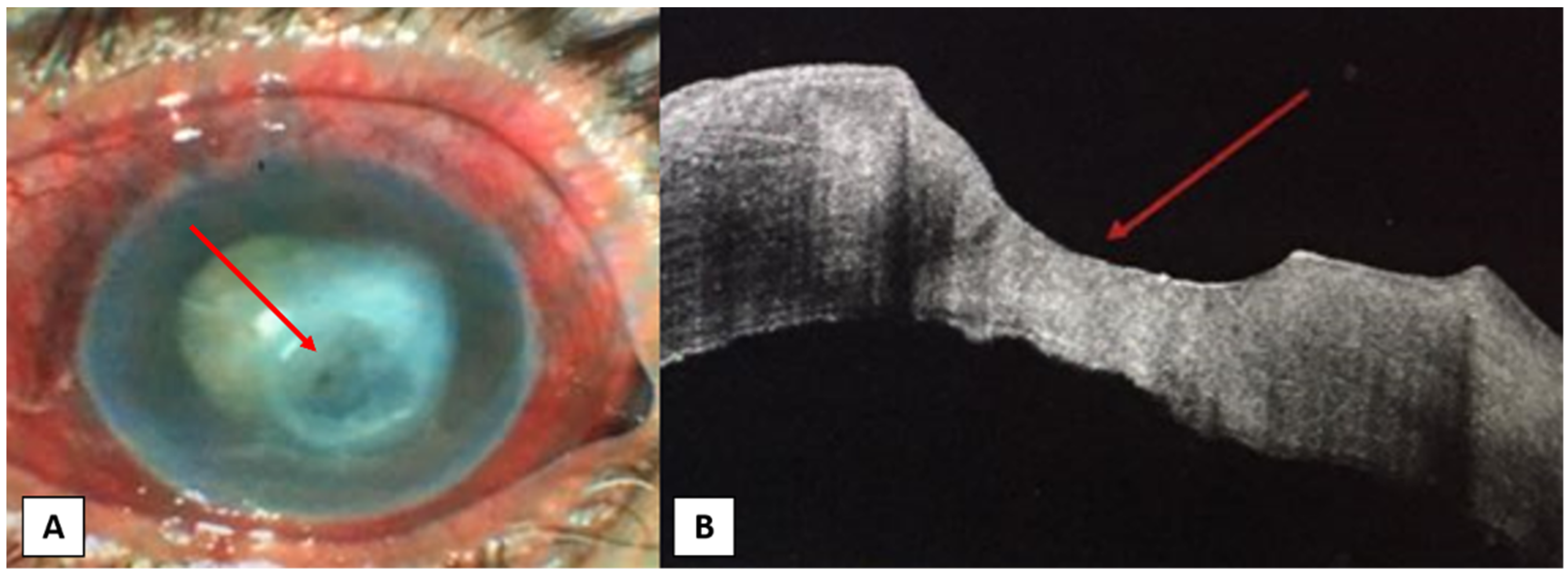
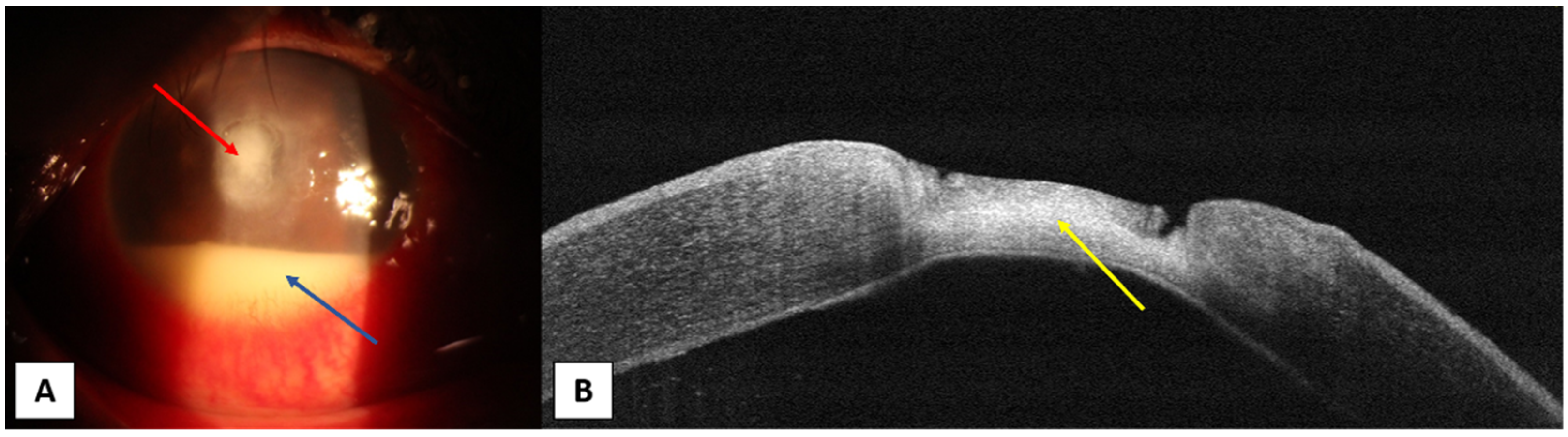
4. Anterior Segment OCT in Different Corneal Infections
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Resnikoff, S.; Pascolini, D.; Etyaale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology. 2017, 124, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, F.; Bruttin, O.; Zografos, L.; Guex-Crosier, Y. Bacterial keratitis: A prospective clinical and microbiological study. Br. J. Ophthalmol. 2001, 85, 842–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hume, E.B.; Dajcs, J.J.; Moreau, J.M.; Sloop, G.D.; Willcox, M.D.; O’Callaghan, R.J. Staphylococcus corneal virulence in a new topical model of infection. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2904–2908. [Google Scholar] [PubMed]
- Bourcier, T.; Thomas, F.; Borderie, V.; Chaumeil, C.; Laroche, L. Bacterial keratitis: Predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 2003, 87, 834–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shing Ong, H.S.; Corbett, M.C. Corneal infections in the 21st century. Postgrad. Med. J. 2015, 91, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Efron, N.; Morgan, P.B.; Hill, E.A.; Raynor, M.K.; Tullo, A.B. The size, location and clinical severity of corneal infiltrative events associated with contact lens wear. Optom. Vis. Sci. 2005, 82, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, A.; Kuo, J.; Anderson, D.F.; Hossain, P.N. Assessment of the use of anterior segment optical coherence tomography in microbial keratitis. Am. J. Ophthalmol. 2008, 146, 534–542. [Google Scholar] [CrossRef]
- Shah, A.; Sachdev, A.; Coggon, D.; Hossain, P. Geographic variations in microbial keratitis: An analysis of the peer-reviewed literature. Br. J. Ophthalmol. 2011, 95, 762–767. [Google Scholar] [CrossRef]
- Van der Meulen, I.J.; van Rooij, J.; Nieuwendaal, C.P.; Cleijnenbreugel, H.V.; Geerards, A.J.; Remeijer, L. Age-related risk factors, culture outcomes, and prognosis in patients admitted with infectious keratitis to two Dutch tertiary referral centers. Cornea 2008, 27, 539–544. [Google Scholar] [CrossRef]
- Dart, J.K.; Radford, C.; Minassian, D.; Verma, S.; Stapleton, F. Risk factors for microbial keratitis with contemporary contact lenses. Ophthalmology 2008, 115, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Keay, L.; Edwards, K.; Naduvilath, T.; Taylor, H.R.; Snibson, G.R.; Forde, K.; Stapleton, F. Microbial keratitis predisposing factors and morbidity. Ophthalmology 2006, 113, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Loh, R.S.; Chan, C.M.; Ti, S.E.; Lim, L.; Chan, K.S.; Tan, D.T. Emerging prevalence of microsporidial keratitis in Singapore: Epidemiology, clinical features, and management. Ophthalmology 2009, 116, 2348–2353. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sharma, S.; Sahu, S.K.; Nayak, S.S.; Kar, S. Diagnosis, clinical features and treatment outcome of microsporidial keratoconjunctivitis. Br. J. Ophthalmol. 2012, 96, 793–795. [Google Scholar] [CrossRef]
- Dart, J.K.; Saw, V.P.; Kilvington, S. Acanthamoeba keratitis: Diagnosis and treatment update 2009. Am. J. Ophthalmol. 2009, 148, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, M.M. The slit lamp: Applications for biomicroscopy and videography. In History of the Slit Lamp; Gellrich, M.-M., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 189–195. [Google Scholar]
- Allan, D.S.; Dart, J.K.G. Strategies for the management of microbial keratitis. Br. J. Ophthalmol. 1995, 79, 777–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleinen, K.G.; Mohalhal, A.A.; Elmekawy, H.E.; Abdulbaki, A.M.; Sherif, A.M.; El-Sherif, R.H.; Rahman, E.M.A. Polymerase chain reaction-guided diagnosis of infective keratitis—A hospital-based study. Curr. Eye Res. 2012, 37, 1005–1011. [Google Scholar] [CrossRef]
- Wang, S.B.; Cornish, E.E.; Grigg, J.R.; McCluskey, P.J. Anterior segment optical coherence tomography and its clinical applications. Clin. Exp. Optom. 2019, 102, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, M.; Baskaran, M.; Werkmeister René, M.; Chua, J.; Schmidl, D.; Aranha dos Santos, V.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior segment optical coherence tomography. Prog. Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; del Barrio, J.L.A. Atlas of Anterior Segment Optical Coherence Tomography. Essentials in Ophthalmology; Springer: Berlin, Germany, 2021; pp. 31–158. [Google Scholar] [CrossRef]
- Han, S.B.; Liu, Y.C.; Noriega, K.M.; Mehta, J.S. Applications of anterior segment optical coherence tomography in cornea and ocular surface diseases. J. Ophthalmol. 2016, 2016, 4971572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, J.; DeBoer, C.; Xu, B.Y. Anterior Segment Optical Coherence Tomography: Applications for Clinical Care and Scientific Research. Asia Pac. J. Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Ramos, J.L.B.; Li, Y.; Huang, D. Clinical and research applications of anterior segment optical coherence tomography—A review. Clin. Exp. Ophthalmol. 2009, 37, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bald, M.; Li, Y.; Huang, D. Anterior chamber angle evaluation with fourier-domain optical coherence tomography. J. Ophthalmol. 2012, 2012, 103704. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Abou Shousha, M.; Perez, V.L.; Karp, C.L.; Yoo, S.H.; Shen, M.; Cui, L.; Hurmeric, V.; Du, C.; Zhu, D.; et al. Ultra-high resolution optical coherence tomography for imaging the anterior segment of the eye. Ophthalmic Surg Lasers Imaging 2011, 42, S15–S27. [Google Scholar] [CrossRef] [Green Version]
- Shousha, M.A.; Perez, V.L.; Wang, J.; Ide, T.; Jiao, S.; Chen, Q.; Chang, V.; Buchser, N.; Dubovy, S.R.; Feuer, W.; et al. Use of ultrahigh- resolution optical coherence tomography to detect in vivo characteristics of Descemet’s membrane in Fuchs’ dystrophy. Ophthalmology 2010, 117, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.J.; Galor, A.; Nanji, A.A.; Sayyad, F.E.; Wang, J.; Dubovy, S.R.; Joag, M.G.; Karp, C.L. Ultra highresolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul. Surf. 2014, 12, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Abdelghany, A.; D’Oria, F.; Alio, J.L. Surgery of glaucoma in modern corneal graft procedures. Surv. Ophthalm. 2021, 66, 276–289. [Google Scholar] [CrossRef]
- Del Barrio, J.L.A.; D’Oria, F.; Alio, J.L. Visian Implantable Collamer Lens Behavior in Descemet’s Membrane Endothelial Keratoplasty Surgery. Cornea 2021, 40, 113–115. [Google Scholar] [CrossRef]
- D’Oria, F.; Alio, J.L.; Rodriguez, A.E.; Amesty, M.A.; Abu-Mustafa, S.K. Cosmetic Keratopigmentation in Sighted Eyes: Medium and Long-Term Clinical Evaluation. Cornea 2021, 40, 327–333. [Google Scholar] [CrossRef]
- Konstantopoulos, A.; Yadegarfar, G.; Fievez, M.; Anderson, D.F.; Hossain, P. In vivo quantification of bacterial keratitis with optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1093–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, W.; Fathalla, A.M.; El-Sebaity, D.M.; Al-Hussaini, A.K. Spectral domain anterior segment optical coherence tomography in microbial keratitis. Graefes. Arch. Clin. Exp. Ophthalmol. 2013, 251, 549–553. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, F.; Galeone, A.; Pastore, V.; Cardascia, N.; Alessio, G. Multi-drug resistant Enterococcus faecium in late-onset keratitis after deep anterior lamellar keratoplasty: A case report and review of the literature. Medicine 2019, 98, e17140. [Google Scholar] [CrossRef]
- Takezawa, Y.; Suzuki, T.; Shiraishi, A. Observation of Retrocorneal Plaques in Patients with Infectious Keratitis Using Anterior Segment Optical Coherence Tomography. Cornea 2017, 36, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Abbouda, A.; Estrada, A.V.; Rodriguez, A.E.; Alio, J.L. Anterior segment optical coherence tomography in evaluation of severe fungal keratitis infections treated by corneal crosslinking. Eur. J. Ophthalmol. 2014, 24, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Kobayashi, A.; Yokogawa, H.; Ishibashi, Y.; Oikawa, Y.; Tokoro, M.; Sugiyama, K. In vivo imaging of radial keratoneuritis in patients with Acanthamoeba keratitis by anterior-segment optical coherence tomography. Ophthalmology 2014, 121, 2153–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.M.; Lee, J.S.; Yoo, J.M.; Park, J.M.; Seo, S.W.; Chung, I.Y.; Kim, S.J. Comparison of anterior segment optical coherence tomography findings in acanthamoeba keratitis and herpetic epithelial keratitis. Int. J. Ophthalmol. 2018, 11, 1416–1420. [Google Scholar]
- Soliman, W.; Nassr, M.A.; Abdelazeem, K.; Al-Hussaini, A.K. Appearance of herpes simplex keratitis on anterior segment optical coherence tomography. Int. Ophthalmol. 2019, 39, 2923–2928. [Google Scholar] [CrossRef]
- Hixson, A.; Blanc, S.; Sowka, J. Monitoring keratitis resolution with optical coherence tomography. Optom. Vis. Sci. 2014, 91, S40–S45. [Google Scholar] [CrossRef]
- Yokogawa, H.; Kobayashi, A.; Yamazaki, N.; Sugiyama, K. In vivo imaging of coin-shaped lesions in cytomegalovirus corneal endotheliitis by anterior segment optical coherence tomography. Cornea 2014, 33, 1332–1335. [Google Scholar] [CrossRef]
- Kobayashi, R.; Hashida, N.; Soma, T.; Koh, S.; Miki, A.; Usui, S.; Maeda, N.; Nishida, K. Clinical Findings of Anterior Segment Spectral Domain Optical Coherence Tomography Images in Cytomegalovirus Corneal Endotheliitis. Cornea 2017, 36, 411–414. [Google Scholar] [CrossRef]
- Sridhar, M.S.; Shaik, B. Anterior segment optical coherence tomography of microsporidial keratoconjunctivitis. Indian J. Ophthalmol. 2018, 66, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Thanathanee, O.; Laohapitakvorn, S.; Anutarapongpan, O.; Suwan-Apichon, O.; Bhoomibunchoo, C. Anterior Segment Optical Coherence Tomography Images in Microsporidial Keratoconjunctivitis. Cornea 2019, 38, 943–947. [Google Scholar] [CrossRef]
- Ramier, A.; Eltony, A.M.; Chen, Y.; Clouser, F.; Birkenfeld, J.S.; Watts, A.; Yun, S.H. In vivo measurement of shear modulus of the human cornea using optical coherence elastography. Sci. Rep. 2020, 10, 17366. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.R.; Dupps, W.J.; Rollins, A.M.; Roy, A.S.; Hu, Z. Method for optical coherence elastography of the cornea. J. Biomed. Opt. 2011, 16, 016005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Stefano, V.S.; Ford, M.R.; Seven, I.; Dupps, W.J. Live human assessment of depth-dependent corneal displacements with swept-source optical coherence elastography. PLoS ONE 2018, 13, e0209480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Li, J.; Singh, M.; Wu, C.; Liu, C.-H.; Raghunathan, R.; Aglyamov, S.R.; Vantipalli, S.; Twa, M.; Larin, K.V. Optical coherence elastography assessment of corneal viscoelasticity with a modified Rayleigh-Lamb wave model. J. Mech. Behav. Biomed. Mater 2017, 66, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Singh, M.; Aglyamov, S.; Emelianov, S.; Twa, M.D.; Larin, K.V. Air-pulse OCE for assessment of age-related changes in mouse cornea in vivo. Laser Phys. Lett. 2014, 11, 065601. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelghany, A.A.; D’Oria, F.; Alio Del Barrio, J.; Alio, J.L. The Value of Anterior Segment Optical Coherence Tomography in Different Types of Corneal Infections: An Update. J. Clin. Med. 2021, 10, 2841. https://doi.org/10.3390/jcm10132841
Abdelghany AA, D’Oria F, Alio Del Barrio J, Alio JL. The Value of Anterior Segment Optical Coherence Tomography in Different Types of Corneal Infections: An Update. Journal of Clinical Medicine. 2021; 10(13):2841. https://doi.org/10.3390/jcm10132841
Chicago/Turabian StyleAbdelghany, Ahmed A., Francesco D’Oria, Jorge Alio Del Barrio, and Jorge L. Alio. 2021. "The Value of Anterior Segment Optical Coherence Tomography in Different Types of Corneal Infections: An Update" Journal of Clinical Medicine 10, no. 13: 2841. https://doi.org/10.3390/jcm10132841
APA StyleAbdelghany, A. A., D’Oria, F., Alio Del Barrio, J., & Alio, J. L. (2021). The Value of Anterior Segment Optical Coherence Tomography in Different Types of Corneal Infections: An Update. Journal of Clinical Medicine, 10(13), 2841. https://doi.org/10.3390/jcm10132841








