Impact of Early Pancreatic Graft Loss on Outcome after Simultaneous Pancreas–Kidney Transplantation (SPKT)—A Landmark Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Acquisition
2.2. Immunosuppression
2.3. Surgical Technique
2.4. Anticoagulation Procedures
2.5. Definitions
2.6. Data Analysis
3. Results
3.1. Characteristics of Recipients and Donors
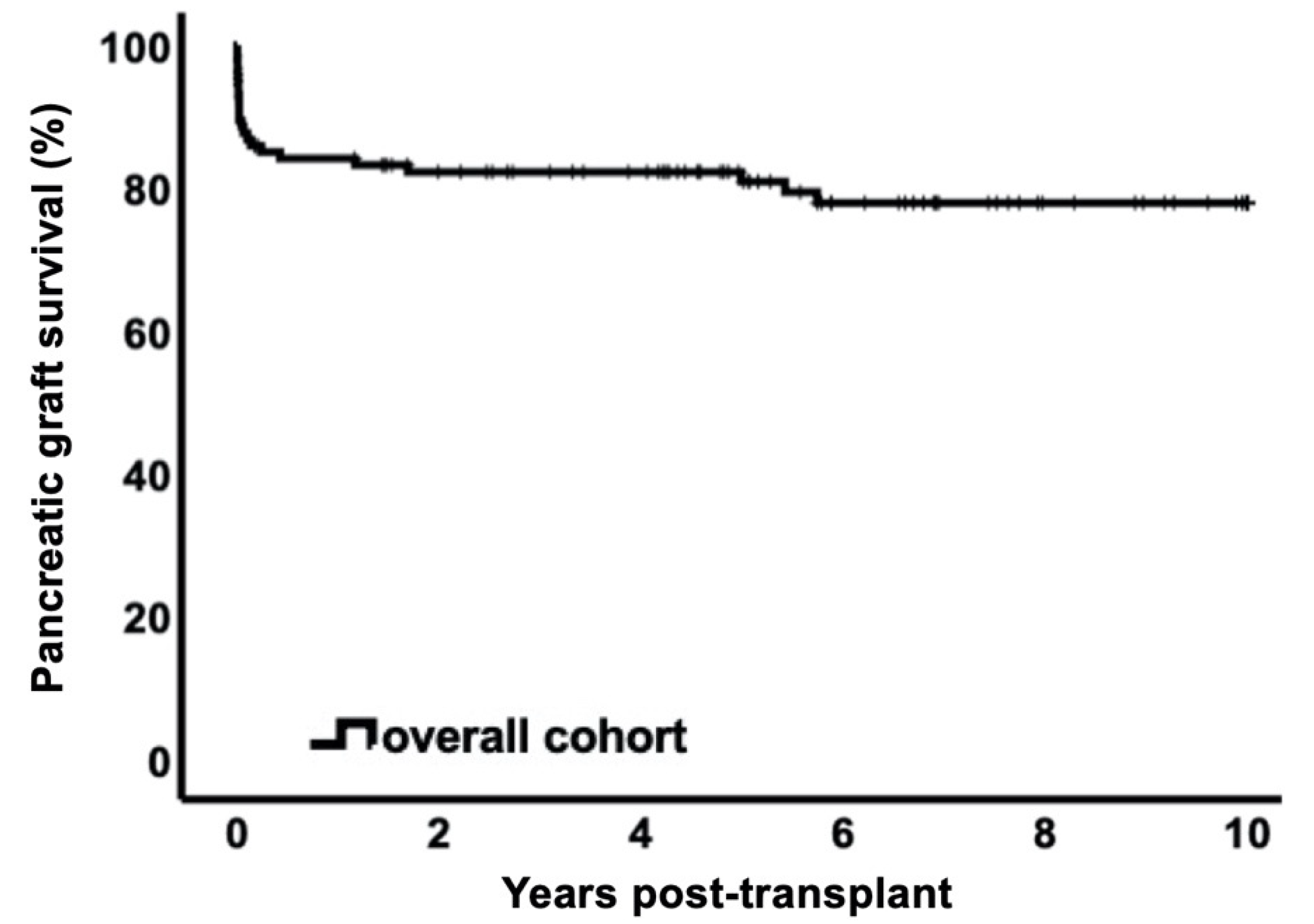
3.2. Pancreas Graft Survival
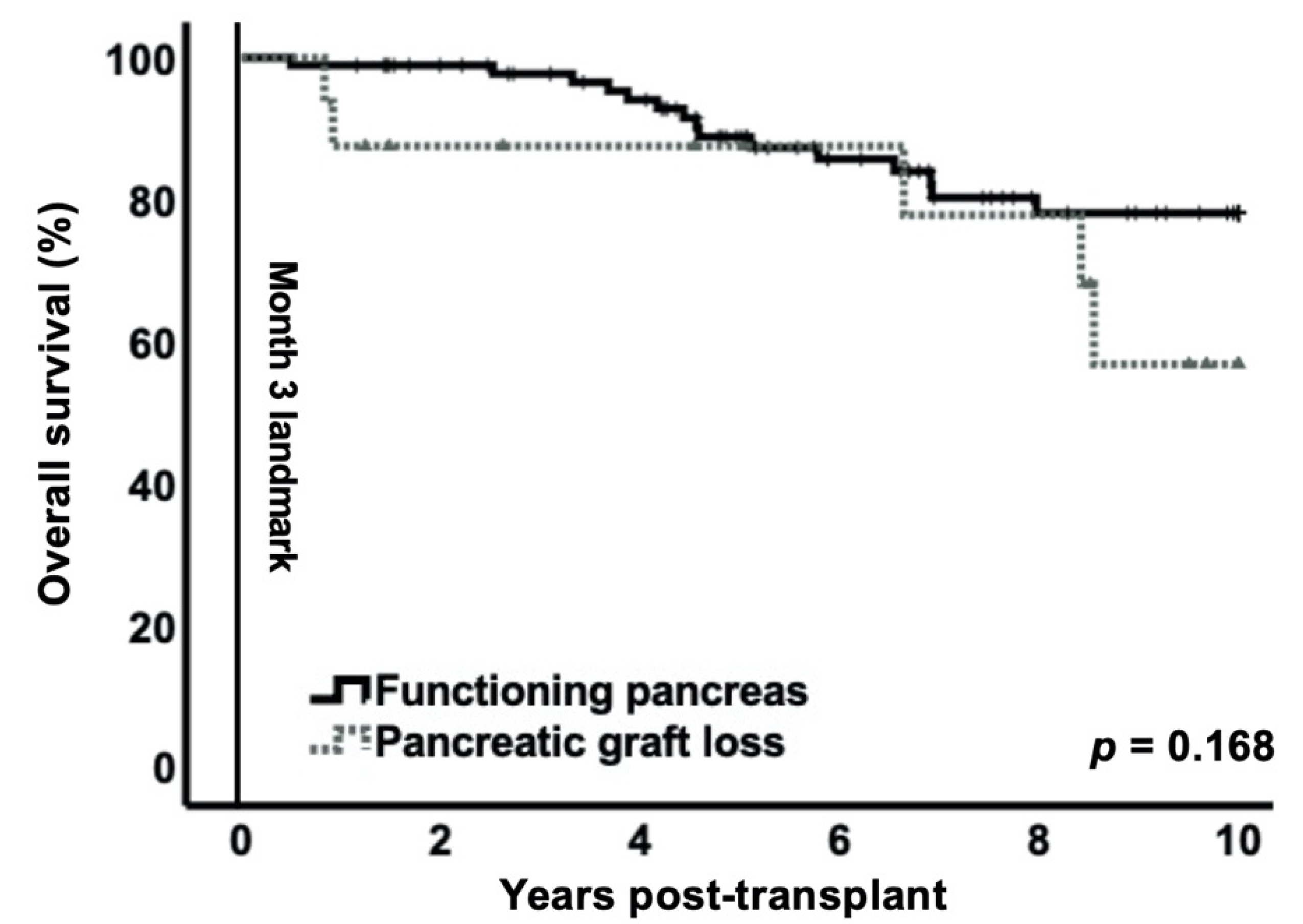
3.3. Impact of Early Pancreas Graft Loss on Patient Survival
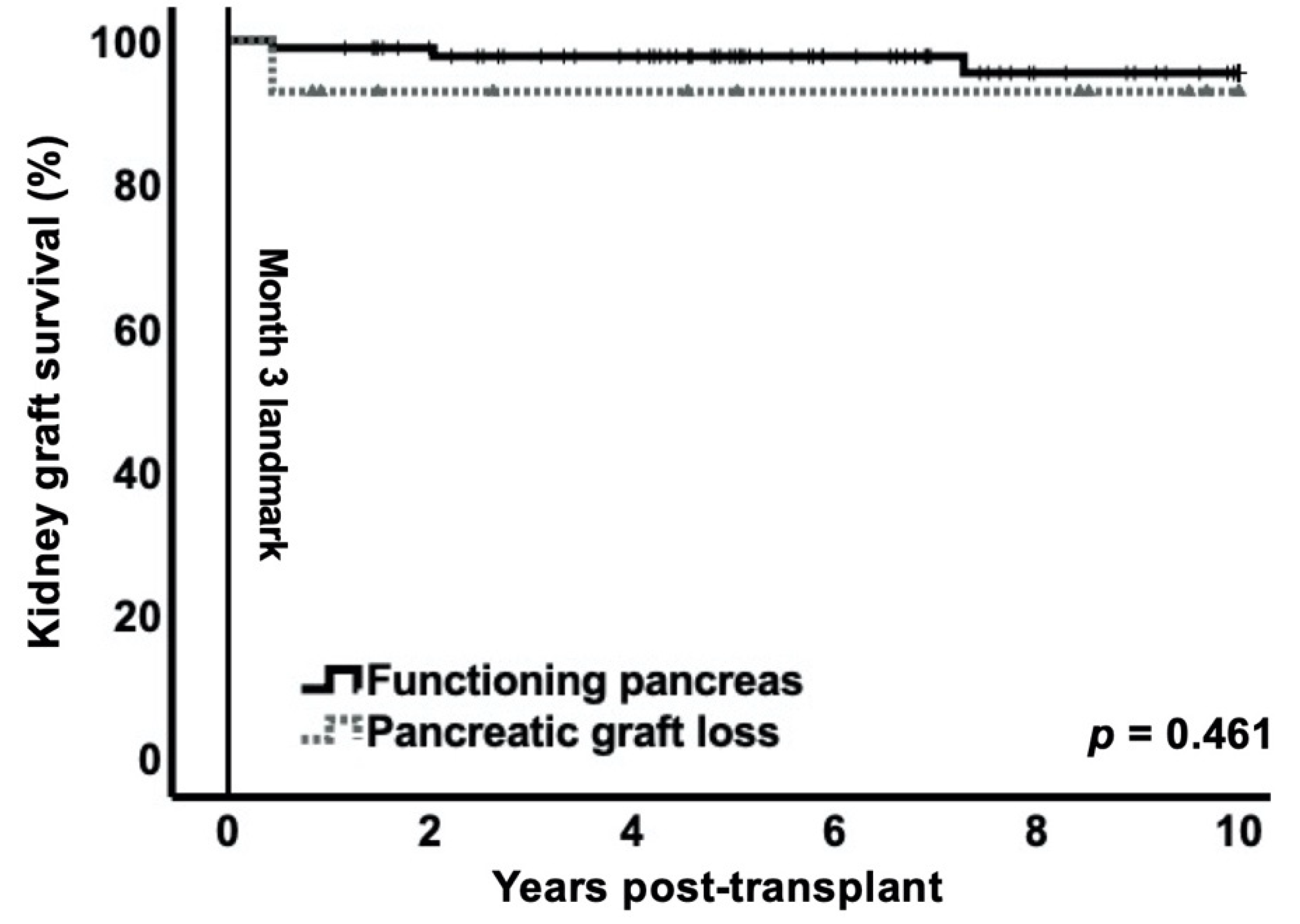
3.4. Impact of Early Pancreas Graft Loss on Kidney Graft Survival
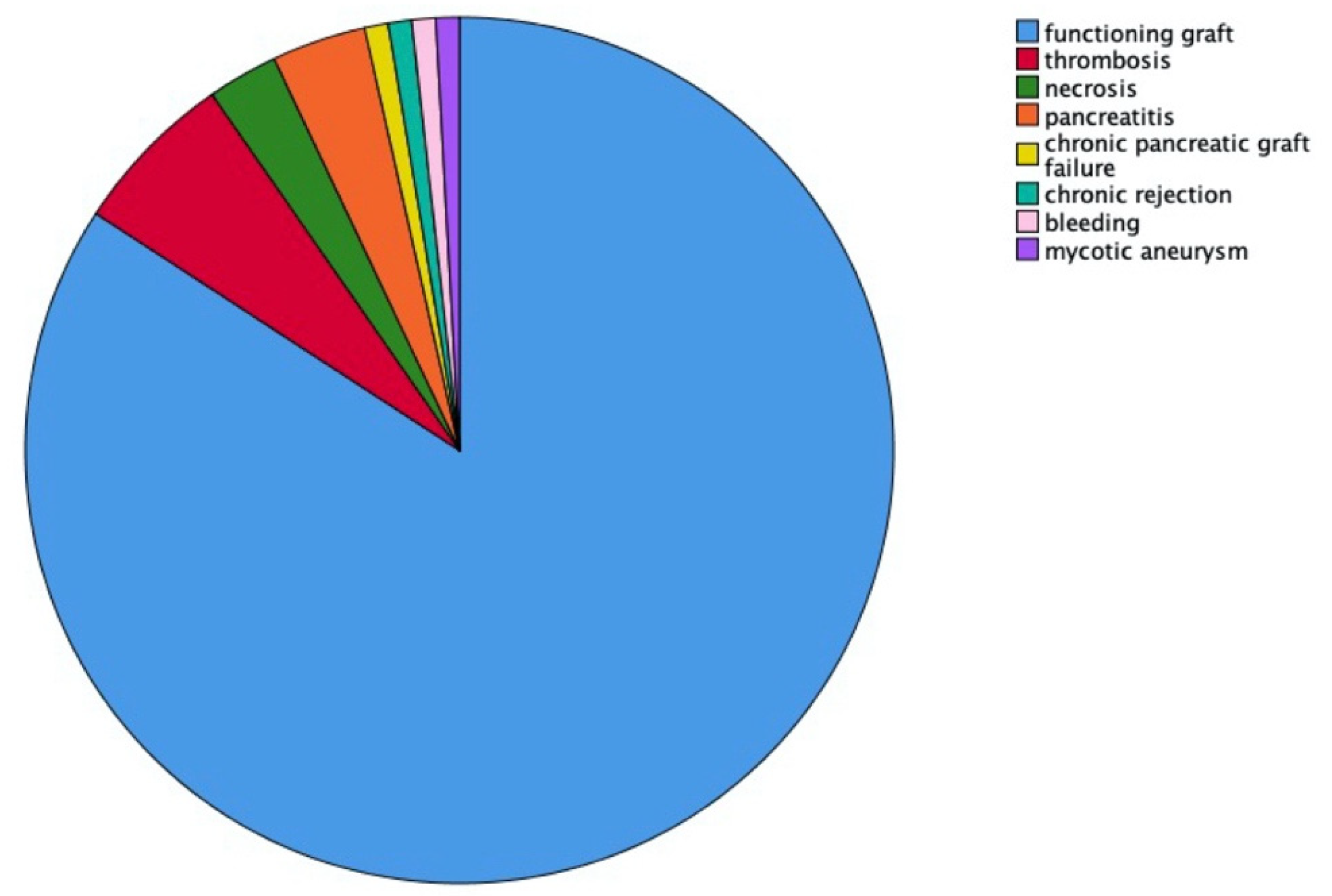
3.5. Impact of Early Pancreas Graft Loss on Other Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kandaswamy, R.; Stock, P.G.; Gustafson, S.K.; Skeans, M.A.; Urban, R.; Fox, A.; Odorico, J.S.; Israni, A.K.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2017 Annual Data Report: Pancreas. Am. J. Transplant. 2019, 19, 124–183. [Google Scholar] [CrossRef]
- White, S.A.; Shaw, J.A.; Sutherland, D.E. Pancreas transplantation. Lancet 2009, 373, 1808–1817. [Google Scholar] [CrossRef]
- Gross, C.R.; Limwattananon, C.; Matthees, B.; Zehrer, J.L.; Savik, K. Impact of transplantation on quality of life in patients with diabetes and renal dysfunction. Transplantation 2000, 70, 1736–1746. [Google Scholar] [CrossRef]
- Rajkumar, T.; Mazid, S.; Vucak-Dzumhur, M.; Sykes, T.M.; Elder, G.J. Health-related quality of life following kidney and simultaneous pancreas kidney transplantation. Nephrology 2019, 24, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Marmanillo, C.G.; Langaro, C.; Nicoluzzi, J.E.; Belila, R.T.; Macri, M.; Zamprogna, R.; Luvizotto, M.; Takahashi, M. Renopancreatic Transplantation: Evaluation of 15 Years in 131 Patients. Transplant. Proc. 2018, 50, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Morath, C.; Zeier, M.; Dohler, B.; Schmidt, J.; Nawroth, P.P.; Opelz, G. Metabolic control improves long-term renal allograft and patient survival in type 1 diabetes. J. Am. Soc. Nephrol. 2008, 19, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Smets, Y.F.; Westendorp, R.G.; van der Pijl, J.W.; de Charro, F.T.; Ringers, J.; de Fijter, J.W.; Lemkes, H.H. Effect of simultaneous pancreas-kidney transplantation on mortality of patients with type-1 diabetes mellitus and end-stage renal failure. Lancet 1999, 353, 1915–1919. [Google Scholar] [CrossRef]
- Esmeijer, K.; Hoogeveen, E.K.; van den Boog, P.J.M.; Konijn, C.; Mallat, M.J.K.; Baranski, A.G.; Dekkers, O.M.; de Fijter, J.W.; Dutch Transplant Centers; Dutch Kidney Transplant Centres. Superior Long-term Survival for Simultaneous Pancreas-Kidney Transplantation as Renal Replacement Therapy: 30-Year Follow-up of a Nationwide Cohort. Diabetes Care 2020, 43, 321–328. [Google Scholar] [CrossRef]
- Heilman, R.L.; Mazur, M.J.; Reddy, K.S. Immunosuppression in simultaneous pancreas-kidney transplantation: Progress to date. Drugs 2010, 70, 793–804. [Google Scholar] [CrossRef]
- Humar, A.; Kandaswamy, R.; Granger, D.; Gruessner, R.W.; Gruessner, A.C.; Sutherland, D.E. Decreased surgical risks of pancreas transplantation in the modern era. Ann. Surg. 2000, 231, 269–275. [Google Scholar] [CrossRef]
- Gruessner, R.W.; Gruessner, A.C. The current state of pancreas transplantation. Nat. Rev. Endocrinol 2013, 9, 555–562. [Google Scholar] [CrossRef]
- Redfield, R.R.; Scalea, J.R.; Odorico, J.S. Simultaneous pancreas and kidney transplantation: Current trends and future directions. Curr. Opin. Organ. Transplant. 2015, 20, 94–102. [Google Scholar] [CrossRef]
- de Kort, H.; Mallat, M.J.; van Kooten, C.; de Heer, E.; Brand-Schaaf, S.H.; van der Wal, A.M.; Roufosse, C.; Roelen, D.L.; Bruijn, J.A.; Claas, F.H.; et al. Diagnosis of early pancreas graft failure via antibody-mediated rejection: Single-center experience with 256 pancreas transplantations. Am. J. Transplant. 2014, 14, 936–942. [Google Scholar] [CrossRef]
- Humar, A.; Ramcharan, T.; Kandaswamy, R.; Gruessner, R.W.; Gruessner, A.C.; Sutherland, D.E. Technical failures after pancreas transplants: Why grafts fail and the risk factors—A multivariate analysis. Transplantation 2004, 78, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Viglietti, D.; Serrato, T.; Abboud, I.; Antoine, C.; Pillebout, E.; Busson, M.; Desgrandchamps, F.; Meria, P.; Godin, M.; Hurault de Ligny, B.; et al. Kidney graft dysfunction in simultaneous pancreas-kidney recipients after pancreas failure: Analysis of early and late protocol biopsies. Clin. Transplant. 2013, 27, E249–E255. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.; Garcia, R.; Dunn, T.; Kandaswamy, R.; Sutherland, D.E.; Humar, A. What happens to the kidney in an SPK transplant when the pancreas fails due to a technical complication? Clin. Transplant. 2008, 22, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Norman, S.P.; Kommareddi, M.; Ojo, A.O.; Luan, F.L. Early pancreas graft failure is associated with inferior late clinical outcomes after simultaneous kidney-pancreas transplantation. Transplantation 2011, 92, 796–801. [Google Scholar] [CrossRef]
- Ojo, A.O.; Meier-Kriesche, H.U.; Hanson, J.A.; Leichtman, A.; Magee, J.C.; Cibrik, D.; Wolfe, R.A.; Port, F.K.; Agodoa, L.; Kaufman, D.B.; et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation 2001, 71, 82–90. [Google Scholar] [CrossRef]
- Das, D.M.; Huskey, J.L.; Harbell, J.W.; Heilman, R.L.; Singer, A.L.; Mathur, A.; Neville, M.R.; Morgan, P.; Reddy, K.S.; Jadlowiec, C.C. Early technical pancreas failure in Simultaneous Pancreas-Kidney Recipients does not impact renal allograft outcomes. Clin. Transplant. 2021, 35, e14138. [Google Scholar] [CrossRef] [PubMed]
- Gleiss, A.; Oberbauer, R.; Heinze, G. An unjustified benefit: Immortal time bias in the analysis of time-dependent events. Transplant. Int. 2018, 31, 125–130. [Google Scholar] [CrossRef]
- Foundation, E.I. ET Pancreas Allocation System (EPAS). Available online: https://www.eurotransplant.org/wp-content/uploads/2020/01/H7-EPAS.pdf (accessed on 15 July 2021).
- Schmidt, D.; Osmanodja, B.; Pfefferkorn, M.; Graf, V.; Raschke, D.; Duettmann, W.; Naik, M.G.; Gethmann, C.J.; Mayrdorfer, M.; Halleck, F.; et al. TBase-an Integrated Electronic Health Record and Research Database for Kidney Transplant Recipients. J. Vis. Exp. 2021. [Google Scholar] [CrossRef] [PubMed]
- El-Zoghby, Z.M.; Stegall, M.D.; Lager, D.J.; Kremers, W.K.; Amer, H.; Gloor, J.M.; Cosio, F.G. Identifying specific causes of kidney allograft loss. Am. J. Transplant. 2009, 9, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Sis, B.; Racusen, L.C.; Solez, K.; Glotz, D.; Colvin, R.B.; Castro, M.C.; David, D.S.; David-Neto, E.; Bagnasco, S.M.; et al. Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am. J. Transplant. 2014, 14, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://optn.transplant.hrsa.gov/media/3420/kdri-to-kdpi-mapping-table-2017.pdf (accessed on 21 July 2021).
- A Guide to Calculating and Interpreting the Kidney Donor Profile Index (KDPI). Available online: https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf (accessed on 21 July 2021).
- Axelrod, D.A.; Sung, R.S.; Meyer, K.H.; Wolfe, R.A.; Kaufman, D.B. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am. J. Transplant. 2010, 10, 837–845. [Google Scholar] [CrossRef]
- Drachenberg, C.B.; Torrealba, J.R.; Nankivell, B.J.; Rangel, E.B.; Bajema, I.M.; Kim, D.U.; Arend, L.; Bracamonte, E.R.; Bromberg, J.S.; Bruijn, J.A.; et al. Guidelines for the diagnosis of antibody-mediated rejection in pancreas allografts-updated Banff grading schema. Am. J. Transplant. 2011, 11, 1792–1802. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Dafni, U. Landmark analysis at the 25-year landmark point. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 363–371. [Google Scholar] [CrossRef]
- Sousa, M.G.; Linhares, M.M.; Salzedas-Netto, A.A.; Gonzalez, A.M.; Rangel, E.B.; Sa, J.R.; Araujo, S.G.; Melaragno, C.S.; Lopes-Filho, G.; Medina Pestana, O.J. Risk factors of pancreatic graft loss and death of receptor after simultaneous pancreas/kidney transplantation. Transplant. Proc. 2014, 46, 1827–1835. [Google Scholar] [CrossRef]
- Gruessner, A.C.; Sutherland, D.E. Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004. Clin. Transplant. 2005, 19, 433–455. [Google Scholar] [CrossRef]
- McCullough, K.P.; Keith, D.S.; Meyer, K.H.; Stock, P.G.; Brayman, K.L.; Leichtman, A.B. Kidney and pancreas transplantation in the United States, 1998-2007: Access for patients with diabetes and end-stage renal disease. Am. J. Transplant. 2009, 9, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; Rimmele, T.; Ber, C.E.; Christin, F.; Badet, L.; Morelon, E.; Ecochard, R.; Allaouchiche, B. Early relaparotomy after simultaneous pancreas-kidney transplantation. Transplantation 2012, 94, 159–164. [Google Scholar] [CrossRef]
- Farney, A.C.; Rogers, J.; Stratta, R.J. Pancreas graft thrombosis: Causes, prevention, diagnosis, and intervention. Curr. Opin. Organ. Transplant. 2012, 17, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Shahrestani, S.; Lendzion, R.; Pleass, H.J.; Hawthorne, W.J. Risk Factors for Early Pancreatic Allograft Thrombosis Following Simultaneous Pancreas-Kidney Transplantation: A Systematic Review. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620942589. [Google Scholar] [CrossRef]
- Malaise, J.; Arbogast, H.; Illner, W.D.; Tarabichi, A.; Dieterle, C.; Landgraf, R.; Land, W.; Van Ophem, D.; Squifflet, J.P.; Grp, E.S. Simultaneous pancreas-kidney transplantation: Analysis of rejection. Transplant. Proc. 2005, 37, 2856–2858. [Google Scholar] [CrossRef] [PubMed]
- Schachtner, T.; Zaks, M.; Otto, N.M.; Kahl, A.; Reinke, P. Factors and outcomes in association with sepsis differ between simultaneous pancreas/kidney and single kidney transplant recipients. Transplant. Infect. Dis. 2018, 20, e12848. [Google Scholar] [CrossRef]
- Kalil, A.C.; Opal, S.M. Sepsis in the severely immunocompromised patient. Curr. Infect. Dis. Rep. 2015, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Syed, A.; Rupp, M.E.; Chambers, H.; Vargas, L.; Maskin, A.; Miles, C.D.; Langnas, A.; Florescu, D.F. Is bacteremic sepsis associated with higher mortality in transplant recipients than in nontransplant patients? A matched case-control propensity-adjusted study. Clin. Infect. Dis. 2015, 60, 216–222. [Google Scholar] [CrossRef]
- Morath, C.; Schmied, B.; Mehrabi, A.; Weitz, J.; Schmidt, J.; Werner, J.; Buchler, M.W.; Morcos, M.; Nawroth, P.P.; Schwenger, V.; et al. Simultaneous pancreas-kidney transplantation in type 1 diabetes. Clin. Transplant. 2009, 23, 115–120. [Google Scholar] [CrossRef]
- La Rocca, E.; Fiorina, P.; di Carlo, V.; Astorri, E.; Rossetti, C.; Lucignani, G.; Fazio, F.; Giudici, D.; Cristallo, M.; Bianchi, G.; et al. Cardiovascular outcomes after kidney-pancreas and kidney-alone transplantation. Kidney Int. 2001, 60, 1964–1971. [Google Scholar] [CrossRef][Green Version]
- Gruessner, A.C.; Gruessner, R.W. Declining numbers of pancreas transplantations but significant improvements in outcome. Transplant. Proc. 2014, 46, 1936–1937. [Google Scholar] [CrossRef]
- Light, J.; Tucker, M. Simultaneous pancreas kidney transplants in diabetic patients with end-stage renal disease: The 20-yr experience. Clin. Transplant. 2013, 27, E256–E263. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.P.; Mangus, R.S.; Powelson, J.A.; Samy, K.P.; Taber, T.E.; Goble, M.L.; Fridell, J.A. Impact of recipient age on whole organ pancreas transplantation. Clin. Transplant. 2013, 27, E49–E55. [Google Scholar] [CrossRef] [PubMed]
- Montagud-Marrahi, E.; Molina-Andujar, A.; Pane, A.; Ramirez-Bajo, M.J.; Amor, A.; Esmatjes, E.; Ferrer, J.; Musquera, M.; Diekmann, F.; Ventura-Aguiar, P. Outcomes of pancreas transplantation in older diabetic patients. BMJ Open Diabetes Res. Care 2020, 8, e000916. [Google Scholar] [CrossRef] [PubMed]
| Variables | Functioning Pancreatic Graft | Early Pancreatic Graft Loss within 3 Months | p |
|---|---|---|---|
| Patients, n | 97 (85.1%) | 17 (14.9%) | |
| Recipient characteristics | |||
| Mean recipient age, years (SD) | 44.4 (8.9) | 44.7 (9.4) | 0.931 |
| Male, n (%) | 58 (59.8%) | 12 (70.6%) | 0.435 |
| Kidney retransplantation, n (%) | 6 (6.2%) | 0 (0%) | 0.589 |
| Pancreas retransplantation, n (%) | 3 (3.1%) | 0 (0%) | 0.462 |
| Median time on dialysis, months (IQR) | 38 (26–53) | 28 (19–46) | 0.188 |
| Dialysis use (hemodialysis), n (%) | 67 (69.1%) | 16 (94.1%) | 0.032 |
| Dialysis use (peritoneal dialysis), n (%) | 21 (21.6%) | 0 (0%) | 0.034 |
| Preemptive transplantation, n (%) | 9 (9.3%) | 1 (5.9%) | 0.651 |
| Mean HLA mismatches (SD) | 4.5 (1.3) | 4.4 (0.9) | 0.875 |
| Median Cold ischemia time (kidney), hours (IQR) | 11 (9.5–12.5) | 11 (9.2–12.3) | 0.589 |
| Median Cold ischemia time (pancreas), hours (IQR) | 9 (7–10.7) | 8 (7–10.7) | 0.336 |
| Body mass index (BMI), kg/m2 (IQR) | 23.4 (21.4–26) | 23.4 (20.6–26) | 0.706 |
| Diabetes Type I (%) | 97 (100%) | 17 (100%) | - |
| Mean time since 1st diagnosis of diabetes, years (SD) | 29.1 (10.3) | 33.0 (9.4) | 0.151 |
| Coronary artery disease, n (%) | 24 (24.7%) | 4 (23.5%) | 0.916 |
| Arterial hypertension, n (%) | 96 (99%) | 16 (94.1%) | 0.163 |
| Prior Stroke, n (%) | 13 (13.4%) | 2 (11.8%) | 0.855 |
| Prior myocardial infarction (%) | 6 (6.2%) | 2 (11.8%) | 0.411 |
| HbA1c at transplantation, (%) (IQR) | 7.1 (6.1–8) | 7 (6.3–7.85) | 0.964 |
| Donor characteristics | |||
| Mean donor age, years (SD) | 32.5 (10.5) | 31.4 (10.2) | 0.681 |
| Donor BMI, kg/m2 (IQR) | 23.1 (21.7–24.7) | 23.4 (21.6–25.7) | 0.741 |
| Recipient–Donor BMI match index (IQR) | 1 (0.91–1.18) | 1.02 (0.88–1.14) | 0.58 |
| Donor creatinine at transplantation, (mg/dL) (IQR) | 0.70 (0.59–0.86) | 0.71 (0.64–0.84) | 0.386 |
| Kidney Donor Risk Index (KDRI) 2017 (IQR) | 0.94 (0.82–1.10) | 0.87 (0.79–1.15) | 0.706 |
| Kidney Donor Profile Index (KDPI) 2017 (IQR) | 23 (10–40) | 16 (7–45) | 0.717 |
| Mean Pancreas Donor Risk Index (SD) | 1.03 (0.26) | 1.02 (0.2) | 0.841 |
| Immunosuppressive Medication | Functioning Pancreatic Graft | Early Pancreatic Graft Loss within 3 Months | p |
|---|---|---|---|
| Tacrolimus | 97 (100%) | 17 (100%) | - |
| Mycophenolate | 97 (100%) | 17 (100%) | - |
| Steroids | 97 (100%) | 17 (100%) | - |
| Anti-thymoglobulin (ATG) | 92 (94.8%) | 16 (94.1%) | 0.524 |
| Basiliximab | 5 (5.2%) | 1 (5.9%) | 0.784 |
| Variables | Functioning Pancreatic Graft n | Early Pancreas Graft Loss within 3 Months n |
|---|---|---|
| Graft thrombosis | 1 | 1 |
| Acute rejection (BANFF IIb) | 0 | 1 |
| Chronic transplant glomerulopathy | 1 | 0 |
| Pyelonephritis | 1 | 0 |
| Infected hematoma | 0 | 1 |
| Septic shock | 1 | 0 |
| Unknown | 1 | 0 |
| Primary non-function | 1 | 2 |
| Variables | Functioning Pancreatic Graft | Early Pancreas Graft Loss within 3 Months | p |
|---|---|---|---|
| Patients, n | 97 (85.1%) | 17 (14.9%) | |
| Delayed graft function | 19 (19.6%) | 5 (29.4%) | 0.364 |
| Kidney biopsy-proven acute rejection (≥BANFF Ia) | 12 (12.4%) | 2 (11.8%) | 0.945 |
| Mean estimated glomerular filtration rate at 1 year post-transplant by CKD-EPI formula censored for death and kidney graft failure (SD) | 68.8 (19.2) | 64.9 (15.7) | 0.494 |
| Imputed eGFR (GFR = 0) at 1 year post-transplant for patients with kidney graft loss by CKD-EPI formula censored for death (SD) | 68.1 (20.4) | 59.9 (23.4) | 0.184 |
| Median HbA1c at 1 year post-transplant censored for death | 5.4 (5.1–5.6) | 8.1 (6.9–8.6) | <0.001 |
| Major adverse cardiac or cerebrovascular event (MACCE) at 10 years post-transplant, n | 8 (8.2%) | 1 (5.9%) | 0.741 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehner, L.J.; Öllinger, R.; Globke, B.; Naik, M.G.; Budde, K.; Pratschke, J.; Eckardt, K.-U.; Kahl, A.; Zhang, K.; Halleck, F. Impact of Early Pancreatic Graft Loss on Outcome after Simultaneous Pancreas–Kidney Transplantation (SPKT)—A Landmark Analysis. J. Clin. Med. 2021, 10, 3237. https://doi.org/10.3390/jcm10153237
Lehner LJ, Öllinger R, Globke B, Naik MG, Budde K, Pratschke J, Eckardt K-U, Kahl A, Zhang K, Halleck F. Impact of Early Pancreatic Graft Loss on Outcome after Simultaneous Pancreas–Kidney Transplantation (SPKT)—A Landmark Analysis. Journal of Clinical Medicine. 2021; 10(15):3237. https://doi.org/10.3390/jcm10153237
Chicago/Turabian StyleLehner, Lukas Johannes, Robert Öllinger, Brigitta Globke, Marcel G. Naik, Klemens Budde, Johann Pratschke, Kai-Uwe Eckardt, Andreas Kahl, Kun Zhang, and Fabian Halleck. 2021. "Impact of Early Pancreatic Graft Loss on Outcome after Simultaneous Pancreas–Kidney Transplantation (SPKT)—A Landmark Analysis" Journal of Clinical Medicine 10, no. 15: 3237. https://doi.org/10.3390/jcm10153237
APA StyleLehner, L. J., Öllinger, R., Globke, B., Naik, M. G., Budde, K., Pratschke, J., Eckardt, K.-U., Kahl, A., Zhang, K., & Halleck, F. (2021). Impact of Early Pancreatic Graft Loss on Outcome after Simultaneous Pancreas–Kidney Transplantation (SPKT)—A Landmark Analysis. Journal of Clinical Medicine, 10(15), 3237. https://doi.org/10.3390/jcm10153237







